Physiological and histological responses of Phascolosoma esculenta (Sipuncula: Phascolosomatidea) to acute heat stress*
Xinming GAO , Haiyan YANG , Daojun TANG , Chen DU , Shan JIN ,Congcong HOU , Chundan ZHANG , Junquan ZHU ,, Jianping WANG ,
1 Key Laboratory of Applied Marine Biotechnology by the Ministry of Education, School of Marine Sciences, Ningbo University,Ningbo 315211, China
2 Ningbo Academy of Oceanology and Fisheries, Ningbo 315012, China
Abstract Temperature is an important factor aff ecting the growth, development, and survival of organisms. The eff ects of temperature stress on aquatic organisms have received increasing attention, as these organisms are mostly poikilotherms and their body temperature are directly corresponding changes with ambient temperature, resulting in they are easily exposed in temperature stress. However, little is known about the eff ects of high temperature on Sipuncula. In this study, we investigated the eff ects of acute heat stress on malondialdehyde (MDA) concentration, the activities of antioxidant (superoxide dismutase and glutathione peroxidase) and immunity-related (acidic and alkaline phosphatase) enzymes, heat shock protein 70 ( hsp70) and hsp90 gene expression, and the histological structure of the sipunculid Phascolosoma esculenta. Within the coelom f luid, the MDA concentration and all detected antioxidant enzyme activities increased during high temperature stress; signif icant increases were also observed here and in the intestine in the hsp70 and hsp90 mRNA expression levels. These results indicated that acute heat stress caused oxidative stress; antioxidants and heat shock proteins probably act to protect P. esculenta against oxidative damage, constituting part of its physiological mechanism for adaptation to high temperatures. In addition,the increased activity of the acidic and alkaline phosphatases indicated eff ects on its nonspecif ic immune system. Furthermore, damaged tissue structures were observed in the body wall, retractor muscle, intestine,and nephridium after 96 h of 40-°C stress. The damaged cells of these tissues showed obvious condensed chromatin around the nuclear membrane. This histological damage suggests that heat stress could aff ect movement, food absorption, digestion, and excretion in P. esculenta. These results elucidate the eff ects of temperature stress on P. esculenta and its physiological response mechanisms and provide practical indicators for assessing heat stress status and determining suitable culture temperatures for P. esculenta.
Keyword: heat stress; oxidation stress; enzyme activity; heat shock proteins; nonspecif ic immunity;histological damage
1 INTRODUCTION
Temperature is an important factor aff ecting the physiology, energy expenditure, growth, development,and survival of organisms, especially aquatic organisms, which are mostly poikilotherms (Selong et al., 2001). In such organisms, water temperature f luctuations result in directly corresponding changes in body temperature, which result in they are easily exposed in temperature stress, and the eff ects of high temperature on these organisms have received increasing attention. To date, physiological responses and tissue structures are reported to be aff ected in aquatic organisms such as f ish (Liu et al., 2014; Yan et al., 2017), shrimp (Madeira et al., 2015), crabs (Meng et al., 2014), mollusks (An and Choi, 2010; Jiang et al., 2016), and echinodermata (Xu et al., 2015).
It has been reported that aquatic organisms exposed to heat stress overproduce endogenous reactive oxygen species (ROS; including O2ˉ, H2O2, and -OH;Meng et al., 2014; Cheng et al., 2015). Under normal physiological conditions, ROS are in dynamic equilibrium, their generation balanced by their elimination through antioxidant systems. However,under stress, including high temperature stress, this dynamic equilibrium fails as excess ROS is generated,causing oxidative stress. This is lethal for cells, as excess ROS can damage cell DNA, proteins, and lipids (Halliwell, 1999; Simon et al., 2000). Under these conditions, oxidized lipids decompose and f inally generate malondialdehyde (MDA; Farmer and Muelle, 2013; Jiang et al., 2016). MDA was therefore deemed a suitable biomarker for the assessment of cellular and histological oxidative damage and organism-level stress. The key physiological response is the increase in antioxidant activities, including O2ˉ removal by superoxide dismutase (SOD) and catalysis of H2O2reduction by glutathione peroxidase (GPX).These activities, at the appropriate level, eliminate excess ROS, protecting cells and preventing damage to cellular functions (Kashiwagi et al., 1997; Verlecar et al., 2007). The expression of heat shock proteins(HSPs) is also upregulated, protecting other newly synthesized proteins, restoring impaired proteins, and regulating the degradation of unrecoverable damaged proteins (Mosser et al., 1997; Kiang and Tsokos,1998; Chen et al., 2018). The levels of antioxidant activity andhspgene expression can therefore ref lect the adaptability of an organism to high temperature,acting as biomarkers of stress. Furthermore, the exposure of aquatic organisms to heat stress aff ects their immunological defense capacity, ref lected by changes in immune-related enzyme activity, such as that of acidic phosphatase (ACP) and alkaline phosphatase (AKP; Liu et al., 2004; Chen et al.,2007a, b).
Although animals have diverse physiological mechanisms enabling adaptation to heat stress,cellular and histological damage will occur when the stress exceeds the organism’s coping ability. Examples of damage resulting from heat stress include: nuclear shrinkage and pyknosis in the columnar epithelial cells of the intestine inApostichopusjaponicus(Xu et al., 2015); damage to gill tissue involving lamellar fusion, lamellar aneurism, epithelial lifting, and hyperplasia inParalichthysolivaceus(Liu et al.,2014); and swelling of muscle cells and increased space between muscle cells inLophiosilurusalexandri(Martins et al., 2014). These pathological changes in the histological structure represent further detectable biomarkers for stress assessment. Further, they could provide pointers for the screening of genes for resistant forms that prevent histological damage.
The phylum Sipuncula contains worm-like marine invertebrates that are widely distributed globally,from polar to tropical oceans and from the intertidal zone to the deep sea (Lan et al., 2007). To our knowledge, the physiological and histological responses of sipunculids to high temperature stress are currently unknown. This limits our understanding of the eff ects of temperature stress on these organisms.The sipunculidPhascolosomaesculentais commonly found in the intertidal zone around Southeast China and farmed in aquaculture operations around the Zhejiang, Fujian, and Guangxi regions for consumption as traditional seafood with high nutritional and medicinal value. However, suitable culture temperatures and an understanding of the physiological mechanisms underlying the response ofP.esculentato temperature stress remain unclear.
In this study, we selectedP.esculentaas a model to investigate the eff ects of acute high temperature stress on sipunculids. Specif ically, we measured its physiological responses in terms of MDA concentration, activities of antioxidant and immunityrelated enzymes, andhsp70andhsp90gene expression as well as its histological structure, with the aim of uncovering elements of its physiological mechanisms for heat stress adaptation. The results are novel in terms of the phylum studied and provide a series of usable indicators for practical heat stress testing for ecology and aquaculture purposes.
2 MATERIAL AND METHOD
2.1 Experimental animal
Phascolosomaesculentaindividuals were captured from the Xizhou of Ningbo (Zhejiang Provinces,China). They had a mean body weight of 4.3±0.9 g;they were acclimated for 1 week in f iltered and aerated seawater at a temperature of 20 °C and salinity of 23.0.
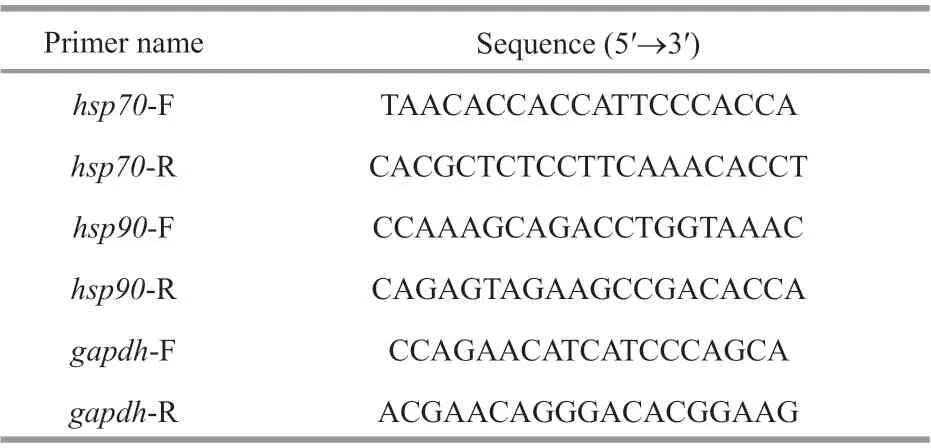
Table 1 List of all primers used in this study
2.2 Experimental design and sample collection
Four experimental groups and a control group consisted of 50P.esculentaindividuals each contained in separate 12.5-L temperature-controlled tanks f illed with f iltered seawater. Control tanks were maintained at 20 °C, and experimental tanks were maintained at 25 °C, 30 °C, 35 °C, or 40 °C; each condition was simultaneously maintained in triplicate.OnlyP.esculentaexhibiting normal behavior were used. During the experiments, the seawater in the tank was exchanged daily with isothermal fresh seawater.Six randomly-selected individuals were collected from each tank after 3, 6, 12, 24, 48, 72, and 96 h. The coelom f luid, intestine, body wall, retractor muscle,and nephridium were extracted by dissection and immediately transferred into RNAse-free centrifuge tubes for rapid freezing in liquid nitrogen and storage at -80 °C until later detection of enzyme activity and gene expression. The intestine, body wall, retractor muscle, and nephridium fromP.esculentain the control and 40-°C groups were dissected from individuals collected after 96 h, cut into cubes, and f ixed for 24 h in Bouin’s f ixative (containing 75 mL of saturated picric acid buff er solution, 25 mL of 40%formaldehyde, and 5 mL of 100% acetic acid).Thereafter, the samples were rinsed in 70% ethanol and stored until use.
2.3 Determination of MDA content and enzyme activities
Coelom f luid was homogenized using an Ultra Turrax Homogenizer (IKA, Staufen, Germany),combining 1 g of tissue with 9 mL of 0.9% normal saline in an ice bath, and then centrifuged at 956 ×gfor 10 min at 4 °C. The supernatant was collected and used to measure the content of MDA and the activity levels of SOD, GPX, ACP, and AKP. MDA concentrations and enzyme activity levels were measured using assay kits from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.4 Histological analysis
The f ixed and rinsed samples described in Section 2.2 were embedded in paraffi n and cut into 7-μm sections. These sections were stained with standard hematoxylin-eosin and then observed and photographed under a BX51 light microscope(Olympus, Tokyo, Japan) to analyze the cellular and histological damage.
2.5 Real-time quantitative PCR (qPCR) analysis
Total RNA was extracted from the frozen samples described in Section 2.2 using TRIzol reagent(Invitrogen, Carlsbad, USA) and reverse-transcribed using the Prime Script™ RT reagent kit with gDNA Eraser (TaKaRa, Japan) to obtain f irst-strand cDNA.The primers used in this study are listed in Table 1.Thegapdhgene was used as the internal control (Su et al., 2010). Thehsp70-F/R primers were synthesized based on Su et al. (2010) and thehsp90-F/R primers were designed based on thehsp90cDNA sequence(GenBank accession No: GQ503177.1). The Expression levels ofhsp70andhsp90mRNA were quantif ied by qPCR using SYBR Green Master I(Roche, Basel, Switzerland) and analyzed on the Roche LightCycler480 System. The thermal cycling program was as follows: activation at 95 °C for 3 min,followed by 40 cycles of 95 °C for 20 s, 60 °C for 30 s, and 72 °C for 20 s. Three biological replicates were measured for each group, and all samples were examined in triplicate on the same plate. The expression levels ofhsp70andhsp90were calculated by the 2-ΔΔCtmethod. mRNA expression levels were calculated relative to the control group samples treated with 20 °C.
2.6 Statistical analysis
All data are expressed as the mean±standard deviation of three replicates. Statistical analysis was performed using SPSS v19.0 software (IBM Corp.,Armonk, USA). The diff erences were analyzed using one-way analysis of variance (ANOVA).P<0.05 were considered statistically signif icant.
3 RESULT
3.1 Coelom f luid MDA concentration
As shown in Fig.1, the coelom f luid MDA concentration initially increased and then decreased in all four experimental groups; at 48 h, the MDA concentration was 2.51-, 4.41-, 5.34-, and 6.06-fold greater than that in the control group for the 25-°C,30-°C, 35-°C, and 40-°C stress groups, respectively(P<0.01). The MDA concentrations in all of the experimental groups remained signif icantly higher than that in the control group after 96 h of heat stress(P<0.05). This result indicates that acute heat stress results in oxidative stress inP.esculenta.
3.2 Coelom f luid SOD and GPX activities
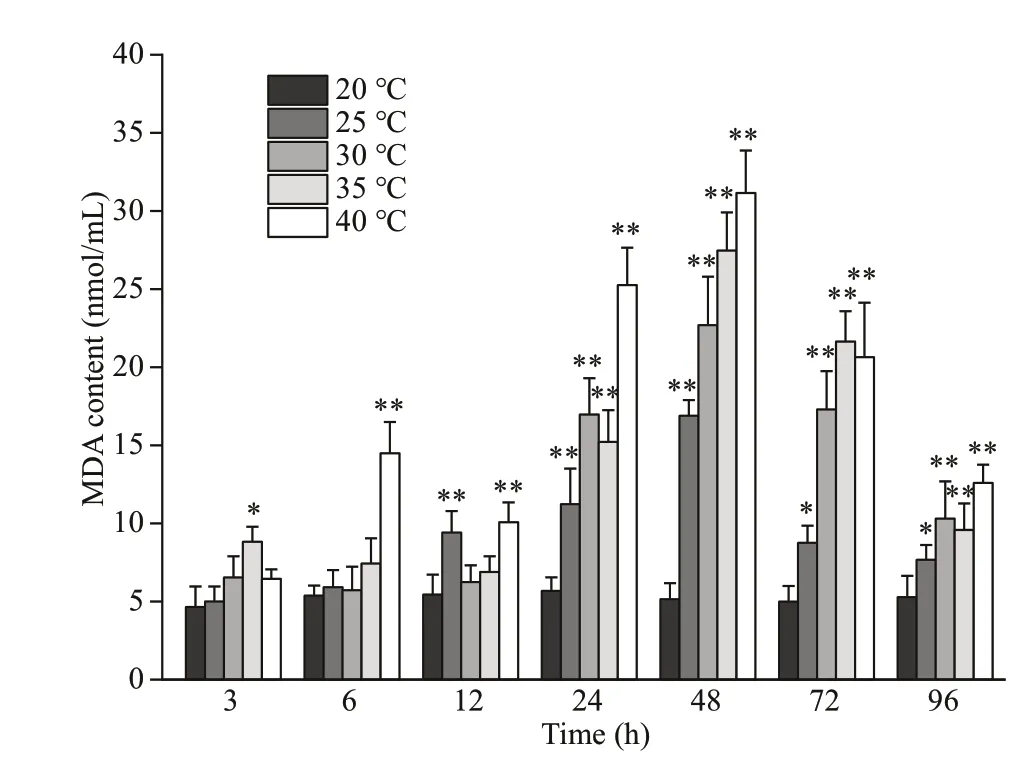
Fig.1 Malondialdehyde (MDA) content of the coelom f luid of P. esculenta under diff erent temperature conditions
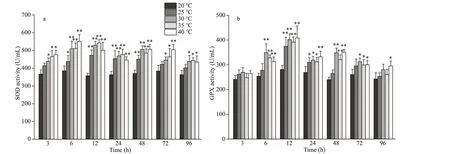
Fig.2 Changes of superoxide dismutase (SOD) (a) and glutathione peroxidase (GPX) (b) activity in the coelom f luid of P. esculenta under diff erent temperature conditions
As shown in Fig.2, the coelom f luid SOD activity increased f irst and then decreased in the experimental groups. In the 40-°C group, it peaked after 6 h (1.43-fold greater than that in the control,P<0.01) and decreased thereafter. In the other three experimental groups, peak activity levels of SOD were observed after 12 h (1.32-, 1.48-, and 1.52-fold greater than that in the control for the 25-°C, 30-°C, and 35-°C experimental groups, respectively;P<0.01). Similarly,GPX activity also f irst increased and then decreased in all the acute heat stress groups. Peak activity levels of GPX were observed after 12 h (1.33-, 1.41-, 1.38-,and 1.45-fold greater than that in the control for the 25-°C, 30-°C, 35-°C, and 40-°C experimental groups,respectively;P<0.01). Antioxidant enzymes were thus demonstrated to have increased; they may play a role during acute heat stress inP.esculenta.
3.3 Tissue-specif ic expression of hsp70 and hsp90 genes
The qPCR results showed thathsp70andhsp90mRNA were present in the coelom f luid, intestine,retractor muscle, nephridium, and body wall (Fig.3).The expression level ofhsp70mRNA in the coelom f luid was the highest, followed in order by those of the intestine, retractor muscle, nephridium, and body wall (Fig.3a). The expression ofhsp90mRNA in the coelom f luid was the highest; it was lower in the intestine and retractor muscle and the lowest in the body wall and nephridium (Fig.3b).
3.4 Eff ects of acute heat stress on hsp70 and hsp90 expression
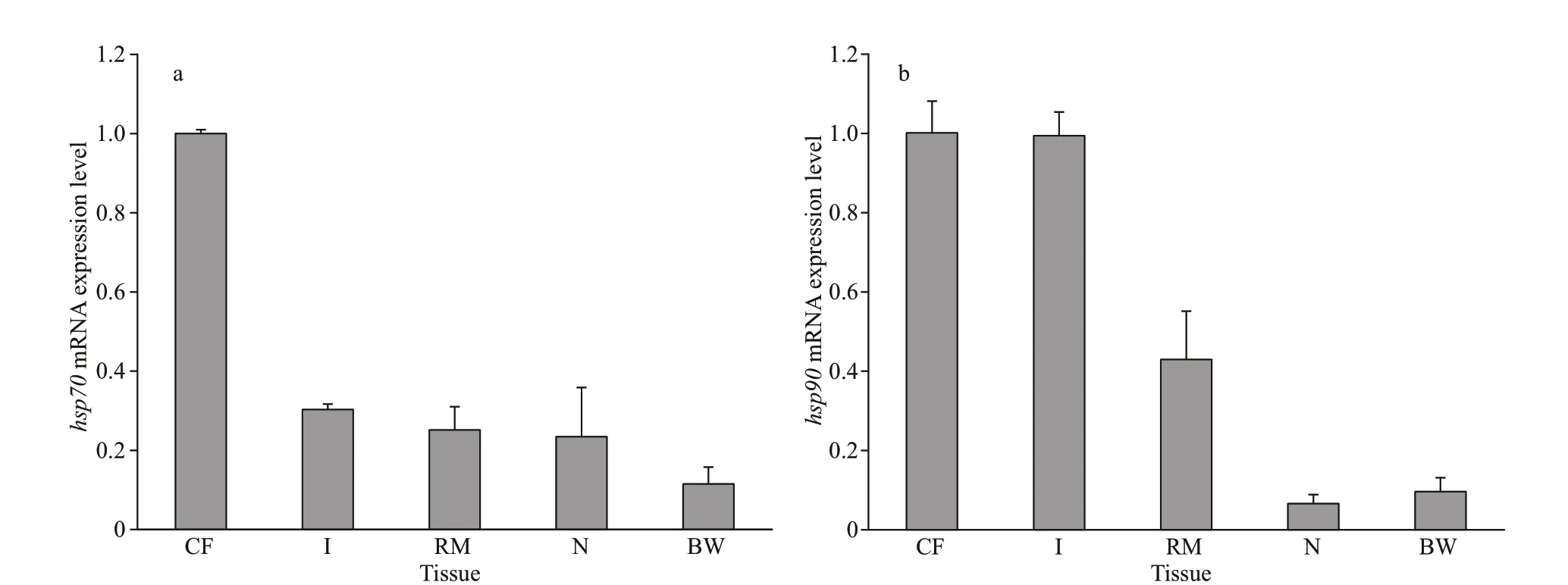
Fig.3 Expression of hsp70 (a) and hsp90 (b) mRNA in diff erent tissues of P. esculenta
As shown in Fig.4, upon exposure to high temperature, thehsp70andhsp90mRNA expression in all the experimental groups was signif icantly upregulated in the coelom f luid and intestine.Compared to that in the control, the expression ofhsp70mRNA in the coelom was 1.59-fold higher after 6 h at 25 °C, 3.09-fold higher after 48 h at 30 °C,3.56-fold higher after 48 h at 35 °C, and 4.87-fold higher after 48 h at 40 °C (Fig.4a). Compared to that in the control, the expression ofhsp70mRNA in the intestine was 1.62-fold higher after 6 h at 25 °C, 2.37-fold higher after 6 h at 30 °C, 2.96-fold higher after 24 h at 35 °C, and 3.16-fold higher after 24 h at 40 °C(Fig.4c). In the 35-°C and 40-°C groups, the expression ofhsp70mRNA was still signif icantly higher than that in the control group in both the coelom f luid and intestine after 96 h of stress (Fig.4a& c;P<0.01). Similarly, the coelom f luid and intestinalhsp90mRNA expression in all the experimental groups was signif icantly upregulated following exposure to acute heat stress. Compared to that in the control, thehsp90mRNA expression in the coelom was 1.69-fold higher after 6 h at 25 °C, 2.68-fold higher after 6 h at 30 °C, 2.45-fold higher after 24 h at 35 °C, and 2.78-fold higher after 24 h at 40 °C(Fig.4b). Compared to that in the control, thehsp90mRNA expression in the intestine was 1.37-fold higher after 48 h at 25 °C, 1.81-fold higher after 48 h at 30 °C, 3.33-fold higher after 48 h at 35 °C, and 3.90-fold higher after 48 h at 40 °C (Fig.4d). In the 35-°C and 40-°C groups, the coelom f luid and intestinal expression ofhsp90mRNA was still signif icantly higher than that in the control group after 96 h of stress (Fig.4b & d;P<0.01). These increases suggest that heat shock protein (HSP) have an important role in the response to acute heat stress inP.esculenta.
3.5 Coelom f luid ACP and AKP activities
As shown in Fig.5, the ACP and AKP activity levels in coelom f luid f irst increased and then decreased in all the experimental groups. In the 40-°C experimental group, ACP and AKP activity peaked after 24 h (2.41- and 3.49-fold greater values than that in the control, respectively (P<0.01)). In the other three treatment groups, peak activities of ACP and AKP were observed after 48 h. ACP activity peaks were 1.77-, 1.91-, and 2.2-fold greater than that in the control in the 25-°C, 30-°C, and 35-°C groups,respectively (P<0.01). For AKP, these were 2.29-,2.89-, and 3.17-fold greater than that in the control for the 25-°C, 30-°C, and 35-°C experimental groups,respectively (P<0.01). This result indicates that acute heat stress aff ects the nonspecif ic immunity ofP.esculenta.
3.6 Histological structure
3.6.1 Body wall and retractor muscle
The body wall ofP.esculentais composed of the cuticle, epithelial layer, and muscularis (Fig.6a & b).Its retractor muscle consists of smooth muscle f ibers(Fig.6e & f). In the control group, the muscle cells were bundled tightly in the muscularis of the body wall and retractor muscle (Fig.6a & e); the nuclei of the columnar cells in the body wall epithelium and the muscle cells in the retractor muscle were oval,with the chromatin in the form of small, scattered plaques in the nucleus (Fig.6c & g). However, after 96 h of stress at 40 °C, crevices were observed between the muscle cells in the body wall and retractor muscle (Fig.6b & f). In addition, the chromatin was condensed around the nuclear membrane, and a round basophilic granule was present in the nuclear core of the columnar cells in the body wall epithelium and the retractor muscle cells (Fig.6d & h).
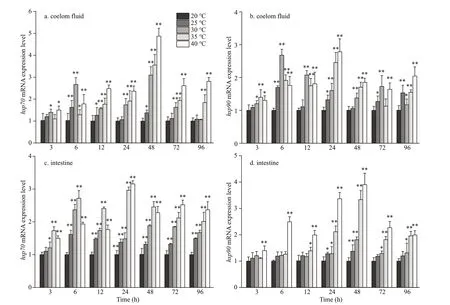
Fig.4 Expression of hsp70 (a & c) and hsp90 (b & d) mRNA in the coelom f luid and intestine of P. esculenta under diff erent temperature conditions
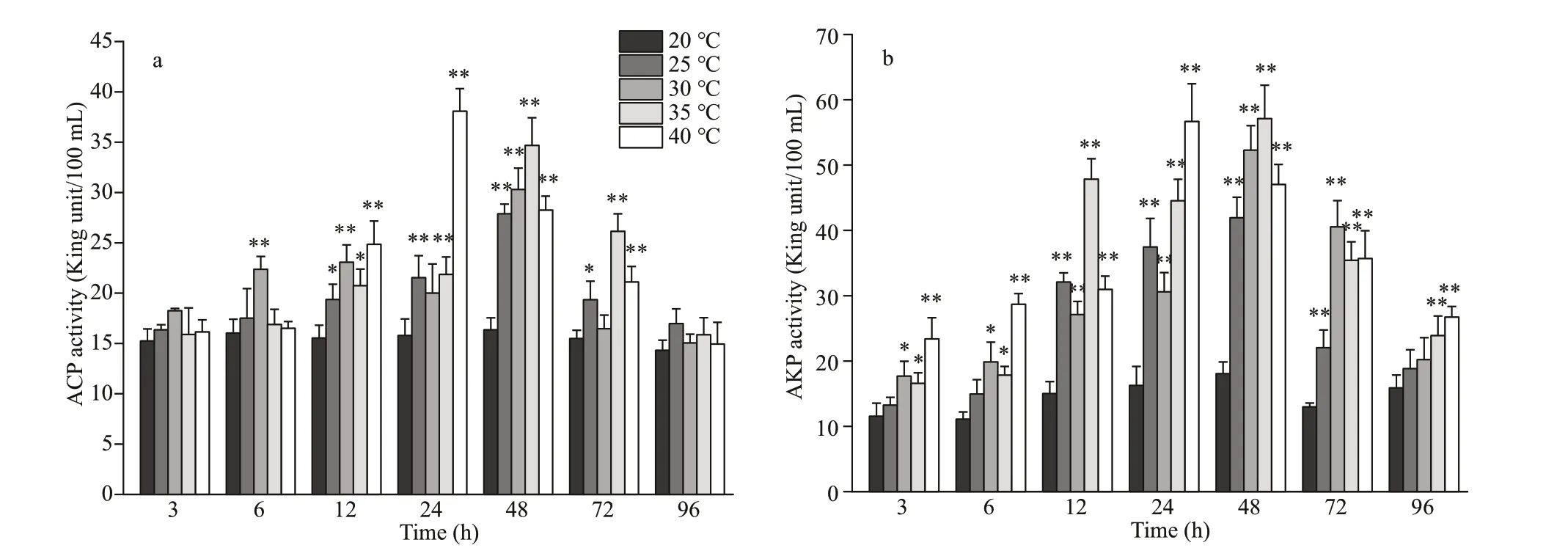
Fig.5 Changes in acidic phosphatase (ACP) (a) and alkaline phosphatase (AKP) (b) activity in the coelom f luid of P. esculenta under diff erent temperature conditions
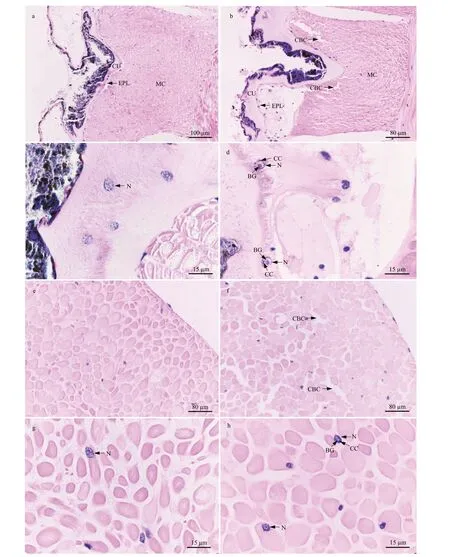
Fig.6 Light micrograph showing the histological structure of the body wall and retractor muscle
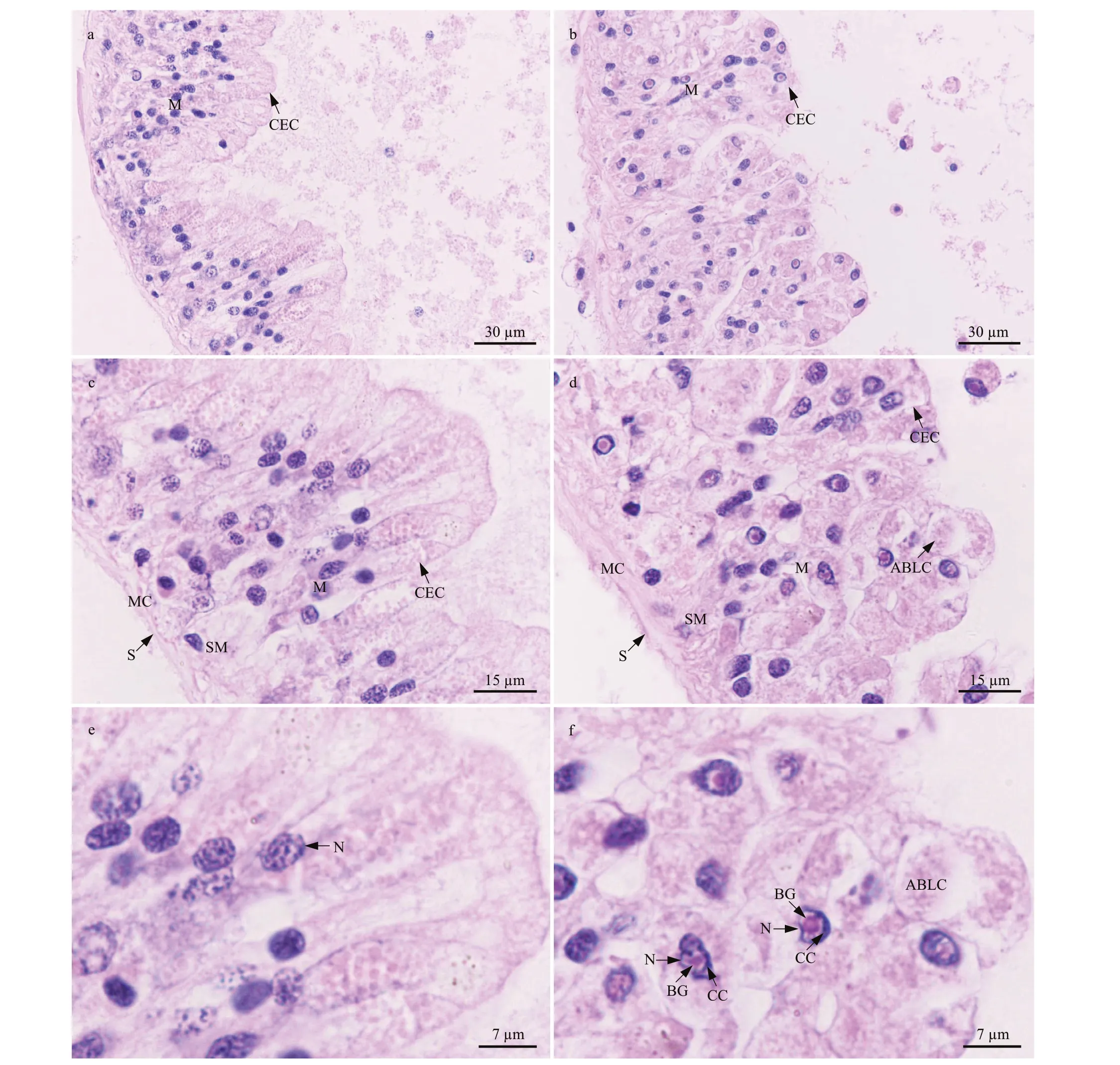
Fig.7 Light micrograph showing intestinal histological structure
3.6.2 Intestine
The intestinal wall ofP.esculentais composed of serosa, muscularis, submucosa, and mucosa. In the control group, the columnar epithelial cells in the mucosa were columnar; their nuclei were round or oval and located near the base of the cells, with distributed small plaques of chromatin (Fig.7a, c, &e). However, after 96 h of stress at 40 °C, the shape of the columnar epithelial cells in the mucosa was irregular, and some apoptotic body-like components were observed (Fig.7b, d, & f). Furthermore, the chromatin was condensed around the border of the nuclear membranes, and a round basophilic granule was present in the nuclear core of the columnar epithelial cells (Fig.7f). This was similar to the pattern observed in the body wall and muscle cells described in Section 3.6.1.
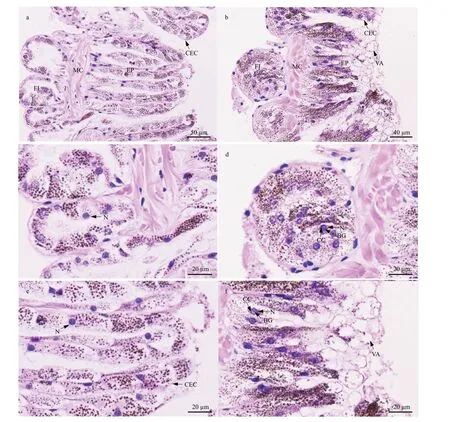
Fig.8 Light micrograph showing nephridial histological structure
3.6.3 Nephridium
The nephridial wall ofP.esculentais composed of f lask-shaped infoldings, muscularis, and epithelium. In the control group, the columnar epithelial cells in the epithelium and the cuboidal epithelial cells in the f laskshaped infoldings were fascicled, with substantial amounts of particulate matter in the cytoplasm (Fig.8a,c, & e); their nuclei were ovate with chromatin plaques(Fig.8c & e). However, after 96 h of stress at 40 °C, the boundary between some epithelial cells was blurred(Fig.8b, d, & f); damage to the cell membrane resulted in the outf low of granular matter with vacuolization at the top of some columnar epithelial cells (Fig.8b & f).In addition, some cells had irregularly shaped nuclei in which condensed chromatin was gathered around the nuclear membrane; a basophilic granule was also observed in the nuclear core (Fig.8d & f).
4 DISCUSSION
4.1 MDA content and SOD and GPX activity
Heat stress can lead to excess ROS generation,causing oxidative stress and damage to biological molecules, including the polyunsaturated fatty acids in the cell membranes (Park et al., 2009). The f inal product of lipid oxidation is MDA, which has therefore become a commonly used indicator to evaluate lipid peroxidation and oxidative stress. The antioxidant defense system plays an important role in scavenging ROS to reduce such oxidative stress(Kashiwagi et al., 1997; Verlecar et al., 2007). The data in our study showed that MDA concentrations were universally higher in the coelom f luid after 24 h of heat stress, compared to the control, demonstrating that lipid peroxidation and oxidative stress occurred under these conditions inP.esculenta. Similar results have been reported in crabs, mollusks, and f ish,namelyEriocheirsinensis(Hong et al., 2007),Crassostreagigas(Park et al., 2009), andOreochromisniloticus(Qiang et al., 2012), following exposure to high temperature stress. The cascade of events is considered to start with a raised body temperature,caused by higher water temperatures, which, in turn,increases oxygen consumption. This then triggers mitochondrial damage and disturbances to the oxidation-reduction processes, resulting in overproduction and accumulation of ROS (Verlecar et al., 2007). It is noteworthy that the raised MDA concentrations were not sustained; the levels decreased following prolonged stress inP.esculenta.This phenomenon has also been reported inScapharcabroughtonii(An and Choi, 2010). This reduction in oxidative stress may indicate the involvement of the antioxidant system.
In the present study, we observed that the activities of the antioxidant enzymes SOD and GPX were signif icantly increased in coelom f luid under acute heat stress (Fig.2). SOD functions during the f irst step of ROS elimination, converting intracellular O2ˉ to H2O2and O2(Downs et al., 2001). H2O2is then broken down by GPX, thus reducing its toxic eff ect (Verlecar et al.,2007). Our results therefore ref lect the important roles of SOD and GPX in heat stress: scavenging the ROS(in the forms of O2ˉ and H2O2) to protectP.esculentaby reducing the cellular damage caused by heat stress. In fact, in many aquatic organisms in both vertebrate and invertebrate taxa, such asChlamysfarreri(Chen et al.,2007a, b),Takifuguobscurus(Cheng et al., 2015), andS.broughtonii(An and Choi, 2010), similar increased activities of SOD and GPX induced by heat stress have been detected. Antioxidant enzymes could therefore be universally involved in protecting against heat stress in animals. The genes for such enzymes have potential as heat-resistance genes and are worth investigating in this regard in future research.
4.2 Expression of hsp70 and hsp90
Heat shock proteins are important molecular chaperones that play critical roles in preventing protein denaturation, refolding denatured protein, and removing irreversibly damaged proteins (Peng et al.,2016). Among the HSP families, HSP70 and HSP90 are generally reported to be signif icantly upregulated under heat stress. For example, the upregulated expression ofhsp70orhsp90or both was reported inMisgurnusanguillicaudatus(Yan et al., 2017),Husodauricus(Peng et al., 2016),T.obscurus(Cheng et al.,2015), andMegalobramaamblycephala(Zhang et al.,2014) under high temperature stress. In the present study, we detected the upregulated expression ofhsp70andhsp90in the coelom f luid and intestine of heatstressedP.esculenta(Fig.4). The widespread reporting of this observation indicates that it plays an important role in the adaptation of animals to heat stress.
Given that heat stress causes excess ROS generation and oxidative stress, which damage proteins, and considering the chaperone functions of HSP70 and HSP90, we infer that high expression levels of HSPs could enable the repair of denatured proteins and the removal of irreversibly damaged proteins. These functions are undoubtedly important for cell homeostasis and the protection of cells from damage.The HSP70 and HSP90, therefore, complement the antioxidant system to make the organism adapting to the oxidative stress. Furthermore, HSP70 is also reported to be a negative regulator of apoptosis, acting to restrain the signaling cascade initiating the apoptotic process (Beere and Green, 2001; Ravagnan et al., 2001). Cellular apoptosis in response to heat stress was reported in aquatic animals (Cheng et al.,2015); thus, this could be the mechanism by which high HSP70 expression levels are associated with heat tolerance in animals. Future testing of the heat tolerance ofhsp70- andhsp90-knockout animals will reveal the exact function of these molecular chaperones under heat stress.
4.3 Eff ect on the nonspecif ic immune system
It has been reported that heat stress can inf luence immunity-related enzyme activity; this is commonly tested in aquatic organisms using ACP and AKP (Liu et al., 2004; Hu et al., 2015). ACP is a typical lysosomal enzyme, involved in killing and digesting microbial pathogens as a component of innate immunity; AKP, usually located in the membrane system, participates in carbohydrate metabolism,growth, and diff erentiation (Qiang et al., 2012). Both can catalyze the hydrolysis of various phosphatecontaining compounds and participate in the degradation of foreign proteins, carbohydrates, and lipids (Liu et al., 2004; Qiang et al., 2012). They are important indices for the assessment of nonspecif ic immune system function in animals. Typically, high activity represents high function. In our study,increased activities of AKP and ACP were detected under high temperature stress, especially after 24 h and 48 h (Fig.5). A similar phenomenon was reported in bothMytiluscoruscus(Hu et al., 2015) andHaliotisdiscushannai(Jiang et al., 2017). However, inProcambarusclarkii, heat stress resulted in reduced activity (Wang et al., 2012). This variation may be related to the stress intensity and duration or ref lect interspecif ic diff erences. Regardless, these results ref lect the sensitivity of ACP and AKP to temperature variations; inP.esculenta, high temperature aff ects the nonspecif ic immune system. Furthermore, it is worth noting that SOD, GPX, HSP70, and HSP90 were also reported to participate in nonspecif ic immunity (Spallholz, 1990; Fu et al., 2011; Lu et al.,2015; Yan et al., 2017). The observed high activities and abundances of these proteins were likely associated with the nonspecif ic immune response.Our results certainly suggest that this was the case inP.esculenta. However, to our knowledge, no studies have yet shown that organisms show enhanced resistance to pathogens after high temperature stress.An investigation of the changes in the actual immune capacity under these conditions may be an interesting and fruitful future study.
4.4 Tissue damage
High temperatures, exceeding the coping ability of animals, will cause cell and tissue damage. Studying the resulting pathological changes can help us understand the failure of animals to adapt successfully to high temperatures at a histological level.Theoretically, the genes that prevent such tissue damage are potential heat-resistance genes, off ering a route to identify these through screening. Furthermore,tissue damage can function as a quantitative indicator of the degree of damage. In aquatic organisms, it has been reported that heat stress causes oncotic problems in muscle f ibers in the form of distension of the spaces between muscle cells inL.alexandri(Martins et al.,2014), and leads to epithelial cytoplasmic shrinkage,organelle reduction, and condensed chromatin in the intestinal cells ofA.japonicusand others (Xu et al.,2015). These results reveal that heat stress can aff ect the functions of organs involved in movement, food absorption, and digestion. In the present study, we detected tissue damage in the body wall (Fig.6),retractor muscle (Fig.6), intestine (Fig.7), and nephridium (Fig.8) under 40-°C stress. The body wall and retractor muscle are related to movement; the intestine functions in food absorption and digestion(Lei et al., 2013); the nephridium functions in excretion and osmotic pressure regulation (Long et al., 2014). The observed damage to these structures conf irms such eff ects inP.esculenta. Notably, a signif icant feature of damaged cells is condensed chromatin around the nuclear membrane (Figs.6-8).This is also reported to be a morphological feature of apoptosis (Van Cruchten and Van den Broeck, 2002).Its presence suggests that apoptosis had taken place.However, we did not observe the ultrastructure of the damaged cells, and molecular biological indicators that reveal apoptosis were not evaluated. Therefore,we cannot conclusively state whether apoptosis occurred.
As described above, we detected increased MDA levels, ref lecting excess ROS generation. The ROS was, in turn, the primary factor causing lipid peroxidation, resulting in protein denaturation, DNA damage, and f inally apoptosis (Simon et al., 2000). It is therefore likely that the probable apoptosis detected in tissues may be related to the ROS. In the future, we will analyze the relationship between ROS level and apoptosis to explore the molecular mechanism underlying tissue damage in depth and thus provide reference information for the screening of heat-stress resistance genes inP.esculenta.
5 CONCLUSION
We found that exposure to high temperature stress caused oxidative stress, evidenced by the increased activity of the antioxidant enzymes SOD and GPX and the upregulated expression ofhsp70andhsp90mRNA. These results suggest that the antioxidant system could play an important role in eliminating the ROS generated owing to heat stress. Protein protection could be provided by HSP70 and HSP90 to enhance the tolerance ofP.esculentato high temperatures,forming part of its physiological mechanism of adaptation to such conditions. In addition, the high temperature aff ected the activity of the immunerelated enzymes ACP and AKP, indicating that the nonspecif ic immunity ofP.esculentawas aff ected.Furthermore, acute high temperature stress caused tissue damage to its body wall, retractor muscle,intestine, and nephridium, indicating that heat stress could aff ect the movement, absorption and digestion of food, and excretion process. This study thus contributes to our understanding of the physiological responses of other sipunculids when adapting to high temperatures, usingP.esculentaas a model for this phylum. As the biochemical indicators andhspexpression levels used in this study were shown to be sensitive to high temperatures and the cellular damage, such as condensed chromatin around the nuclear membrane, was easy to observe, these could function as useful indicators for evaluating heat stress status and screening optimal culture temperature ofP.esculentain the future.
6 DATA AVAILABILITY STATEMENT
The data of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
