Impact of ocean acidif ication on physiology and microbiota in hepatopancreas of Pacif ic oyster Crassostrea gigas*
Lingshuai ZHANG , Xiudan WANG , Weiqian ZHANG , Xiaoting YIN ,Qing LIU , Limei QIU ,**
1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
2 Qingdao University of Science and Technology, Qingdao 266071, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
Abstract The hepatopancreas is an important tissue involved in various biological metabolism for mollusks, but its responses to ocean acidif ication (OA) have not been well evaluated. In this study,the oysters were cultured in simulated conditions by continuously bubbling with ambient air (pH=8.10)or air-CO 2 (pH=7.50) for up to two months, and the variations on the antioxidant capacity, digestive ability, and microbiota composition in hepatopancreas of Crassostrea gigas were analyzed. The results show that although superoxide dismutase and glutathione responded quickly to OA stress, the antioxidant capacity of the hepatopancreas was inhibited, as revealed by the decrease of the total antioxidant capacity,which led to an upward trend of the malondialdehyde, demonstrating that the oxidative damages were accumulated under the OA process. The determination of the digestive ability manifested as the decrease of pepsin activity and the recovery of lipase and amylase activity after long-term acidif ication, which may be helpful to improve the adaptability of oysters. In addition, analysis on 16S rDNA amplicon revealed that the total species abundance and diversity of the hepatopancreas microbiota experienced a dynamic change, but f inally it decreased greatly after long-term acidif ication. The structure of the hepatopancreas microbiota was changed drastically with the change of the dominant species from aerobic to the anaerobic and facultative anaerobic bacteria, and the abnormal proliferation of some species, such as genus of Mycoplasma and order Clostridiales, which may aggravate the adverse eff ects of OA on the physiological functions of the hepatopancreas. As a result, our f indings enrich our understanding of the accumulated oxidative damage and adaptive digestive ability in oyster hepatopancreas caused by OA. For the f irst time,the changes of the hepatopancreas microbiota under long-term acidif ication conditions are described,proving a good reference for the study of the response and adaptation mechanisms of bivalve mollusks in a wide range of oceans OA.
Keyword: ocean acidif ication; oyster; hepatopancreas; antioxidant defense; digestive enzymes; microbiota
1 INTRODUCTION
The phenomenon that the ocean absorbs excess CO2in the atmosphere, causes a decrease in pH value of seawater and changes in the carbonate balance system, is called ocean acidif ication (OA) (Caldeira and Wickett, 2003). At the end of this century, the atmospheric CO2concentration is expected to be 0.55‰-0.8‰ (Mackay, 2008), which is much higher than the current concentration of 0.4‰. Accordingly,the pH value of the ocean surface will fall by 0.3-0.4(Hoegh-Guldberg et al., 2007), which is sure to have a profound impact on marine biodiversity and ecosystem functions (Doney et al., 2009, 2012), and challenge the survival of many marine organisms.The most direct and key impact of ocean acidif ication on organisms is the acid-base imbalance, which will increase the diffi culty of calcif ication of marine organisms (Fitzer et al., 2014). Bivalves, as the important marine calcif iers, will spend more energy for biomineralization and adjustment of acid-base balance (Beniash et al., 2010). This will further inf luence the growth and survival of bivalves(Gutowska et al., 2010; Parker et al., 2013), so the full understanding about the responses of bivalves to OA is of critical importance to the future development of these species.
Many researches have revealed that OA can impact a series of biological processes in bivalves, such as energy metabolism (Beniash et al., 2010; Gu et al.,2019), calcif ication process (Melzner et al., 2011; Liu et al., 2020a), immune response (Wang et al., 2016),growth (Rodolfo-Metalpa et al., 2011; Amaral et al.,2012), development (Kurihara, 2008; Talmage and Gobler, 2010; Liu et al., 2020b), and genetic reproduction (Gobler and Talmage, 2013; Zhao et al.,2020). These observational data are mainly obtained by describing their physiological and biochemical changes in the larvae of diff erent developmental stages, or in some important tissues, such as shell and hemolymph (Parker et al., 2013; Clements and Hunt,2017). The hepatopancreas, an important functional tissue of mollusks (Zhang et al., 2012), plays major roles in the body’s nutritional intake, hormone synthesis, energy metabolism, and immune defense(Canesi et al., 2007a, b; Khan et al., 2018), but their reactions under OA conditions have not been well studied. Recently, an increasing interest has been attracted to the study of the impact of OA on hepatopancreas functions. For example, elevated CO2concentration (2‰) had an inhibitory eff ect on some antioxidant activities and glutathione levels decreased in hepatopancreas of Pacif ic oysters (Wang et al.,2016). The hepatopancreas structure of bivalves could also be prominently damaged after 21 days of OA exposure (pH=7.1), which might further reduce the activity of digestive enzymes (Kong et al., 2019;Wang et al., 2020; Xu et al., 2020). If the marine environment is continuously deteriorated, as most of the bivalves are attaching organisms, they are inclined to permanently survive in the acidif ied environment after the pH value of seawater drops. However,previous researches focus mainly on the short-term(within a month) response of hepatopancreas to OA,the impact of long-term OA on hepatopancreas should be further emphasized and evaluated.
Shellf ish are f ilter feeders and can f ilter up to 109bacterial cells per hour, many of them may appear to be transients or opportunists in the microbiota(Paillard et al., 2004; Pruzzo et al., 2005). Studies have shown that hepatopancreas of healthy shellf ish usually harbors species-rich microbial communities(Green and Barnes, 2010; Khan et al., 2018). Evidence reveals that the stable structure of microbiota in the digestive glands may contribute importantly to the maintenance of the normal function of shellf ish (Khan et al., 2018). However, studies on this aspect are few at present. In addition, some researchers have proved that elevated CO2level in seawater could lead to the change in the structure of bacterial communities of some marine organisms. For example, spongesDysideaavaraacquired 255 new Operational Taxonomic Units (OTUs) under the acidif ied condition (pH=7.8) (Ribes et al., 2016). Variations in the composition of microbiota may adversely aff ect hosts in the acidif ied environment. The intestinal bacterial community of sea breamSparusaurataunderwent signif icant dysbiosis after exposure to 121.59-kPa CO2for one month due to the absence of Firmicutes phylum and increase of Proteobacteria abundance. The changes in the microbial community might be related to the digestion ability, for example,impaired digestion was observed in f ish under hypercapnia (Fonseca et al., 2019). In mollusks, the hepatopancreas microbial community is also closely related to the host’s digestion, nutrient absorption,and immune functions (Khan et al., 2018; Butt and Volkoff , 2019). Therefore, exploring the variations of hepatopancreas microbiota will help us to better understand the changes in physiological activities of hepatopancreas in an acidif ied environment.
The Pacif ic oysterCrassostreagigas, known as ecosystem engineers, plays an important role in the ecological environment (Coleman and Williams,2002), and their response and adaptation to OA have received increasing attention (Wei et al., 2015). In this study, we investigated the eff ects of long-term OA on antioxidation, digestion, and structure of microbiota in hepatopancreas of oysters. The results of the research will provide a reference for the study of the response and adaptation mechanisms of bivalve mollusks in acidif ication seawater and provide a scientif ic basis for strategies to deal with OA.
2 MATERIAL AND METHOD
2.1 Animal
OystersC.gigaswith an average shell length of 13.0 cm were collected from a local farm in Qingdao,China. The oysters were fed every other day withSpirulinaplatensis, and were kept in aerated seawater about 15 °C for two weeks before the experiment.Animal experiments were approved by the Qingdao Animal Care and Use Committee, which complied with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2 Experimental design and tissue collection
All animal tanks with a volume of 100 L were continuously bubbled with ambient air or air-CO2mixtures as appropriate, the water was changed every other day to ensure adequate water quality, and the oysters were fed withSpirulinaplatensisafter changing the water. The pH value controlled by an acidometer (AiKB, Qingdao, China) was 7.50 in the OA group, the pH value predicted for the year of 2250(Caldeira and Wickett, 2003), and was 8.10 in the control group, which was the mean pH value of the normal seawater. The temperature was controlled at 14.9±0.5 °C and 15.0±0.4 °C for the control group and OA group, respectively. The salinity was relatively stable, and it was 32.8±0.04 and 32.6±0.05 in the control group and OA group, respectively. The dissolved oxygen was monitored and adjusted by controlling bubbling of air and the dissolved oxygen in the control group and OA group was 7.73±0.04 and 7.83±0.02 mg/L, respectively.
In the experiments, oysters used for the detection of the biochemical parameters were grouped as following: 10 oysters kept in the seawater with pH 7.50 for 56 days were designated as O56 group, and the same number of oysters placed in the normal seawater with pH 8.10 for 14 days, then transferred to seawater with pH 7.50 for 42 days were designated as O42 group. The remaining OA groups (O28, O21,O14, and O7) were processed similarly, that was the oysters were maintained in normal seawater (28, 35,42, and 49 days) f irstly, and then kept in the acidif ied environment at the appointed time (28, 21, 14, and 7 days). As a comparison, 10 oysters in normal seawater with pH 8.10 for 56 days were set as the control group. The fresh hepatopancreas tissues from f ive random oysters were cut into pieces on ice, and the portions in the order of cutting with the weight of~2 g were digested immediately and used for the reactive oxygen species (ROS) determination. The rest hepatopancreas of each group were then divided into several aliquots with the weight of ~100 mg. The aliquots were snap-frozen in liquid nitrogen and then stored at -80 °C for other detections.
To determine the microbiota of hepatopancreas,oysters were kept in the acidif ied seawater for 7, 28,and 56 days (O7, O28, and O56), and the control groups were set at the corresponding time points and designated as C7, C28, and C56. In the meantime, a population of 100 oysters was cultured in the normal seawater environment. During the whole process, the death rate of the population was lower than 2%,indicating that the oysters have attempted to adapt to this laboratory culture conditions, as well as excluded the possibility of infection from bacteria or virus,which might disturb their microbiota. Three oysters were randomly sampled in each experimental condition for the amplicon sequencing. About 50 mg of hepatopancreas samples from each oyster were harvested by dissection under sterile conditions and stored at -80 °C for genomic DNA extraction.
2.3 D etermination of intracellular reactive oxygen species (ROS) level
The prepared tissues were minced and incubated with Pronase (20 μg/mL) in 1 100-mOsm/L Hank’s buff er containing no Ca2+or Mg2+with gentle shaking at room temperature for 30 min (Jemaà et al., 2014).The supernatant was f iltered (48-μm mesh) and then centrifuged at 800×g, 4 °C for 10 min. The obtained pellet was resuspended in Hank’s buff er to adjust the cell concentration to 106cells/mL. The intracellular ROS content was determined by incubating the cells with 2′,7′-dichlorof luorescein diacetate (DCFH-DA)at a f inal concentration of 10 μm at 37 °C for 20 min,and ROS level was quantif ied by the median f luorescence intensity of 10 000 cells by FACS Arial II f low cytometer.
2.4 D etermination of antioxidant capability, lipid peroxidation, and digestive enzyme activities
Antioxidant is the basic and important function of hepatopancreas, so superoxide dismutase (SOD)activity, glutathione (GSH) level, and total antioxidant capacity (T-AOC) were applied together to assess the change of antioxidant capacity during the acidif ication for 56 days. malondialdehyde (MDA) content was often used for the evaluation of the lipid peroxidation(Valavanidis et al., 2006). To investigate the eff ect of OA exposure on the digestive capacity of the hepatopancreas, the activity levels of three digestive enzymes including pepsin (PES), lipase (LPS), and amylase (AMS) were measured. The SOD activity was estimated by the nitro blue tetrazolium method according to the literature (Beauchamp and Fridovich,1971). The level of GSH activity was measured based on the method of dithionitrobenzoic acid (DTNB) by detecting the absorbance of the yellow compound produced (Ringwood et al, 1999). The T-AOC was estimated by detecting the absorbance of 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid radical cation (ABTS) according to the reference(Miller et al., 1993). Lipid peroxidation was quantif ied by measuring the thiobarbituric acid reactive substances (TBARS) produced during lipid peroxidation, and expressed in terms of MDA content. The activity of PES was estimated by detecting the absorbance of the blue substance reduced by phenol-containing amino acids according to the reference (Rungruangsak and Utne, 1981). The LPS activity was measured based on the method of methyl resoruf in substrate (Steiner, 2005). The AMS activity could be calculated by detecting the absorbance of the blue complex formed by the combination of iodine solution and unhydrolyzed starch (Xiao et al., 2006).
These physiological and biochemical parameters were measured with commercially available kits(Jiancheng, Nanjing, China) according to the manual instruction. For every indictor, three pieces of thawed tissues from each experimental group were randomly applied as the biological replicates. Each pieces of 100-mg tissues (wet weight) were homogenized by adding 9-fold phosphate buff ered saline (PBS) buff er in a weight: volume ratio of 1:9 and the homogenates were then centrifuged at 3 500 r/min, 4 °C for 10 min.The supernatants obtained from each tissue were divided into three equal portions as technical replicates and prepared for detection. The total protein concentration in the supernatant was measured using the BCA assay kit (Tiangen, Beijing, China) to normalize the relevant data. The absorbance values were determined by the microplate reader (BioTek Synergy, Vermont, USA). SOD activity and T-AOC were expressed as U/mg and mmol/g of tissue protein,respectively. GSH and MDA content was expressed as nmol/mg of tissue protein. PES and AMS activity units were expressed as U/mg, whereas LPS activity was expressed in U/g of the tissue protein.
2.5 Genomic DNA extraction and deep amplicon sequencing of the 16S rDNA gene
Genomic DNA extraction and amplicon sequencing were accomplished by the company (Novogene,Beijing, China). Genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB)reference to the literature (Raimundo et al., 2018).The quality and concentration of DNA were detected by agarose gel electrophoresis and NanoDrop®spectrophotometer, respectively. The DNA sample was diluted to 1 ng/μL and used for the amplif ication for the 16S rDNA gene using primers of 515F(5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R(5′-GGACTACHVGGGTWTCTAAT-3′) targeting the variable regions 16S V4. The obtained PCR products were then purif ied with the Gel recovery Kit of Qiagen Gel Extraction Kit (Qiagen, Germany). The library was constructed using the Tru Seq®DNA PCR-Free Sample Preparation Kit (Illumina,California, USA). The constructed library was quantif ied by Qubit 4.0 Fluorometer (Thermo Fisher Scientif ic, Massachusetts, USA) and ABI 7500 Real time Thermal Cycler (Thermo Fisher Scientif ic).After the library was qualif ied, NovaSeq6000 sequencing platform (Illumina, California, USA) was used for on-line sequencing.
2.6 Data processing and analysis
The results of biochemical parameters were expressed as means±SD (n=3) and analyzed by Statistical Package for Social Sciences (SPSS) 17.0.The signif icant diff erences among groups were tested by one-way analysis of variance (ANOVA) and multiple comparisons and the assumptions of ANOVA(normality and homogeneity of variances) were met.The relationship was analyzed by the Pearson test.Diff erences were considered signif icant atP<0.05.
Raw reads of amplicon sequencing were processed using QIIME (V1.9.1) (Caporaso et al., 2010) to obtain eff ective tags. After stitching and f iltering, all the eff ective tags were clustered using Uparse (Uparse v7.0.1001) software (Haas et al., 2011). The eff ective sequences were clustered into Operational Taxonomic Units (OTUs) based on 97% sequence identity. The Mothur method and SILVA’s SSUr RNA database were used for species annotation and classif ication analysis (Edgar, 2013). Samples were raref ied to the maximum depth of the sample with less sequencing depth, and rarefaction curves were plotted. Alpha diversity was evaluated using the Chao1 and Shannon Diversity Index. The Wilcox test of the agricolae package was used to analyze the sample α-diversity.
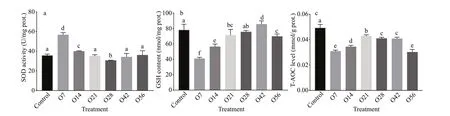
Fig.1 SOD (a), GSH (b), and T-AOC (c) level in the hepatopancreatic after OA exposure
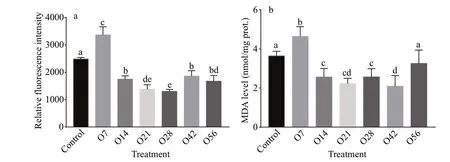
Fig.2 ROS (a) and MDA (b) level in the hepatopancreatic of oyster after OA exposure
3 RESULT
3.1 Eff ects of OA exposure on antioxidant system capability
The antioxidant for SOD activity, GSH level, and T-AOC were presented in Fig.1. Compared with the control group, SOD activity increased signif icantly on day 7 after OA exposure, which was about 1.59-fold of the control group. Then it decreased to the lowest concentration on day 28 with 30.49 U/mg, and gradually returned to normal level on the 42ndday(34.06 U/mg) and the 56thday (35.94 U/mg) (P<0.05)(Fig.1a). The GSH level decreased signif icantly from 78.04 μmol/g of the control group to 40.88 μmol/g of the O7 group, then it displayed an increasing tread until it reached the highest level with 85.56 μmol/g on day 42, followed by a bit drop with the concentration of 69.70 μmol/g on day 56 (P<0.05) (Fig.1b). The content of T-AOC decreased signif icantly during the whole acidif ication process, especially on day 56 when it decreased by 38.88% compared to the control group (P<0.05) (Fig.1c).
3.2 Eff ects of OA exposure on oxidative stress
The indicators of oxidative stress, ROS level, and MDA content were shown in Fig.2. In this study, ROS level quantif ied by the relative f luorescence intensity of the hepatopancreas of the O7 group increased signif icantly to 1.35-fold (P<0.05) compared with the control group, while the intensity decreased signif icantly after that (P<0.05), with the lowest level on day 28 and reduced by 47.67% (Fig.2a). Similarly,the MDA content increased signif icantly from 3.64 nmol/mg in the control group to 4.64 nmol/mg in the O7 group, and then it decreased to a signif icantly lower level in O14, O21, O28, and O42 groups, but it recovered to the control level on day 56 after OA exposure (Fig.2b).
3.3 Eff ects of OA exposure on digestive enzyme activities
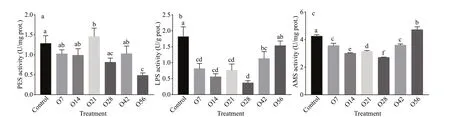
Fig.3 PES (a), LPS (b), and AMS (c) activity in the hepatopancreatic after OA exposure
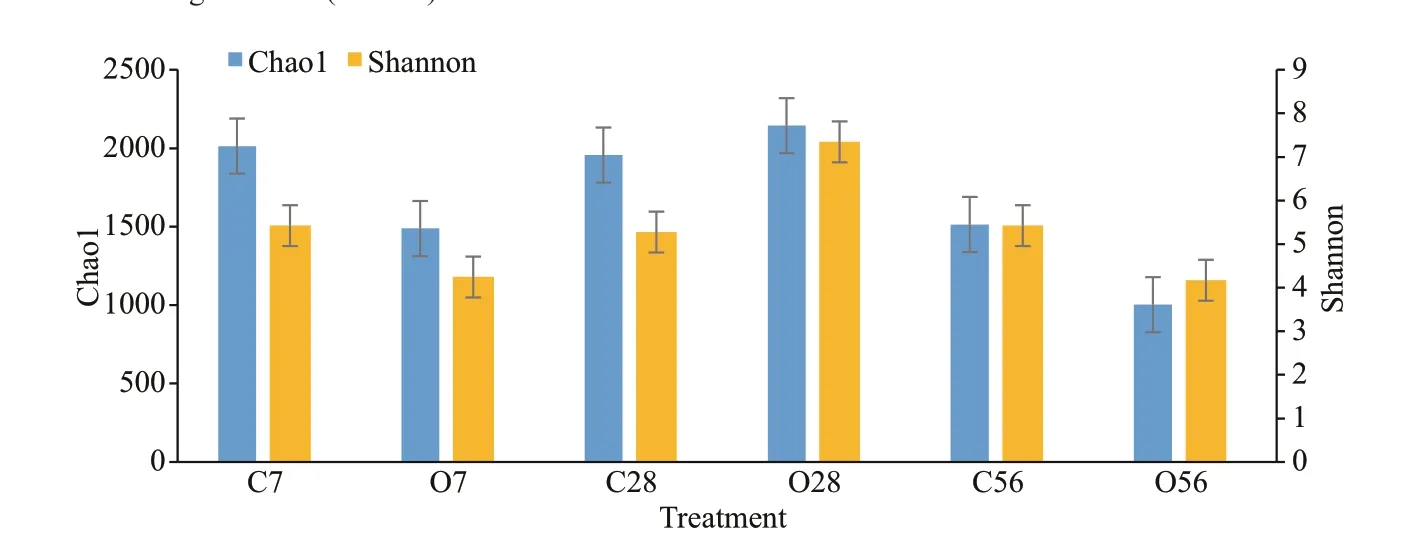
Fig.4 Chao1 and Shannon index of microbiota in the control groups and OA-treated groups
The enzymatic activities for PES, LPS, and AMS in all treatments were presented in Fig.3. The activity of PES in the hepatopancreas displayed no signif icant change during 42 days of OA exposure but decreased by 62.47% on day 56 (P<0.05) (Fig.3a). LPS activity was signif icantly reduced since day 7 after OA exposure, and reached a minimum level on day 28 at the level of 0.367 U/g. After that, it recovered gradually and almost reached the control level of 1.53 U/g on day 56. The change of AMS activity was similar to LPS that AMS activity decreased to the minimum level with 2.69 U/mg in the O28 group, and then increased to a signif icantly higher level on day 56 with 4.62 U/mg than that of the control group(P<0.05) (Fig.3b & c).
3.4 Characterization of bacterial communities by 16S rDNA deep amplicon sequencing analysis
The DNA samples from six groups including C7,O7, C28, O28, C56, and O56 were applied for the analysis of 16S rDNA deep amplicon sequencing, and a total of 1 145 895 eff ective tags were obtained(Supplementary Table S1). All Eff ective Tags were clustered into 5 007 OTUs based on 97% identity.Rarefaction analysis was performed to standardize and compare the taxon richness between samples. As shown in Supplementary Fig.S1, the rarefaction curve of diff erent samples approached the saturation plateau,indicating that the data amount was reasonable, which met the requirements of subsequent analysis.
Alpha diversity metrics of the total species abundance index (Chao1) and species diversity index(Shannon) were presented in Fig.4. The two indexes showed the same change tendency which was more clearly for the Shannon index. Taken Shannon index for details, it maintained no obvious change in the control groups, but in OA groups, it f irstly reduced to a signif icantly lower level than that of the C7 group,and then it increased a bit in O28 group, but f inally the Shannon index in O56 group decreased to the lowest level of the six groups. Brief ly, the variations of Chao1 and Shannon indexes both suggested that the microbial community experienced a dynamic change after OA exposure, and the abundance of microbiota reduced greatly at the end of the two months of acidif ication.
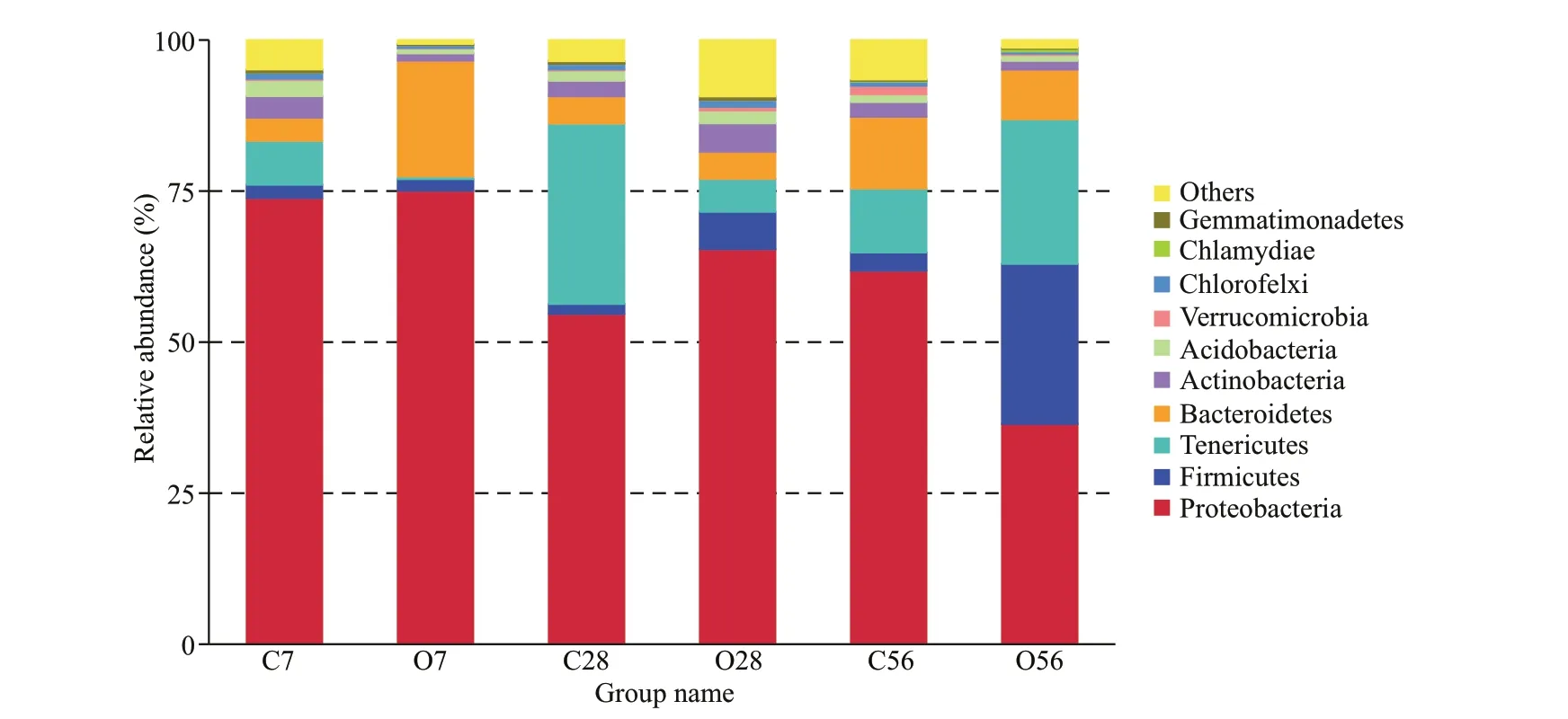
Fig.5 Relative abundance of bacterial phyla in the control groups and OA groups
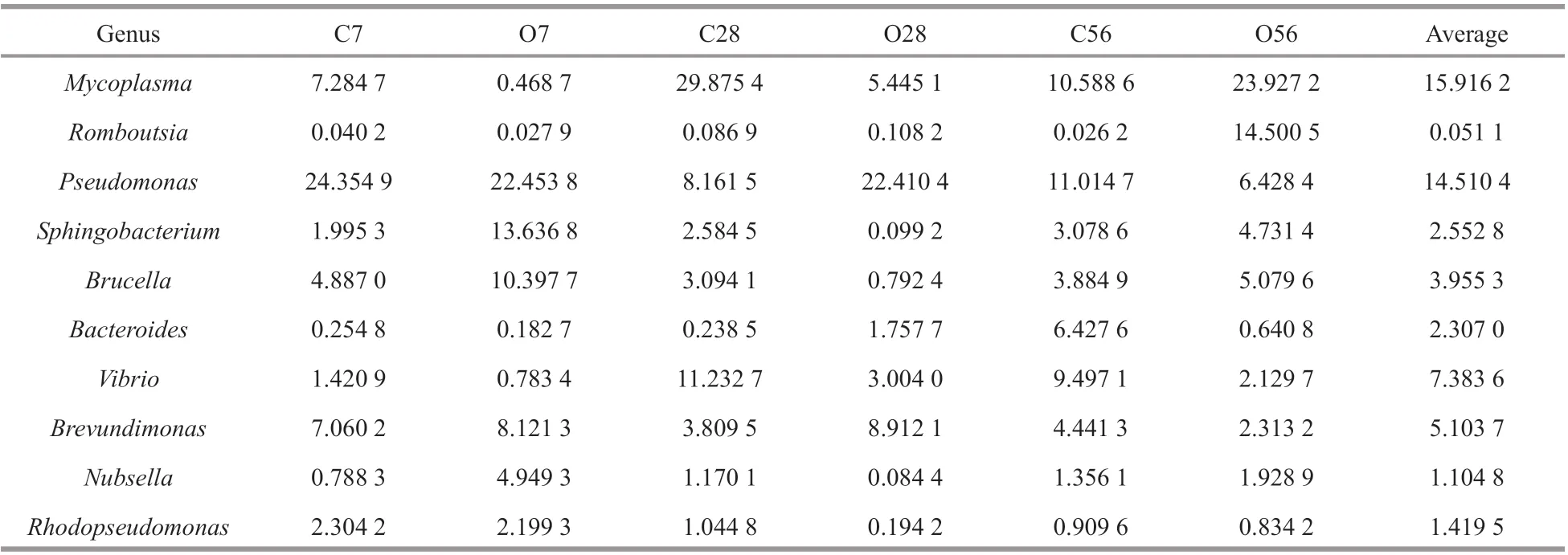
Table 1 The abundance percentage (%) of bacterial genus in the control groups and OA-treated groups
The relative abundance of microbial communities at the phylum level was analyzed and the relative abundance of the top 10 phyla was presented in Fig.5.The dominant phylum in the hepatopancreas microbiota included Proteobacteria, Firmicutes,Tenericutes, and Bacteroidetes, all of which accounted for 81.52%-96.58% of the reads in the six groups, but the four phyla bacteria showed diff erent change tendency during the acidif ication. Among them, the phylum Proteobacteria, which accounted for the largest ratio in all the groups, did not change after 7 days of acidif ication and increased from 54.64% in C28 to 65.36% in O28, while its abundance reduced from 61.81% in C56 to 36.44% in O56 group. The phylum of Firmicutes also did not change signif icantly after 7 days of acidif ication, while its abundance increased to 3.73 times in O28 and 8.74 times in O56 than that in the control group, which f inally accounted for 26.50% of the reads in the O56 group. The abundance of Tenericutes decreased obviously after 7 and 28 days of acidif ication, but it increased from 10.59% in C56 to 23.94% in O56. For the Bacteroidetes, although its abundance increased to a much higher level of 19.14% after 7 days of CO2treatment than the C7 group (3.81%), it recovered to normal levels in the O28 and O56 groups compared with C28 and C56 groups, respectively.
The abundance percentage of microbial communities at the genus level was further analyzed and the relative percentage of the top 10 genera were presented in Table 1. According to the mean value of the dominant species in the group of C7, C28, and C56, the three dominant genera in the control groups wereMycoplasma(15.9%),Pseudomonas(14.5%),andVibrio(7.4%). To describe the change of the main species of microbiota, the abundance percentage of the three dominant genera were compared during the OA exposure. On day 7, the abundance percentage of genusMycoplasmain the O7 group decreased from 7.284 7% to 0.468 7%, andVibriodecreased from 1.420 9% to 0.783 4%, while there was no much diff erence forPseudomonas. Compared with the control, the abundance percentages ofMycoplasmaandVibrioin the O28 group decreased by 24.430 3%and 8.228 7%, respectively, whilePseudomonasgenus increased from 8.161 5% to 22.410 4%. On the contrary, the abundance percentage ofMycoplasmain the O56 group increased from 10.588 6% to 23.927 2%, andRomboutsiaincreased from 0.026 2%to 14.500 5% relative to the control group, whilePseudomonasreduced from 11.014 7% to 6.428 4%.

Fig.6 Abundance heap map of bacterial genus in the control groups and OA-treated groups
The top 35 most abundant genera in the control groups and OA-treated groups were selected for heatmap analysis (Fig.6). The 35 genera belonged to the phyla of Actinobacteria, Bacteroidetes, Firmicutes,Proteobacteria, Tenericutes, and Verrucomicrobia,which were the most abundant as shown in Fig.5.After 7-day OA exposure, f ive genera includingCoxiella,Nubsella,Sphingobacterium,Brucella, andDevosiabecome more abundant than that in the C7 group. For the O28 group,Paracoccus,Enhydrobacter,Acinetobacter, andLimnobacterwere more enriched than that in the C28 group. Notably, the enriched genera all belonged to microaerobic bacteria. After long-term OA exposure of 56 days, Order Clostridiales(unidentif ied Clostridiales, unidentif ied Lachnospiraceae, unidentif ied Ruminococcaceae,Romboutsia, andFaecalibacterium), which was famous for the anaerobic property, was more abundant than that of the C56 group.
4 DISCUSSION
Environmental stress can cause oxidative damage and elevate the steady-state level of ROS (Rada and Leto, 2008; Lushchak, 2011). Antioxidants, such as SOD and GSH are important substances that prevent ROS-mediated oxidative damage in bivalves(Soldatov et al., 2007). In this study, the activity of SOD increased signif icantly and the content of GSH decreased signif icantly after 7 days of acidif ication(Fig.1a & b), indicating that the enhanced antioxidant response of the hepatopancreas might be an early adaptation mechanism that eliminated excess ROS under acidif ication stress. The similar results were observed in the hepatopancreas of clamChameleagallinaand the musselMytilusgalloprovincialis,which showed that the OA stress generally increased the activity of antioxidant enzymes after 7 days of acidif ication (Matozzo et al., 2013). Nevertheless, the T-AOC of the hepatopancreas reduced signif icantly after OA (Fig.1c), indicating that OA would weaken the antioxidant capacity of the hepatopancreas.Correspondingly, the ROS and MDA content increased signif icantly after 7 days of acidif ication(Fig.2), which indicated that the oysters suff ered severe oxidative damage. The antioxidant system plays an important role in resisting environmental pressure, and its weakening will not be conducive to the long-term survival of oysters.
The activities of digestive enzymes experienced a dynamic process during the OA period. Some evidence showed that OA could inhibit the digestive enzyme activity of bivalves (Kong et al., 2019; Wang et al., 2020). For example, the hepatopancreas structure of blue mussel,Mytiluseduliswas destroyed and the activities of AMS, protease, and LPS reduced signif icantly after 21 days of treatment in seawater with a pH of 7.10 (Xu et al., 2020). It was same that we found the AMS and LPS activities of oysters reduced signif icantly, but the diff erence was that the PES had no signif icant diff erence under short-term OA stress, and its activity reduced signif icantly only after long-term OA stress (Fig.3a). In addition, the enzyme activities of LPS and AMS tended to return to the control levels or even increased after long-term OA (Fig.3b & c). Digestive enzyme patterns can ref lect the digestive capacity and indirectly ref lect the metabolic prof iles of various substances in oysters,and the metabolic effi ciency determines the energy output (Debnath et al., 2007). The recovery of some certain digestive enzymes may be related to the energy metabolism of hepatopancreas and the adaptability to acidif ication. The previous studies have found that the main metabolic substrate in the energy metabolism ofPetrolisthescinctipesin OA was converted from lipid to proteins (Carter et al.,2013). However, after long-term OA treatment,protein digestion might not be sustainable, and the hepatopancreas metabolic pathways inclined to restore the activities of lipase and amylase to increase energy input. Especially for AMS, the enzyme activity was signif icantly higher than that of the control group after 56 days of acidif ication. The adaptation strategy of diff erent species might be diff erent. The previous study found that the activity of trypsin in the hepatopancreas of the thick shell musselsMytiluscoruscuswas signif icantly inhibited after acidif ication for 7 days, but there was an obvious upward trend at day 14, which presumably due to the adaptive mechanism ofM.coruscusunder acidif ication conditions (Wang et al., 2020). In short, the dynamic course of the activities of the three digestive enzymes indicates that acidif ication can cause signif icant changes in the substrates of energy metabolism on hepatopancreas, which may be benef icial to the adaptation of oysters to the acidif ication environment.
The analysis of microbiota composition revealed that the microbiota of hepatopancreas had a high level of abundance and diversity, and more than 1 000 OTUs were found in the hepatopancreas microbiota under the normal condition (Supplementary Table S1). Among them, Proteobacteria, Firmicutes,Tenericutes, and Bacteroidetes were the dominant phyla, accounting for 81.52%-96.58% of the reads in the six groups, and other species were extremely low in abundance which might coexist harmlessly with the host in a normal environment (Fig.5). Similar results were observed in oysterCrassostreasikameaand scallopArgopectenpurpuratus(Bernal et al.,2017; Muñoz et al., 2019), which showed that the rich diversity and the same dominant phyla. In the genus level,Mycoplasma(15.9%),Pseudomonas(14.5%),andVibrio(7.4%) were the three-dominant genus of the hepatopancreas microbiota in normal oyster(Table 1). Traces of the three genera have been found in various bivalve microbiota, such asCrassostreavirginica(Li et al., 2019) and Pearl oysterPinctadamazatlanica(Tremaroli and Bäckhed, 2012). Studies have shown thatMycoplasmaappears abundant in the digestive gland of the healthy Sydney rock oystersSaccostreaglomerataand the intestines of the abaloneHaliotisdiscushannai(Green and Barnes, 2010;Roeselers et al., 2011). Some species ofMycoplasmasare only part of the natural microbiota and harmlessly symbiotic with the host, which use the substrate produced by the host or other microorganisms during the digestion process to proliferate (Pitcher and Nicholas, 2005).Pseudomonasproduces a wide range of secondary metabolites, such as antibiotics,hydrogen cyanide or iron-chelating siderophores, and can inhibit a wide range of pathogenic bacteria(Campa-Córdova et al., 2009). The genusVibriois also a natural part of the host microbiota (Fernandez-Piquer et al., 2012; Vezzulli et al., 2018), which can provide chemical defense and produce digestive enzymes (AMS, LPS, and chitinase) to decompose dietary components for the host (Engel et al., 2002;Ray et al., 2012). The stable structure of microbiota is essential to maintain the homeostasis of the host(Sokolova et al., 2011), and a good composition and high diversity of hepatopancreas microbiota is indispensable for the physiological functions of oysters.
However, further analysis displayed that the structure of the hepatopancreas microbiota showed an obvious dynamic change during the process of acidif ication, and f inally was changed greatly by OA treatment. At the beginning, the diversity of microbiota was greatly decreased compared with the control on day 7 of acidif ication (Fig.4), and the abundance of some species, such asMycoplasmaandVibriowas reduced greatly (Table 1), indicating that the hepatopancreas microbiota was sensitive to the change of the external environment, and the OA treatment might lead to apparent changes in the composition of the dominant genus and disrupt the dynamic balance of the original microbiota. The imbalance of bacterial community structure would be conducive to the growth of potential pathogenic components and the invasion of external pathogens(García-Bayona and Comstock, 2018), as revealed in the O28 group, a noticeable increase was observed in the abundance ofPseudomonas,Paracoccus,Enhydrobacter, andAcinetobacter, which were known as pathogens in marine aquaculture (Ferguson et al., 2004; Li et al., 2017). The higher abundance level of these genus bacteria might damage the immune defense function of the bacterial community and stimulate the invasion of the external bacteria,resulting in a temporarily richer diversity of microbiota in the O28 group. However, under the long-term OA treatment, the abundance and diversity of hepatopancreas microbiota were irreversibly decreased, as revealed by the decreased the Chao1 and Shannon index in the O56 group. To be detailed,manyVibriospecies could help the host to decompose dietary components by producing digestive enzymes(AMS, LPS, and chitinase) (Ray et al., 2012). The abundance ofVibriodecreased signif icantly after long-term OA, which might be detrimental to the digestive process of the host in this study. In addition,it was found that the bacterial community was dominated by anaerobic or facultative anaerobic bacteria in the O56 group, which might be the consequence of increased hypoxia due to OA induced reduction of f iltration activity (Xu et al., 2020). Taken an example, the abundance of order Clostridiales(unidentif ied Clostridiales, unidentif ied Lachnospiraceae, unidentif ied Ruminococcaceae,Romboutsia, andFaecalibacterium), which was famous for anaerobic, increased signif icantly (Fig.6).These bacteria can produce lactic acid, formate, and other acidic products through anaerobic fermentative,which further promotes the acidif ication of the host internal environment (Mountfort et al., 1998;Bosshard et al., 2002; Duncan et al., 2002; Aujoulat et al., 2014; Gerritsen et al., 2014). In addition, the genusMycoplasma, for example, which was a wellknown pathogen, was increased by 13.34% in the O56 group relative to the control (Table 1). Studies have shown that lower microbiota diversity in invertebrate is related to the presence of pathogens,and poor species richness is related to the sensitivity to pathogen invasion (Edgar, 2018), so long term acidif ication may threaten the survival of oysters due to the excessive proliferation of some potential pathogenic bacteria (Rottem, 2003; Pitcher and Nicholas, 2005). In short, the structure of the hepatopancreas microbiota was drastically changed after long-term OA, causing the decline of microbial diversity and abnormal proliferation of pathogenic and anaerobic bacteria, which in turn might further interfere with the digestive system, the immune reactions, and even the survival of oysters.
According to our result, it is worth noting that some of the responses and reactions in oyster hepatopancreas, such as the SOD activity, GSH content, MDA level, and the activities of LPS and AMS, tended to return to the control level after drastic changes, which indicated that oysters could partially adapt to the acidif ied environment through the changes of some enzymes or substances after longterm acidif ication. Taken the enzymes in the digestive system as an example, studies have found that bivalves can alleviate or even off set the adverse eff ects of OA under adequate food conditions, which fully demonstrates the importance of digestive capacity to adapt to acidif ied environments for bivalves (Melzner et al., 2011; Thomsen et al., 2013;Pansch et al., 2014). In this study, the activity of AMS increased signif icantly relative to control after longterm acidif ication, supposing that feeding high-starch content might be conducive to the survival of oysters in an acidif ied environment. On the contrary, some other reactions have shown an obvious diff erence between short-term and long-term OA conditions. For instance, ROS level increased signif icantly after acidif ication for 7 days but decreased signif icantly after long-term acidif ication. The abundance and diversity of hepatopancreas microbiota increased obviously after acidif ication of 28 days but decreased in O56 group. The available data not only remind us that two months or even longer time are required for the purpose to better understand the adaptability of shellf ish to the elevated CO2, but also suggest the possibility to improve the adaptability of oysters to OA to some extent by adding antioxidants and the abundance and variety of probiotics to oyster feed.
5 CONCLUSION
Having characterized the responses on the antioxidant capacity, the digestive ability, and the microbiota composition in the hepatopancreas of oysterC.gigasunder OA conditions, we revealed that the recovery of LPS and AMS activity might be benef icial to the adaptation of oysters to the acidif ication environment, while the inhibition of T-AOC could cause the accumulation of oxidative damage during the long time OA condition.Meanwhile, the inevitable decline of microbial diversity and the abnormal proliferation of pathogenic and anaerobic bacteria caused by OA may aff ect the normal physiological function of the hepatopancreas,which would be harmful to the survival of oyster. The data are helpful for the understanding of the potential implications of OA for shellf ish physiological functions, and provide some scientif ic basis to improve the adaptability to the elevated CO2.
6 DATA AVAILABILITY STATEMENT
The data used to support the f indings of this study are available from the corresponding author upon request.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
