Quantitative proteomics provides insight into the response of the marine dinof lagellate Prorocentrum donghaiense to changes in ambient phosphorus*
Shufeng ZHANG , Chunjuan YUAN , Ying CHEN , Lin LIN , Dazhi WANG ,2,**
1 State Key Laboratory of Marine Environmental Science/College of the Environment and Ecology, Xiamen University, Xiamen 361102, China
2 Key Laboratory of Marine Ecology & Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Dinof lagellates are the major causative agents of harmful algal blooms in the global ocean and they usually form blooms under conditions of very low dissolved inorganic phosphorus (DIP). However, the mechanisms underpinning the dinof lagellate blooms remain unclear. Here, we quantitatively compared protein expression prof iles of a marine dinof lagellate, Prorocentrum donghaiense, grown in inorganic P-replete,P-def icient, and DIP- and dissolved organic phosphorus (DOP)-resupplied conditions by employing a Tandem Mass Tag (TMT)-based quantitative proteomic approach. Proteins involved in intracellular P reallocation,organic P, and non-P lipid utilization were up-regulated under the P-def icient condition, while inorganic phosphate transporters varied insignif icantly. In response to the P resupplementation, nitrogen metabolism,ribosome, porphyrin, and chlorophyll metabolism were up-regulated, while lysosome, and starch and sucrose metabolism were down-regulated. Notably, photosynthesis was up-regulated and secondary metabolism was down-regulated only in the DIP-resupplied cells, whereas amino acid metabolism and vitamin B6 metabolism were up-regulated in the DOP-resupplied cells, indicating diff erential response mechanisms of P. donghaiense to DIP or DOP resupplementation. Our results indicated that P. donghaiense initiated multiple strategies in response to an ambient inorganic P-def iciency, and its effi cient DOP assimilation by providing both P and carbon sources might be a key factor driving bloom formations of P. donghaiense in a low DIP environment.
Keyword: marine dinof lagellates; harmful algal blooms; Prorocentrum donghaiense; phosphorus;quantitative proteomics
1 INTRODUCTION
Dinof lagellates are the major agents responsible for harmful algal blooms (HABs) in the global ocean,which cause serious ecological, environmental, and health problems (Anderson et al., 2002; Wells et al.,2015, 2020). Studies have shown that dinof lagellates usually form blooms in the low-nutrient conditions or environments (Zhou et al., 2017; Xiao et al., 2018),but the underlying molecular mechanisms remain poorly understood. As a limiting nutrient in the ocean,phosphorus (P) is recognized as one of the key factors regulating cell growth and bloom formation of marine dinof lagellates (Do Rosário Gomes et al., 2014;Fricke et al., 2015; Shi et al., 2017). Dissolved inorganic P (DIP) and dissolved organic P (DOP) are the two major available sources of P for marine dinof lagellates (Dyhrman, 2016). However, DIP concentrations are usually low, at less than 0.5 μmol/L,which cannot fulf ill the needs of dinof lagellates’growth, let alone their bloom formation (Benitez-Nelson, 2000; Cañellas et al., 2000; Ou, 2006; Ou et al., 2015; Dyhrman, 2016). DOP comprises a signif icant portion of the total P in both oceanic and coastal waters and it can be utilized by some dinof lagellate species (Ou et al., 2015; Dyhrman,2016; Shi et al., 2017; Zhang et al., 2019a, b). It has been postulated that the capacity of dinof lagellates to utilize DOP in DIP-def icient ambient conditions is essential to their success and bloom formation in the ocean (Zhang et al., 2014, 2018, 2019a, b; Ou et al.,2015; Shi et al., 2017). Yet a comprehensive understanding of the dinof lagellates’ response to changing ambient DIP or DOP is still limited,especially at the molecular level.
Prorocentrumdonghaienseis a typical dinof lagellate species that causes extensive HABs in the coastal East China Sea, which seriously threatens the marine ecosystem, mariculture, and environmental health (Li et al., 2014; Zhou et al., 2017; Yu et al.,2018). The strong ability ofP.donghaienseto utilize DOP in a low DIP environment is reportedly an important reason for why it can gain a competitive advantage and form blooms (Ou, 2006; Ou et al.,2015; Zhang et al., 2018, 2019b). However, our understanding ofP.donghaiensein response to changes in either ambient DIP or DOP is surprisingly limited. Our previous study showed that the physiological responses of the P-def icientP.donghaienseto DIP and DOP resupply are similar(Zhang et al., 2019b). Building on that f inding, this study compared global protein expression prof iles ofP.donghaienseunder P-replete, P-def icient, and DIPand DOP-resupplied conditions by using a Tandem Mass Tag (TMT)-based quantitative proteomic approach that combined the corresponding transcriptomic database and characterized the diff erentially expressed proteins. Our results reveal a signif icantly diff erent proteomic response ofP.donghaienseto DIP versus DOP resupply, and that DOP could provide both P and carbon sources for sustained cell growth which triggers bloom formation ofP.donghaienseunder the low DIP environment.
2 MATERIAL AND METHOD
2.1 Marine organism and culture conditions
TheProrocentrumdonghaiensestrain was isolated from the East China Sea, in May 2014, and routinely maintained in K-medium at 20 °C under a 14-h:10-h light:dark cycle (Keller et al., 1987), with a light intensity of approximately 100 μmol/(m2·s) provided by f luorescent lamps. Before the experiment, the culture was treated with an antibiotic cocktail to minimize bacterial contamination and then inoculated into fresh K-medium after rinsing it with autoclaved seawater (Zhang et al., 2016).
2.2 Experimental design
To investigate the response ofP.donghaienseto ambient P-def iciency and the utilization mechanism of DOP in a low DIP environment, four treatments were set up as described in our previous study (Zhang et al., 2019b), which consisted of the P-replete,P-def icient, DIP-resupplied, and DOP-resupplied groups with diff erent ecological relevance(Supplementary Table S1). Each treatment had triplicate biological repeats and the initial cell density was 8.0×103cells/mL. At the beginning of the experiment, cultures with 10.0-μmol/L and 0.2-μmol/L Na2HPO4were respectively def ined as the P-replete and P-def icient groups. According to the results of physiological response (Zhang et al., 2019b), the P-replete cells entered the exponential phase on day 4, while the P-def icient cells exhibited P limitation on day 6 according to the result of alkaline phosphatase activity (APA) (Supplementary Fig.S1). At this point,a f inal concentration of 10.0-μmol/L Na2HPO4or Glucose-6-phosphate (G-6-P) was added to the three P-def icient cultures, respectively, for recovery,constituting the DIP- and DOP-resupplied groups.
2.3 Protein preparation
According to the physiological responses ofP.donghaiensepreviously reported on (Supplementary Fig.S1) (Zhang et al., 2019b), there is no signif icant change in cell density on day 4 and 6 in the P-def icient group, but the result of APA shows that the cells are limited by P on day 6 but not on day 4 (Supplementary Fig.S1). Therefore, in order to ref lected their real P status and the diff erence between the P-replete group and the P-def icient group, samples of the P-replete cells on day 4, the P-def icient cells on day 6, and the DIP- and DOP-resupplied-28 h cells were collected for quantitative proteomic analysis, using three replicates.
Protein extraction was conducted using the lysisbuff er extraction method (Zhang et al., 2015). Brief ly,the samples were f irst ground by liquid nitrogen and then transferred to a 1.5-mL centrifuge tube containing a lysis buff er (8-mol/L urea, 1% Triton-100,65-mmol/L dithiothreitol, and 0.1% protease inhibitor cocktail). Total protein was obtained after sonication,centrifugation, acetone precipitation, and air-drying.Finally, protein content was quantif ied using a 2D Quant Kit (GE Healthcare, USA).
2.4 Peptide labeling and fractionation
In this study, 100 μg of protein was taken from each sample, and 100-mmol/L borane-triethylamine complex (TEAB) was added to dilute the urea concentration to less than 2 mol/L. Trypsin was added in a mass ratio of trypsin:protein=1:50, and this solution was further enzymatically hydrolyzed at 37 °C; then trypsin was added once more(trypsin:protein=1:100), and the enzymatic hydrolysis continued for 4 h at 37 °C. After trypsin digestion, the peptide was desalted by a Strata X C18 (Phenomenex,USA) and vacuum-dried. Peptides were redissolved in 0.5-mol/L TEAB and then labeled with the 6-plex TMT kit according to its manual: Tag126, P-replete;Tag127, P-def icient; Tag128, DIP-resupplied; Tag129,DOP-resupplied. The sample was subjected to liquid phase separation by high pH reversed-phase highperformance liquid chromatography (RP-HPLC),using an Agilent 300 liquid phase system (C18 separation column, 5-μm particle size, 4.6-mm inner diameter, 250 mm in length). Peptides were f irst separated into 80 fractions, by mixing with 2%-60%acetonitrile in a 10-mmol/L ammonium bicarbonate solution of pH 10 for 80 min, and then the peptides were combined into 20 fractions and vacuum-dried.
2.5 LC-MS/MS analysis
Each fraction was dissolved in 0.1% formic acid(FA) and then loaded onto a reversed-phase precolumn (Acclaim PepMap 100, Thermo Scientif ic,USA), and the peptide was separated via a reversedphase analytical column (Acclaim PepMap RSLC,Thermo Scientif ic). The elution procedure went as follows: each sample was f irst eluted with 0.1% FA and 98% acetonitrile for 45 min, then the linear gradient was increased from 6% to 23%, and increased again, from 23% to 36% within 15 min, after which it rose to 85% within 5 min. The elution was carried out on an EASY-n LC1000 ultra-performance liquid chromatography (UPLC) system (Thermo Scientif ic),at a constant f low rate of 280 mL/min, and the ensuing peptides were analyzed by a Q Exactive TM Plus hybrid quadrupole-Orbitrap mass spectrometer(Thermo Scientif ic, USA). Peptide fragments were separated by the UPLC system and ionized by implantation into the nanospray ionization (NSI) ion source, after which they were coupled to the online UPLC system using tandem mass spectrometry(MS/MS) in a Q Exactive TM Plus.
2.6 Bioinformatics analysis
Raw data for mass spectrometry was converted to the materials and geometry format (MGF) format,using Proteome Discoverer software, and the protein identif ication conducted with the Mascot search engine (v.2.3.0, Matrix Science, UK). In this study,the corresponding transcriptome of each of the four treatments was also analyzed. After de novo assembly with the Trinity software (Release-201302251),overall 220 440, 222 259, 229 711, and 225 876 unigenes were obtained from the P-replete, P-def icient,DIP-resupplied and DOP-resupplied cells,respectively (Zhang et al., 2019b). All datasets were deposited in the SRA database (BioProject ID:PRJNA522720). The putative amino acid sequences translated from the coding sequence (CDS) of unigenes served as the protein database. The following search parameters were designated: the enzyme was set to Trypsin, the maximum allowable number of missed sites was set to 2, the f ixed modif ier was set to carbamidomethyl (C), the variable modif ier was set to oxidation (M), TMT 6-plex (N-term), and TMT 6-plex (K); the mass error setting peptide precursor ion mass tolerance was 10×10-6, with a fragment mass tolerance of 0.02 Da; theP-value was set to <0.05 and the peptide cutoff (Score cutoff ) was set to >20; the false discovery rate (FDR) of the peptide was controlled (to be less than 1%). According to a previous study (Zhang et al., 2015), peptides at the 95% conf idence interval were used for protein identif ication, for which every conf ident designation involved at least one unique peptide. The quantif ication of protein was performed using Mascot and normalized by the median ratio in that software.Diff erentially expressed proteins (DEPs) were distinguished by pairwise comparisons, withP-values≤0.05 and a fold change ≥1.3 (up-regulated) or ≤0.77(down-regulated) used as the threshold criteria.
To further understand the function and characteristics of the identif ied proteins, each was annotated accordingly into four broad categories: Gene Ontology(GO), protein domain, Kyoto Encyclopedia of Genes and Genomes (KEGG), and subcellular location. GO annotation was derived from the UniProt-GOA database (http://www.ebi.ac.uk/GOA/). Firstly,converting identif ied protein ID to UniProt ID and then mapping to GO IDs by protein ID. If some identif ied proteins were not annotated by UniProt-GOA database, the InterProScan soft was used to annotate GO function of proteins based on protein sequence alignment method. Domain functional description of identif ied proteins was annotated by InterProScan (a sequence analysis application) based on protein sequence alignment method, and the InterPro domain Database (http://www.ebi.ac.uk/interpro/) was used. KEGG database was used to annotate protein pathway. Firstly, KEGG online service tool KAAS was used to annotate proteins based on KEGG database descriptions. Then the annotation results were mapped to the KEGG pathway database using KEGG online service tool KEGG mapper. The subcellular localization was predicted using subcellular localization predication soft wolfpsort(version: PSORT/PSORT II) (Feng et al., 2017).
GO and KEGG pathway functional enrichment analysis was performed by the Fisher’s exact test, and a correctedPvalue <0.05 was considered as signif icant(Feng et al., 2017). Brief ly, the numbers of DEPs and all quantitative proteins in each GO term or pathway were calculated, and then the total numbers of DEPs annotated by GO terms or pathway and all quantitative proteins annotated by GO terms or pathway were calculated, f inally, the four numbers were used to calculate Fisher’ exact testPvalues and enrichment folds (Feng et al., 2017). For further hierarchical clustering analysis based on DEPs functional classif ication, we f irst collated all the categories obtained after enrichment along with theirPvalues,and then f iltered for those categories which were at least enriched in one of the clusters withPvalue<0.05. This f ilteredPvalue matrix was transformed by the functionx=-log10 (Pvalue).
3 RESULT
3.1 Proteome overview
A total of 351 739 mass spectra were generated, of which 43 882 spectra were matched to 19 903 peptides with a spectrum-utilizing rate of about 12.5%. The mass error distribution of all peptides identif ied was near zero and most of them were less than 0.02 Da,which meant the mass accuracy of the MS data met the requirements for robust analysis (Fig.1a), and the length of most peptides was distributed between 8 and 16 amino acids, being consistent with the known properties of tryptic peptides (Fig.1b).
Overall, 8 348 proteins were identif ied from 15 553 unique peptides inP.donghaiense. To ascertain the repeatability of the experiment, Pearson’s correlation coeffi cient and the relative standard deviation (RSD)were calculated to evaluate the repeatability of each protein’s relative quantif ication (Fig.1). There was a signif icant positive correlation between the replicates,with negative or no correlations found between the diff erent treatments, thus indicating the quantitative repeatability was sound (Fig.1c). The average RSD of the biological replicates was <0.05, and all the quantiles were <1, further indicating that the quantitative repeatability of the experimental samples was very good (Fig.1d).
3.2 Diff erentially expressed proteins
According to the screening criteria for DEPs, the data of three replicates were combined according to the protein ID, to calculate three mean values: that of each protein’s relative expression among the diff erent comparison groups, the RSD, and the diff erentially expressedt-test statistic. In all, 1 182 proteins were signif icantly altered among the four treatments.Compared with the P-replete cells, 138 and 213 proteins were signif icantly up-regulated and downregulated in the P-def icient cells, respectively(Supplementary Fig.S2). After the recovery of DIP and DOP, 141 and 125 proteins were up-regulated and 175 and 101 proteins were down-regulated,respectively, relative to the P-def icient cells(Supplementary Fig.S2). Despite no signif icant diff erence in the dinof lagellate’s physiological response between the DIP- and DOP-resupplemented cultures (Zhang et al., 2019b), its proteomic responses nonetheless varied remarkably, including 129 upregulated proteins and 114 down-regulated proteins(Supplementary Fig.S2).
In further exploring the biological processes associated with DEPs, the up-regulated DEPs were mainly associated with lysosome, f lavone and f lavonol biosynthesis, taurine and hypotaurine metabolism,and oxidative phosphorylation in the P-def icient cells when compared with the P-replete cells, while the down-regulated DEPs were found mainly involved in porphyrin and chlorophyll metabolism, ribosome,terpenoid backbone biosynthesis, chloroalkane and chloroalkene degradation, and steroid biosynthesis(Fig.2). After the DIP resupply, processes such as porphyrin and chlorophyll metabolism, nitrogen metabolism, photosynthesis, and ribosome were upregulated compared with the P-def icient cells, whereas taurine and hypotaurine metabolism, lysosome, and f lavonoid biosynthesis were down-regulated (Fig.2).Yet some processes, such as ribosome, nitrogen metabolism, vitamin B6 metabolism and alanine,aspartate and glutamate metabolism, were upregulated, while lysosome, starch and sucrose metabolism, and taurine and hypotaurine metabolism were down-regulated, following the DOP resupply when compared with the P-def icient cells (Fig.2).
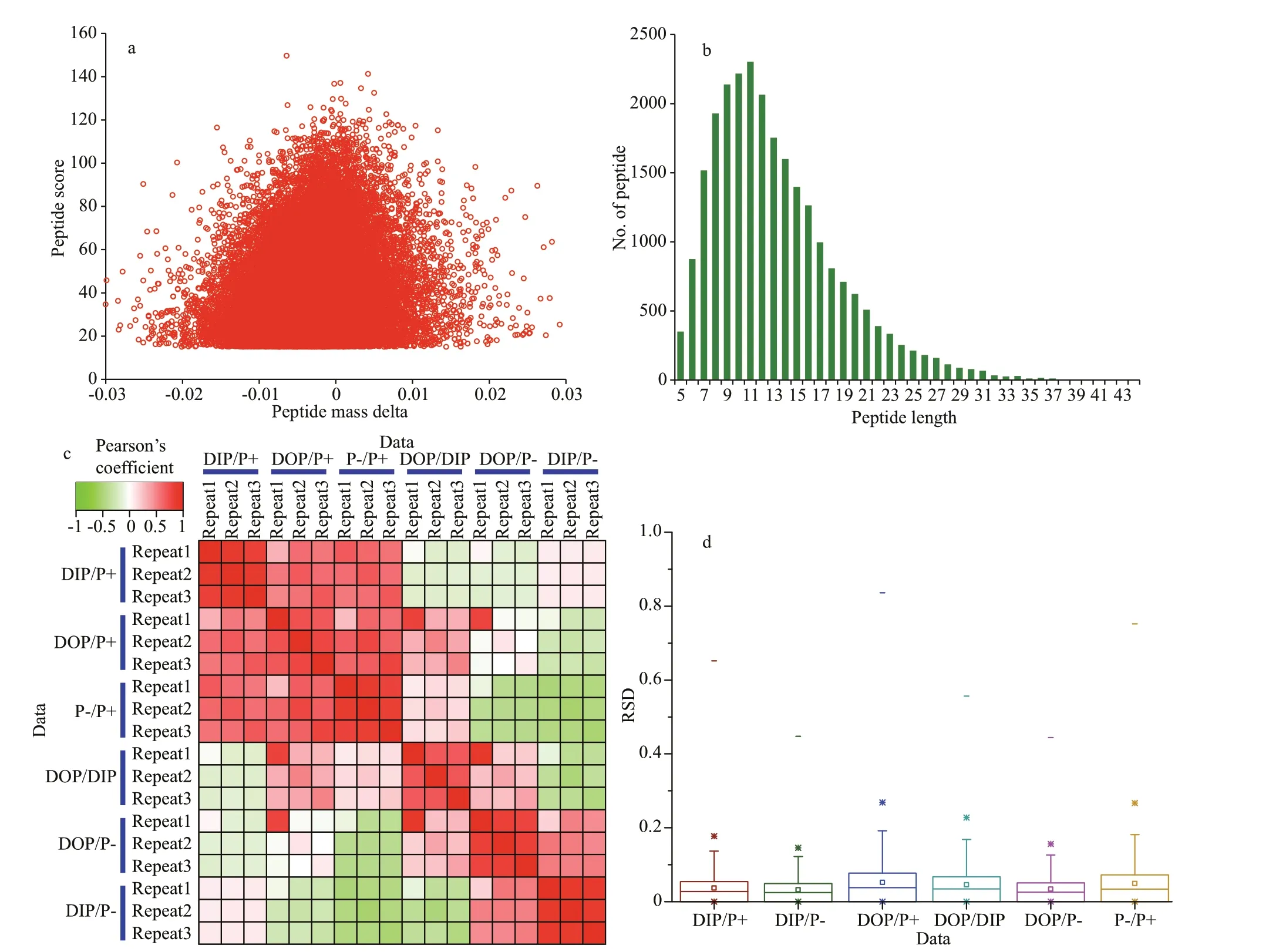
Fig.1 Quality control validation of MS data and validation of data reproducibility
Diff erent biological responses were also discerned between the DIP- and DOP-resupplied cells. These processes were mainly related to vitamin B6 metabolism, biosynthesis of stilbenoid,diarylheptanoid, and gingerol, as well as f lavonoid biosynthesis and photosynthesis (Fig.2). This pointed to diff erent utilization mechanisms of DIP and DOP byP.donghaiense.
3.3 P metabolism-related proteins
A total of 20 P metabolism-related proteins involved in phosphate transport, P reallocation,organic P utilization, and non-P lipid utilization were identif ied. Two synaptogenesis protein 1/phosphate system positive regulatory protein 81/xenotropic and polytropic retrovirus receptor 1 (SPX), N-terminal proteins related to intracellular P reallocation were up-regulated, by about 2.2-fold, in the P-def icient cells compared with the P-replete cells, but they were down-regulated by 0.7-fold after the both DIP- and DOP-resupply that lasted for 28 h (Table 1). Organic P utilization enzymes, glycerophosphodiester phosphodiesterase, and a cyclic phosphodiesteraselike enzyme were all up-regulated about 1.4-fold in the P-def icient cells; however, no signif icant change was detected after the P resupplementation.Additionally, two arylsulfatases involved in non-P lipid utilization were signif icantly up-regulated in the P-def icient cells but down-regulated signif icantly after the DIP resupply (Table 1).
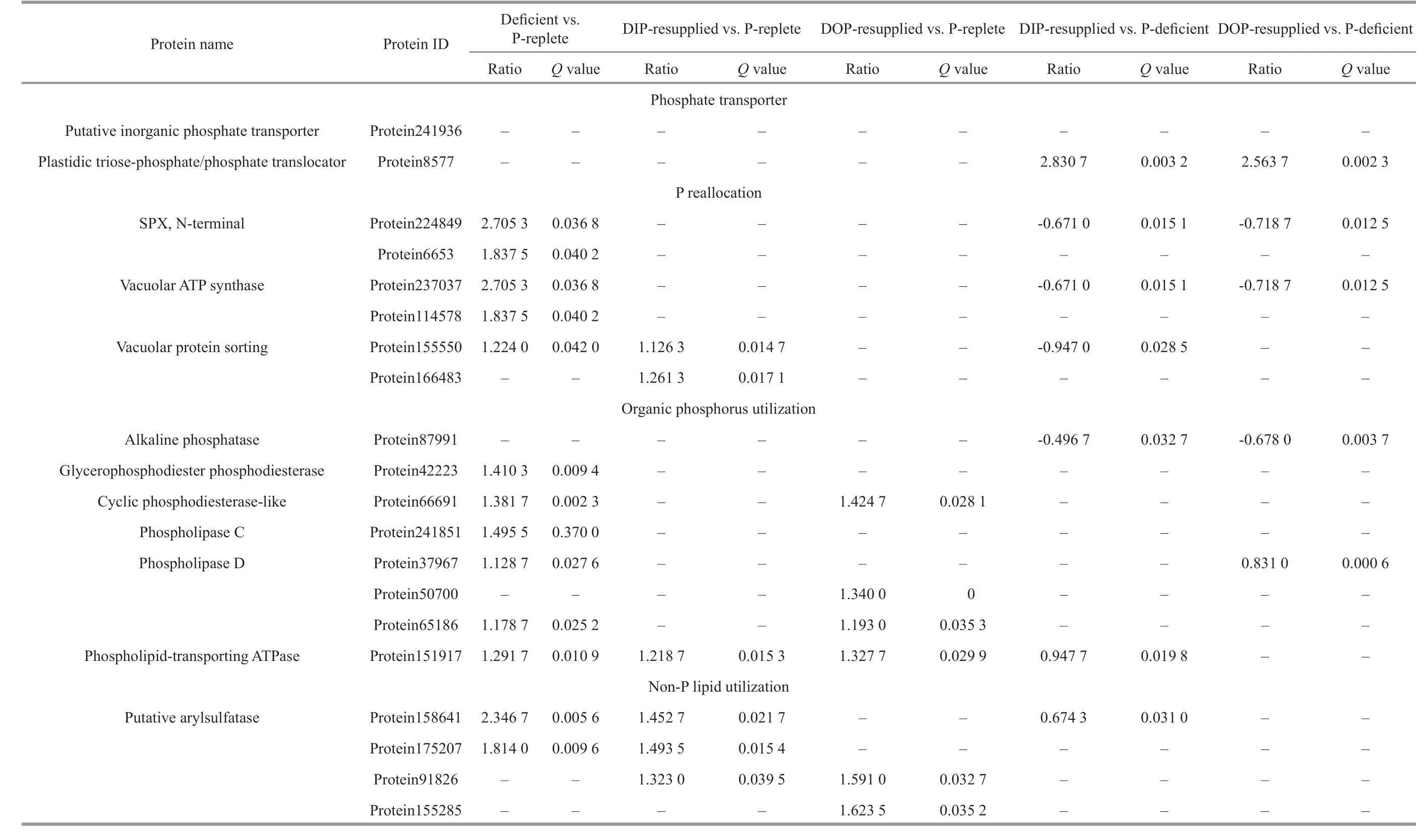
Table 1 Diff erentially expressed proteins related to phosphorus (P) metabolism in Prorocentrum donghaiense
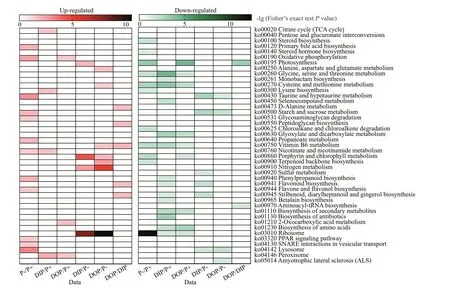
Fig.2 Proteome responses of P. donghaiense to changing ambient P
4 DISCUSSION
4.1 Responses of P. donghaiense to ambient P def iciency
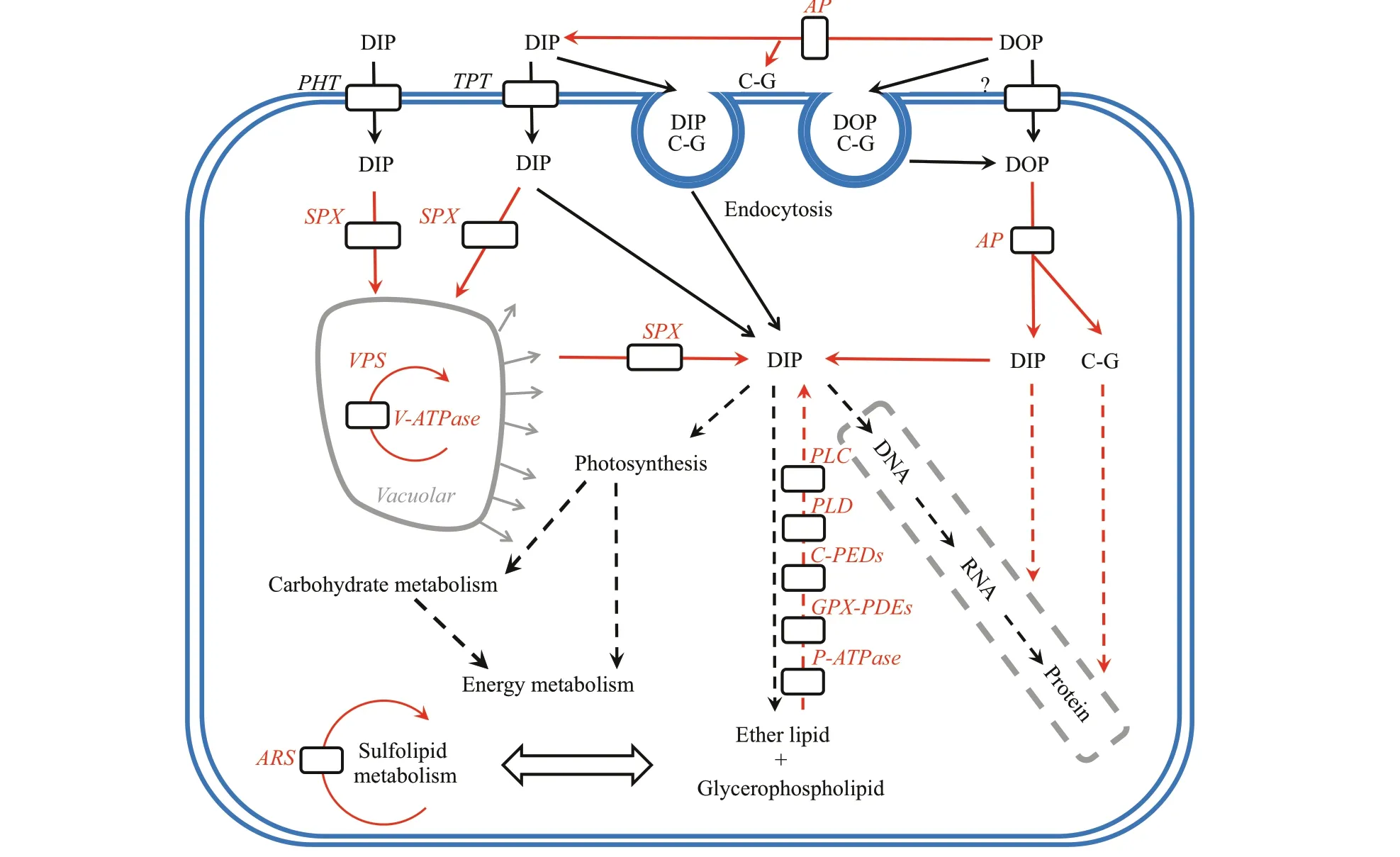
Fig.3 The mechanisms of P utilization in the P-def icient P. donghaiense cells
Phosphate is generally considered to be the only P form that can be directly utilized by phytoplankton(Cembella et al., 1982, 1984; Currie et al., 1986).Under P-def icient conditions, many phytoplankton species, such as the diatomsSkeletonemacostatumandThalassiosirapseudonana, enhance phosphate transport to capture more P from the surrounding environment and/or reallocate their intracellular P(Dyhrman et al., 2012; Zhang et al., 2015; Dyhrman,2016; Lin et al., 2016). Two inorganic phosphate transporters were identif ied inP.donghaiense, but they varied insignif icantly under the P-def icient condition (Fig.3, Table 1), while DIP transport was not enhanced in the P-def icient cells, similar to the dinof lagellateKareniamikimotoi(Lei and Lü, 2011).Some phytoplankton species, such asAlexandriumcatenella,S.costatum, andT.pseudonana, can luxuriously absorb P from the environment and store it in vacuoles in the form of polyphosphate when the ambient P is not limiting, so that later this P can be released and utilized under P-def icient conditions(Jauzein et al., 2010; Dyhrman et al., 2012; Secco et al., 2012; Fu et al., 2013; Zhang et al., 2016). SPXdomain-containing proteins play an important role in the maintenance of phosphate homeostasis in plants(Secco et al., 2012). In our study, the SPX proteins were signif icantly up-regulated in the P-def icient cells(Fig.3, Table 1), indicating that intracellular P reallocation was enhanced and intracellular P was an important P source for P-def icient cells, similar to the diatomsS.costatumandT.pseudonana(Dyhrman et al., 2012; Zhang et al., 2016). Organic P, another vital P source in the ocean, can also be used by most phytoplankton species (Dyhrman et al., 2012; Lin et al., 2016; Zhang et al., 2016, 2018). Under P def iciency,many phytoplankton species can use intracellular and extracellular organic P (Dyhrman et al., 2012; Zhang et al., 2016, 2018; Gong et al., 2017; Luo et al., 2017).However, the utilization mechanisms of organic P among diff erent phytoplankton species are poorly understood. Phospholipids, including glycerophospholipids and sphingolipids, are crucial components of cell membranes (Paolo and Suzanne,2009). In our study, glycerophosphodiester phosphodiesterase was up-regulated in the P-def icient cells (Fig.3, Table 1). The most prominent function of phospholipase is the degradation of membrane lipids;yet phospholipase is also involved in vesicle traffi cking and signal transduction, and it may act as a link between cell membranes and proteins (Munnik and Musgrave, 2001; Bargmann and Munnik, 2006). Our results suggested that membrane phospholipids might be a critical intracellular organic P source for P-def icient cells (Fig.3), similar toS.costatum(Zhang et al., 2016). Some phytoplankton species, such asA.catenella,S.costatum, andT.pseudonana, can use non-P lipids instead of phospholipids to reduce the P requirement of their cells under P-def icient conditions;for example, some diatoms and cyanobacteria can utilize sulfolipid and betaine lipids (Yu et al., 2002;Van Mooy et al., 2009; Jauzein et al., 2010; Dyhrman et al., 2012; Fu et al., 2013; Zhang et al., 2016). P def iciency signif icantly increases the sulfolipid metabolism ofP.donghaienseat both the transcriptional (Shi et al., 2017) and protein level(Zhang et al., 2019b), which implies thatP.donghaiensecould reduce its P requirement by utilizing sulfolipid in response to an ambient P def iciency. Furthermore, our results indicated that the cells’ P requirement under the ambient P-def iciency condition was mainly derived from intracellular polyphosphates, phospholipids, and organic P, with organic P becoming the main P source once intracellular P has been exhausted (Fig.3). In this study, however,we did not detect signif icant changes in endocytosis at the protein level (Fig.3) when compared with the transcriptional level (Zhang et al., 2019b).
Energy fuels the the life activities of all organisms.In this study, ATP synthase subunits (such as β and δ)were down-regulated in the P-def icientP.donghaiensecells compared with the P-replete cells (Supplementary Table S2), similar toA.catenella(Zhang et al., 2014,2019a). Glycolysis can release a large amount of ATP,which is the oldest and most primitive way for organisms to obtain energy (Harris, 2013).Phosphofructokinase (PFK), phosphoglycerate kinase(PGK), and pyruvate kinase (PK) are all involved in glycolysis and they were down-regulated under P def iciency (Supplementary Table S2). PFK is a ratelimiting enzyme for glycolysis (TeSlaa and Teitell,2014), while PGK and PK catalyze two reactions to produce ATP (Plaxton, 1996; Harris, 2013). However,we found that the V-type H+ATPase was signif icantly up-regulated (P<0.05) under P def iciency(Supplementary Table S2). In plants, the V-type H+ATPase obtains energy by hydrolyzing ATP,transferring H+across the membrane to form electrochemical gradients inside and outside the membrane, which provides electrochemical potential energy for the membrane transport system of other substances (Beyenbach and Wieczorek, 2006). Our results show that P def iciency inhibited the production of ATP inP.donghaiense, which might have led to insuffi cient energy for protein, lipid, and nucleic acid synthesis, and cell growth (Zhang et al., 2018, 2019b).In addition, enzymes localized in the lysosome, such as deoxyribonuclease II and iduronate 2-sulfatase,were signif icantly up-regulated in the P-def icient cells(Supplementary Table S2). Under nutrient-def icient conditions, plant autophagy is induced, and macromolecular substances such as intracellular proteins are degraded in the lysosome, while intracellular nitrogen and carbon sources are reused to sustain biosynthesis, energy metabolism, and the reactivation of nutrients (Doelling et al., 2002;Bassham, 2009). Our results indicated thatP.donghaienseengaged in multiple strategies to adapt to the ambient DIP def iciency and mainly used organic P as its P source under a P-def icient condition.
4.2 Diff erent response of P. donghaiense to DIP and DOP resupply
Our previous study revealed that the physiological responses of P-def icientP.donghaienseto DIP and DOP resupply were not signif icantly diff erent, but their transcriptome responses did diff er (Zhang et al.,2019b). In the current study, signif icant diff erences were also observed at the proteome level (Fig.2).
4.2.1 Photosynthesis
The paramount feature of phytoplankton is their ability to synthesize organic matter using light energy via photosynthesis (Falkowski and Raven, 2013).After the DIP or DOP resupply, cytochromeb6andfinP.donghaiensewere up-regulated only in the DIPresupplied cells (Fig.4, Supplementary Tables S3-S6), a result opposite to that found forA.catenella(Zhang et al., 2015). However, there was no signif icant diff erence in porphyrin and chlorophyll metabolism between cells in the DIP and DOP resupply groups(Fig.4, Supplementary Tables S3-S6). The cytochromeb6fcomplex is a membrane protein that is essential for the light reaction in plants (Cramer et al., 2013). Light energy harvested by chlorophyll and other pigments during the light reaction drives the transfer of photosynthetic electrons through photosystems I and II (Gooch, 2011). Once the electrons are f inally transferred to nicotinamide adenine dinucleotide phosphate (NADPH) and ATP,this completes conversion of light energy to chemical energy and this energy is now available for the carbon f ixation reaction (Gooch, 2011; Walker et al., 2014).Both NADPH and ATP synthesis require P, and DIP is more easily utilized by phytoplankton than DOP, soP.donghaienseexpressed more cytochromeb6andfto sustain the electron transfer. Our results showed that DIP is more conducive than DOP for recovering photosynthetic energy production inP.donghaiense.
4.2.2 Secondary metabolism
Plant secondary metabolism is a metabolic process derived from primary metabolism, which f igures prominently in plants’ environmental adaptation,especially for their interaction with and defense response against bacterial pathogens (Erb and Kliebenstein, 2020). Compared with the P-def icientP.donghaiensecells, the secondary metabolites, such as f lavonoid, stilbenoid, diarylheptanoid and gingerol,which are related to plant defense response and scavenging free radicals, were signif icantly downregulated after the DIP resupply (Fig.2, Supplementary Table S3). However, no signif icant changes were observed in these processes after the DOP resupply.Nitrogen and P def iciency aff ect the content of f lavonoids in plants, and P def iciency increases the content of f lavonoid in tomato at the early stage of fruit ripening (Stewart et al., 2001). In our study, DIP was evidently more conducive than DOP for reducing the content of f lavonoids and other secondary metabolites in P-def icientP.donghaiensecells. Since DIP was more easily utilized byP.donghaiensethan DOP and also restored by cellular environmental defenses, more energy could be saved.
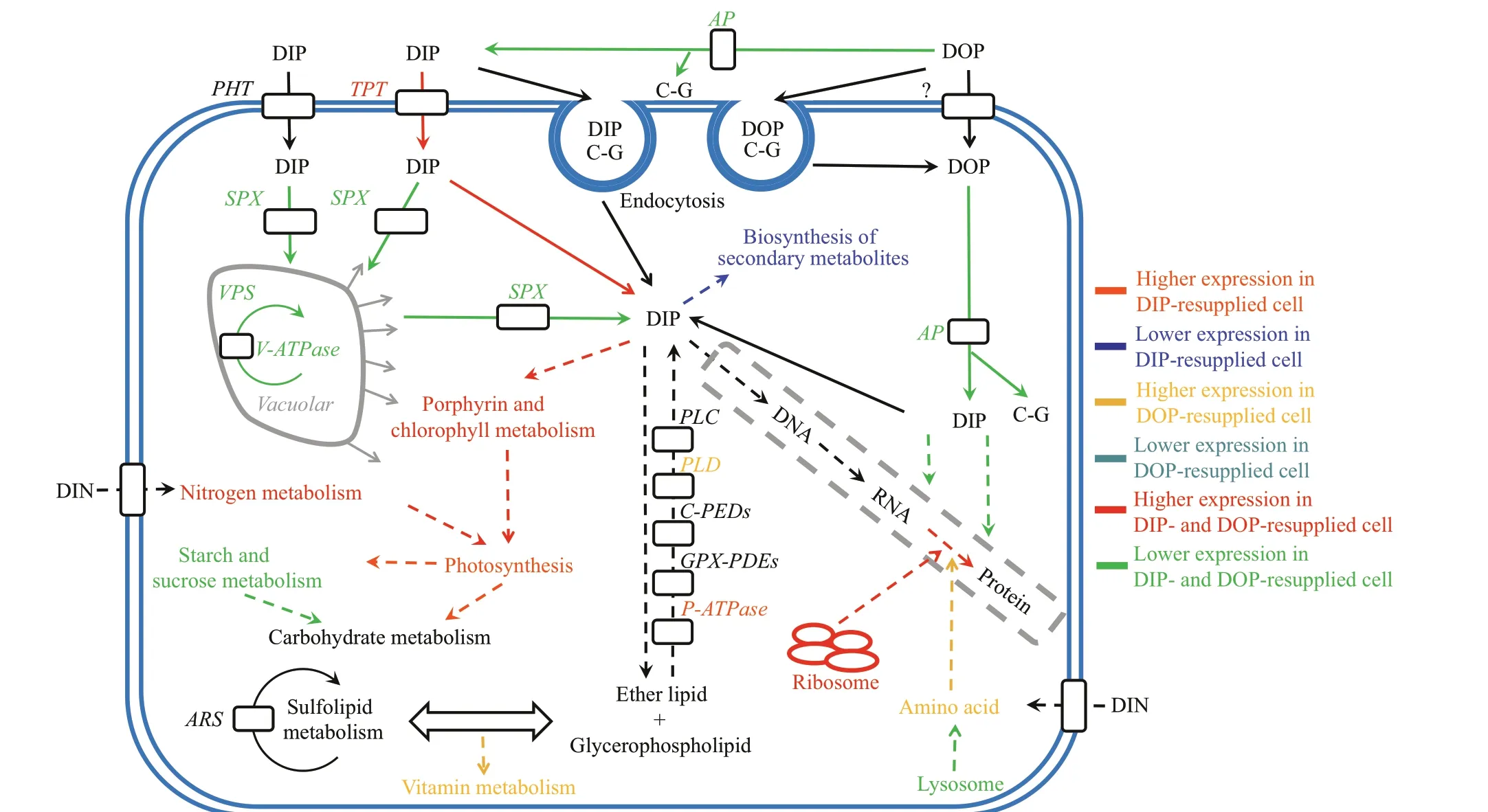
Fig.4 Diff erential responses of P. donghaiense to DIP and DOP resupply
4.2.3 Vitamin B6 metabolism
Vitamin B6 is a cofactor for many metabolic enzymes—especially amino acid metabolism—and a strong biological antioxidant, which can eff ectively quench singlet oxygen and superoxide, and exert antiabiotic stress eff ects in plants (Szydlowski et al.,2013). Pyridoxine biosynthesis protein (PDX), being a rate-limiting enzyme in the synthesis of vitamin B6,is mainly active in the plasma membrane and inner membrane system, where it modulates the functioning of the plant cell membrane (Chen and Xiong, 2005).After the DOP resupply, we found that PDX was down-regulated compared with the P-def icient cells(Fig.4, Supplementary Table S5), whereas the DIPresupplied cells went unchanged. When plants are exposed to strong light, low temperature, or other abiotic stresses, PDX coding genes are usually upregulated (Herrero and Daub, 2007). Overexpression of PDX inArabidopsiscan increase the content of vitamin B6, prolong the growth period of cells,increase cell volume, and augment resistance to oxidative stress and other environmental stresses(Raschke et al., 2011). Therefore, DOP increased the P-def icientP.donghaiensecells’ resistance more than DIP did, by enhancing the synthesis of vitamin B6.
4.2.4 Amino acid metabolism
Protein is the main carrier of biological function,and amino acids are the basic units of all proteins(Morot-Gaudry et al., 2001; Hildebrandt et al., 2015).In our experiment the alanine, aspartate, glutamate,cysteine and methionine metabolisms were each upregulated in the DOP-resupplied cells, yet no signif icant change (P>0.05) was evident after the DIP resupply (Fig.4, Supplementary Tables S4-S6). In comparison with the P-def icient cells,S-adenosylmethionine (SAM) synthetase and methionine S-adenosyl transferase—two key enzymes of SAM synthesis—and glutamate synthase (GS)were all signif icantly up-regulated (P≤0.05) following the DOP resupply (Fig.4, Supplementary Table S5).Because SAM can improve cell metabolism and ensure normal mitochondrial function and membrane f luidity, its accumulation plays a key role in regulating cell membrane stability and reactive oxygen metabolism balance, and for shoring up resistance in plants to P stress (Lu, 2000; Moff att and Weretilnyk,2001). GS, a key enzyme involved in ammonia assimilation, catalyzes ammonia and glutamic acid to form glutamine, which is the f irst step of ammonia assimilation metabolism (Temple et al., 1998; Lea and Mif lin, 2003). Glutamine is a nitrogen donor for the biosynthesis of amino acids, nucleotides, and chlorophyll (Kan et al., 2015), but nitrogen metabolism was up-regulated in both DIP- and DOP-resupplied cells (Fig.4, Supplementary Tables S4-S7). This result indicated that the actual form of P did not aff ect nitrogen metabolism in spite of (or because of) the importance of nitrogen to cells.
Overall, our study shows that those metabolic processes directly requiring P, such as photosynthesis and cellular environmental defense, respond rapidly to DIP resupply while they varied insignif icantly in the DOP-resupplied cells (Supplementary Tables S4-S7). The metabolic processes that responded to DOP resupply were mainly those dependent on C, such as amino acid metabolism (Fig.4). Collectively, these results indicate that intracellular DOP assimilation provides not only P but also C for the biosynthesis of various substances essential for cell growth, and the coupled utilization of P and C triggered by DOP might be an important reason resulting in the occurrence ofP.donghaienseblooms in a P-def icient environment.
5 CONCLUSION
Understanding the adaptation and response of marine dinof lagellates to change in ambient P is always an ecological endeavor. In this quantitative proteomic study, we found thatP.donghaienseinitiated multiple strategies to adjust to an ambient P-def iciency, such as intracellular P reallocation and organic P and non-P lipid utilization, but, interestingly,it did not enhance its inorganic phosphate transport.This suggestsP.donghaiensepreferred to use DOP rather than DIP under ambient P-def icient conditions.Although no signif icant diff erence in their physiological response was observed between the DIP- and DOP-resupplied cells (Zhang et al., 2019b),their proteomic response diff ered remarkably:photosynthesis was up-regulated and secondary metabolism was down-regulated only in the DIPresupplied cells, while alanine, aspartate and glutamate metabolism, cysteine and methionine metabolism, and vitamin B6 metabolism were all upregulated in the DOP-resupplied cells, indicating diff erent utilization mechanisms of DOP and DIP by the dinof lagellate cells. When compared with DIP, a more effi cient utilization of DOP might be an important reason why bloom occurrences ofP.donghaienseoccur under P-def icient conditions.Further in-situ proteomic research is necessary,especially in combination with the analysis of DOP composition and concentration in natural marine environments during the bloom period ofP.donghaiense, to help fully unveil the role of DOP in the bloom formation ofP.donghaiense.
6 DATA AVAILABILITY STATEMENT
The datasets that support the f indings of this study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
