OsNPF5.16, a nitrate transporter gene with natural variation, is essential for rice growth and yield
Jie Wang, Renjing Wan, Haipeng Nie, Shaowu Xue, Zhongming Fang,,*
a Key Laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), College of Agricultural Sciences, Guizhou University, Guiyang 550025, Guizhou, China
b Center of Applied Biotechnology, Wuhan University of Bioengineering, Wuhan 430415, Hubei, China
c College of Life Science and Technology, Huazhong Agricultural University, Wuhan 430070, Hubei, China
Keywords:Rice OsNPF5.16 Nitrate transporter Natural variation Growth Grain yield
ABSTRACT Rice has a large number of nitrate or peptide transporter family(NPF)genes,but the effects of most members on rice growth and development are unknown.We report that OsNPF5.16,a nitrate transporter gene with natural variation in its promoter sequence, is essential for rice growth and yield. The promoter sequence showed various differences between indica and japonica cultivars, and higher expression of OsNPF5.16 was found in indica cultivars with higher plant weight and more tillers than japonica cultivars.OsNPF5.16 was highly expressed in roots,tiller basal parts,and leaf sheaths,and its protein was localized on the plasma membrane.In cRNA-injected Xenopus laevis oocytes,OsNPF5.16 transport of nitrate at high nitrate concentration depended on pH. Overexpression of OsNPF5.16 increased nitrate content and total nitrogen content in leaf sheath as well as biomass and tiller bud length in rice. Elevated expression of OsNPF5.16 increased rice tiller number and grain yield by regulating cytokinin levels. Inhibition of OsNPF5.16 expression showed the opposite effects. Regulating OsNPF5.16 expression has potential for improving rice grain yield.
1. Introduction
Nitrogen (N) is the most basic element of biological proteins,nucleic acids, phospholipids, enzymes, and some growth hormones. When plants are short of N, synthesis of these substances is blocked and plant growth is reduced, with fewer branches and tillers. N fertilization has allowed farmers to increase crop yield greatly over the past century, promoting economic development and allowing larger populations. The projected increase in global population by two to three billion by 2050 implies an increased demand for N fertilizer [1].
Application of excessive N fertilizer causes soil and water eutrophication and atmospheric pollution, because only a limited proportion of the applied fertilizer can be directly used by crops,with most of the applied N escaping into the environment [2,3].Reducing the use of inorganic nitrogen fertilizer while maintaining or increasing rice yield is a desirable strategy for improving crop yields.
In plants, nitrate, ammonium, peptide, and amino acid transporters coordinate the uptake and utilization of nitrogen. Rice has 93 nitrate and peptide transporter family (NPF) members, 4 NRT2 members, and 2 NAR2 members [4], only a few of whose functions have been identified. In the NPF family, OsNPF2.2, a rice vascular-specific transporter, unloads nitrate from the xylem,influencing root-to-shoot nitrate transport and plant development[4]. OsNPF2.4 is a pH-dependent low affinity nitrate transporter,and knockout of OsNPF2.4 weakens nitrate uptake by roots and impedes the transport of K+-coupled NO3-from roots to stems,resulting in reduced K+transport from stems to floral organs,anther atrophy, and reduced pollen fertility [5]. OsNPF4.1 (Sp1) is highly expressed in the phloem of young panicles, affecting their development and thereby controlling panicle size [6]. Functional analysis of the nitrate transporter gene OsNPF4.5 [7] revealed a conserved mycorrhizal pathway of nitrogen acquisition in plants.The low-affinity nitrate transporter gene OsNPF6.1 influences the N use efficiency (NUE) of rice under low N [8]. OsNPF6.5(OsNRT1.1B) is a key gene controlling N uptake and utilization in rice [9] and is involved in regulating root microbiota to facilitate organic N mineralization in soil [10].
In the OsNPF7 and OsNPF8 subfamilies, OsNPF7.1 and OsNPF7.4 showed opposite expression patterns in axillary buds under various N concentrations, and alterations in their expression differentially regulated rice tillering and grain yield[11].Knockdown of the tonoplast-localized low-affinity nitrate transporter OsNPF7.2 retarded rice growth under high nitrate supply, and elevated expression of OsNPF7.2 increased rice tillering and grain yield[12,13]. OsNPF7.3 (OsPTR6) transports the di/tripeptides Gly-His and Gly-His-Gly and OsNPF7.3 overexpressing in rice increased rice growth [14], tiller number, and grain number [15]. OsNPF7.7 has two splicing variants, and elevated expression of either variant in the japonica cultivar ZH11 increased rice shoot branching and NUE [16]. OsNPF8.9 (OsNRT1) has been reported [17] to be a low-affinity NO3-transporter in rice that mediates mainly nitrate uptake in roots. The expression of OsNPF8.20(OsPTR9) is regulated by multiple N sources, and it promoted rice tillering and thus increased yield [18].
Among the NPF members of rice, OsNPF6.5 [9], OsNPF7.1 [11],OsNPF7.2 [13], OsNPF7.3 [15], OsNPF7.4 [11], OsNPF7.7 [16] and OsNPF8.20 [18] can differentially influence rice yield by regulating tillering.Both OsNPF6.1 and OsNPF6.5(NRT1.1B)were reported[8,9]to regulate NUE differently in cultivars.Whether other subfamilies of OsNPFs are responsible for differences in N use and grain yield among cultivars is unknown.
In this study, we analyzed the sequence divergence of OsNPF5.16 between indica and japonica cultivars,investigated characteristics among OsNPF5.16 different expression lines and ZH11 in rice,such as tiller number,grain yield,NO3-content,and cytokinin levels.The objectives of this study were:(1)to clarify the relationship between OsNPF5.16 and yield traits, (2) to identify the transport substrate of OsNPF5.16 protein, (3) to initially reveal the mechanism of OsNPF5.16 regulating rice growth.
2. Materials and methods
2.1. Sequence variation of OsNPF5.16
Based on RiceVarMap 2.0(http://ricevarmap.ncpgr.cn/v2/)[19],SNP and indel variations of OsNPF5.16(LOC_Os01g65200)from 529 cultivars were obtained.The expression of OsNPF5.16 between Hap 1 and Hap 2 were detected by reverse transcription-quantitative(RT-qPCR)in corresponding cultivars.For trait association analysis,haplotypes with a frequency of 10% or higher were selected, and agronomic traits such as tiller number, heading date, and grain yield were obtained from the database RiceVarMap 2.0.
2.2. Acquisition of transgenic lines of OsNPF5.16 and measurement of agronomic traits
To obtain transgenic lines with varying expression levels,1647 bp OsNPF5.16 cDNA was inserted into the pCAM1306 vector to produce the p35S::OsNPF5.16 overexpression (OE) vector and two 300 bp fragments of OsNPF5.16 cDNA were inserted downstream of the Ubi promoter in pTCK303 to construct the OsNPF5.16 RNA interference (Ri) vector. The OE and Ri vectors were transformed into ZH11 by the Agrobacterium tumefaciens-mediated transformation method. Homozygous T2generation transgenic lines were verified with hygromycin and by PCR for further study.The associated primers are listed in Table S1.
To measure agronomic traits, 20 randomly sampled plants of each of the ZH11, OE1-3 and Ri1-3 lines were harvested from rice paddies. Tiller number and filled-grain number per plant were recorded. Yield per plant was scored as the total weight of filled grains per plant.
2.3. GUS staining and subcellular localization
For GUS staining, a 1999 bp promoter sequence of OsNPF5.16 was obtained by PCR amplification from ZH11 and inserted in pCAMBIA1391Z to generate the pOsNPF5.16::GUS vector.Similarly,the pOsNPF5.16::GUS plasmid was inserted into ZH11 by Agrobacterium tumefaciens-mediated transformation. Histochemical GUS staining was performed according to Wang et al. [20]. Samples were first vacuum-infiltrated for 20 min, gently fixed in formalin:acetic acid:70% (v/v) ethanol (1:1:18) at 4 °C for 30 min and then incubated in staining buffer at 37°C for 12 h. The stained samples decolorized with ethanol were observed by a stereomicroscope.Finally, the samples were embedded in Spurr resin and sectioned.The sections were observed with a Zeiss Axio Imager M2 (Zeiss,Oberkochen, Germany).
For subcellular localization,the OsNPF5.16 cDNA was fused with GFP in pCAM1302 to generate the p35S::OsNPF5.16-GFP fusion vector. This vector was transiently expressed in rice protoplasts.OsMCA1,the rice homolog of putative Ca2+-permeable mechanosensitive channels in Arabidopsis,was fused with mCherry as the plasma membrane colocalized marker[21].GFP fluorescence was observed under a confocal microscope(Leica,Weztlar,Germany).
2.4. Measurement of nitrate and total N content
Seedlings at 7 days after germination (DAG) of ZH11, OE lines,and Ri lines were cultured in basic nutrient solution (pH 5.8) for 27 days. The composition of the solution was as follows: 1 mmol L-1NH4NO3, 0.32 mmol L-1NaH2PO4, 0.51 mmol L-1K2SO4,1 mmol L-1CaCl2, 1.65 mmol L-1MgSO4, 8.9 μmol L-1MnSO4,0.5 μmol L-1Na2MoO4,18.4 μmol L-1H3BO3,0.14 μmol L-1ZnSO4,0.16 μmol L-1CuSO4,and 40 μmol L-1FeSO4.Nutrient solution was renewed every three days. At 34 DAG, the roots, leaf sheaths, and leaf blades of seedlings were obtained to detect the nitrate content and the total nitrogen content. Free nitrate content was determined by the colorimetric method according to Cai et al.[22].Total N content was determined by the semi-micro Kjeldahl method with a nitrogen analyzer (Smart Chem 200, Westco, Italy). Three replicates of each assay were performed. At the same time, tiller basal parts (approximately 0.5 cm) of seedlings of ZH11 and OE and Ri lines were acquired for plant hormone detection.
2.5. RNA extraction and RT-qPCR
Total RNA was prepared from fresh tissues using TRIzol reagent(Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using M-MLV reverse transcriptase (TaKaRa, Tokyo, Japan). RT-qPCR was performed using SYBR Green mix (TaKaRa) and the 7500 RT qPCR system(Applied Biosystems,Foster City,USA).The rice ACTIN(LOC_Os03g50885), UBIQUITIN (LOC_Os05g06770), and GAPDH(LOC_Os04g40950) genes were used as internal references, and three biological replicates were tested for each sample.The expression level was calculated using the 2-ΔΔCTmethod and normalized to the geometric mean of the reference genes.All primers used for RT-qPCR are listed in Table S1.
2.6. Functional analysis of OsNPF5.16 in Xenopus laevis oocytes
OsNPF5.16 cDNA was cloned into the pNB1 vector for expression in oocytes following Nour-Eldin et al. [23]. cRNA was transcribed in vitro using a mMessage mMachine T7 kit (Ambion, Austin,USA), and purified with a MEGA clear Transcription Clean-Up kit(Invitrogen). Healthy oocytes at stages IV and V were selected for injection with 50 ng cRNA or the same amount of water. Injected oocytes were incubated in Barth’s solution at 18 °C for 2 days.Barth’s solution contained 96 mmol L-1NaCl, 2 mmol L-1KCl,1 mmol L-1MgCl2, 1.8 mmol L-1CaCl2, and 10 mmol L-1HEPES,adjusted to pH 7.5 with Tris-base.Nitrate uptake assays and kinetic analysis of nitrate uptake in oocytes were performed using various concentrations of15N-NaNO3, following Xia et al. [5].
2.7. Detection of plant hormones
Tiller basal parts excised from seedlings of ZH11 and OE and Ri lines at 34 DAG were quick-frozen in liquid nitrogen. Plant CKs,auxins,and strigolactones(SLs)contents were measured with Met-Ware (http://www.metware.cn/) based on the AB SciexQTRAP 4500 LC-MS/MS platform(AB Sciex,Framingham,USA).Three biological replicates were measured for each assay.
2.8. Statistical analysis
To identify significant differences between two sets of data, a two-tailed t test was performed using SPSS 10 software.For multiple comparisons, one-way ANOVA with Duncan’s multiple range test was performed.
3. Results
3.1. Sequence divergence of OsNPF5.16 between indica and japonica rice
Variation sites in the OsNPF5.16 sequence, which covers the 2-kilobase (Kb) promoter, coding sequence (CDS), and 3′untranslated region (UTR), were retrieved from the rice genome variation database (Rice Variation Map v2.0) for the 529 cultivars. Based on the nucleotide variation, 67 single-nucleotide polymorphisms(SNPs)and 7 insertions/deletions(InDels)were detected in 10 variant types, which were named haplotypes 1–10 (Hap 1–Hap 10;Fig. 1A). Among these variation sites, all of the indels and 55 SNPs were found in the promoter sequence of OsNPF5.16, and 3 SNPs were found in the exons of OsNPF5.16,indicating that the promoter sequence of OsNPF5.16 was more diverse than the CDS (Fig. 1A;Table S2). Hap 1 was present mainly in japonica cultivars and Hap 2 mainly in indica cultivars. Eighteen specific SNPs that only exist in japonica cultivars were found in Hap 1(Fig.1A).A phylogenetic analysis revealed two main clusters (Hap 1 and Hap 2;Fig. 1B), indicating clear differentiation of OsNPF5.16 between indica and japonica sequences.
As variation occurred mainly in the promoter of OsNPF5.16, we speculated that the expression of OsNPF5.16 would differ between Hap 1 and Hap 2. RT-qPCR demonstrated that the expression of OsNPF5.16 in indica cultivars harboring the Hap 2 allele was significantly higher than that in japonica cultivars with the Hap 1 allele(Fig.1C).Dry weight per plant at both the heading stage(Fig.S1A)and mature stage (Fig. 1D), straw weight per plant (Fig. S1B), N content in straw at the mature stage (Fig. 1E), tiller number(Fig. 1F), and grain yield (Fig. 1G) per plant were significantly higher in indica cultivars with Hap 2 than in japonica cultivars with Hap 1. However, no significant differences in heading date(Fig. S1C), N concentrations in straw at either the heading stage(Fig. S1D) or mature stage (Fig. S1E), or N use efficiency (Fig. S1F)were observed. Thus, indica cultivars with Hap 2 showed higher expression of OsNPF5.16 with greater biomass and higher grain yield than japonica cultivars with Hap 1,suggesting a positive association between OsNPF5.16 expression and dry weight per plant of aboveground parts.

Fig. 1. Sequence divergence of OsNPF5.16 in 529 cultivars. (A) SNP and indel divergence in the sequence of OsNPF5.16. Red markers indicate SNPs specific to Hap 1 and triangles indicate indels.(B)Phylogenetic tree of OsNPF5.16 sequences was constructed using the neighbor-joining method with MEGA software(version 5.1).(C)Expression of OsNPF5.16 in rice Hap 1 and Hap 2.Comparison of dry weight(D),N content in straw(E),tiller number(F),and grain yield per plant(G)among all cultivars grouped by rice Hap 1 and Hap 2.**and***indicate significant differences at P <0.01 and P <0.001,respectively.Sets of 151 japonica cultivars with Hap1 haplotype and 179 indica cultivars with Hap 2 haplotype were selected for trait association analysis in D–G.Values are means±SD((C)n=25;(D–G)n=151 in Hap 1 and 179 in Hap 2),and three replications were used for each analysis.
3.2. OsNPF5.16 positively regulated rice tiller number and grain yield
To further validate the positive association between OsNPF5.16 expression and rice growth, we constructed overexpression (OE)lines and RNA interference (Ri) lines of OsNPF5.16 in the japonica variety Zhonghua 11(ZH11)background(Fig.2A),and the expression level of OsNPF5.16 in ZH11 and the OE and Ri lines was confirmed by RT-qPCR (Fig. 2C). Tiller number per plant increased significantly in the three OE lines at the reproductive stage in comparison with that in ZH11 but decreased significantly in the Ri lines(Fig. 2A and D). The filled grain number per plant also increased significantly in the OE lines but decreased significantly in the Ri lines in comparison with that in ZH11 (Fig. 2B and E). Thus, the three OE lines showed higher grain yield per plant, whereas the Ri lines had a lower grain yield per plant in rice paddy than ZH11 (Fig. 2F). These results showed that elevating OsNPF5.16 expression increased rice tiller number, filled-grain number, and grain yield per plant, whereas inhibiting OsNPF5.16 expression led to the opposite result,showing that OsNPF5.16 positively regulated rice growth and grain yield.
3.3. Expression pattern and subcellular localization of OsNPF5.16
RT-qPCR showed that OsNPF5.16 was expressed in the root, tiller basal part, leaf sheath, leaf blade, culm and young panicle, but showed the highest expression in the root and tiller basal part(Fig. 3A). The expression of OsNPF5.16 was up-regulated in both the root and tiller basal parts of ZH11 under the NO3-treatment in comparison with the NH4+treatment (Fig. 3B). Thus, OsNPF5.16 responded mainly to NO3-treatment. With increasing nitrate concentration(0.5 to 5.0 mmol L-1),expression of OsNPF5.16 gradually increased in roots (Fig. S2A) but decreased in the tiller basal part(Fig. S2B), and it initially decreased and then increased in leaves(Fig. S2C). Thus, with increasing nitrate concentration, OsNPF5.16 expression in rice roots and tiller basal parts showed opposite trends.
GUS signal in pOsNPF5.16::GUS transgenic lines was very strong in the root (Fig. 3C), tiller basal part (Fig. 3D), and leaf sheath(Fig. 3E) but weak in the leaf blade (Fig. 3F), culm (Fig. 3G), and young panicle(Fig.3,H and I),in agreement with RT-qPCR expression.GUS activity was abundant in epidermis cells and vascular tissue in the transverse section of the root(Fig. 3J)and was enriched in vascular tissue of the leaf sheath(Fig.3K),but negligible in vascular tissue of the leaf blade (Fig. 3L).

Fig.2. Phenotypes of OsNPF5.16 transgenic plants in japonica ZH11 background.Whole-plant phenotype(A),filled grains per plant(B),expression level of OsNPF5.16 in tiller basal part(C),tiller number per plant(D),filled-grain number per plant(E),and grain yield per plant(F).OE-1~OE-3 indicate three OsNPF5.16 overexpressing lines,and Ri-1–Ri-3 represent three OsNPF5.16-RNAi lines. Values are means ± SD (D–F, n = 20) and three replications were measured in each analysis. *, **, and *** indicate significant difference at P <0. 05, P <0. 01, and P <0. 001, respectively. Scale bars, 10 cm.
OsNPF5.16 contains 4 exons and 3 introns, and the coding sequence is 1647 bp in length(Fig. S3A). Its protein was predicted to have 12 transmembrane domains(Fig.S3B),suggesting that the OsNPF5.16 protein is localized to the membrane system. To test this hypothesis,we transiently expressed OsNPF5.16-GFP under the 35S promoter in rice protoplasts. The green fluorescence protein(GFP) signal of 35S::GFP was widely distributed in the cytoplasm and nucleus (Fig. 4A), whereas coexpression of OsNPF5.16-GFP and mCherry (a plasma membrane marker) showed that the OsNPF5.16-GFP signal was clearly localized to the plasma membrane (Fig. 4B). These results suggest that OsNPF5.16 functions in membrane transport through rice roots and basal parts to aboveground parts.
3.4. Altered expression of OsNPF5.16 influences rice seedling growth and tiller bud outgrowth
To investigate whether changing the expression of OsNFPF5.16 would affect rice seedling growth, seedlings of ZH11 and the OE and Ri lines at 7 DAG were cultured with basic nutrient solution.At 34 DAG, seedling growth of the OE lines was markedly greater than that of ZH11,whereas seedling growth of the Ri lines was less(Fig. 5A). Biomass per plant was significantly increased in the OE lines but decreased in the Ri lines in comparison with ZH11(Fig. 5B). As OsNPF5.16 was highly expressed in roots, the differences in root morphology among ZH11 and the OE and Ri lines may optimize N absorption, thus affecting rice seedling growth.Overexpression of OsNPF5.16 increased root number and root length and inhibition of its expression reduced them (Fig. 5C and D). These results suggested that elevating OsNPF5.16 expression might promote N absorption by promoting more vigorous root growth and biomass accumulation in OE lines.
As OsNPF5.16 expression was induced by NO3-, the content of NO3-taken up by seedlings at 34 DAG among ZH11 and the OE and Ri lines was measured. There was no significant difference in NO3-content in the roots and leaf blades among the different expression lines. However, the total NO3-content in the leaf sheaths of the OE lines became higher than that of ZH11, and the Ri lines showed a lower total NO3-content than ZH11 (Fig. 5E).Total N content in the leaf sheaths of the OE line was also higher than that of ZH11, but total N content in roots and leaves did not differ among the different expression lines(Fig.5F).The expression levels of 6 NPF genes: OsNPF2.1, OsNPF2.2, OsNPF2.3, OsNPF2.4,

Fig.3. Expression pattern of OsNPF5.16.(A)The tissue expression pattern of OsNPF5.16;(B)Expression patterns of OsNPF5.16 from three N sources in root and tiller basal part;GUS staining in the root tip(C),tiller basal part(D),leaf sheath(E),leaf blade(F),culm(G),young panicle at 3–4 cm(H)and 6–8 cm(I)in pOsNPF5.16::GUS transgenic plants.Transverse section of root(J),leaf sheath(K),and leaf blade(L).VT,vascular tissue;Ep,epidermis.Values are means±SD(A and B,n=3)and three replications were assayed in each analysis.Letters above the error bars(A and B)are ranked by Duncan test,the same letters indicate no significant differences,and different letters indicate significant differences at P <0.05. Scale bars, 5 mm (C–I) and 30 μm (J–L).

Fig. 4. Subcellular localization of OsNPF5.16. (A) Free GFP expression in rice protoplasts. (B) OsNPF5.16-GFP expression and co-expression with plasma membrane protein OsMCA1 fused with mCherry. Scale bars, 10 μm (A and B).
OsNPF6.5 and OsNPF8.9,were significantly increased in the OE lines but decreased in the Ri lines relative to those in ZH11 (Fig. S4).Thus,OsNPF5.16 might promote rice seedling growth by increasing N uptake and assimilation from root to sheath.
Rice tiller number is a key trait determining rice grain yield and is determined by tiller bud growth[24].Both first and second tiller buds grew more rapidly in OE lines but less rapidly in Ri lines than in ZH11 (Fig. 6A). Both the first and the second tiller bud length were significantly greater in the OE lines but shorter in the Ri lines than in ZH11 (Fig. 6B and C). Thus, elevating the expression of OsNPF5.16 could promote tiller bud growth of seedlings during the vegetative growth period, thus increasing rice tiller number during the reproductive growth period.
3.5. OsNPF5.16 is a pH-dependent, low-affinity nitrate transporter
To confirm that OsNPF5.16 proteins transport nitrate across plasma membranes, in vitro-synthesized OsNPF5.16 complementary RNAs (cRNAs) were microinjected into X. laevis oocytes and nitrate transport was measured. The nitrate transport activities of OsNPF5.16 were confirmed by measurement of15NO3-uptake activity. We used a well-characterized dual-affinity nitrate transporter, CHL1 (AtNPF6.3), as a positive control in oocytes and assayed high- and low-affinity nitrate transport activity with 0.25 mmol L-1and 10 mmol L-115NO3-, respectively [25,26]. As shown in Fig. 7A and B, compared with water-injected oocytes,CHL1 cRNA-injected oocytes showed a significant increase in15NO3-uptake in both the 0.25 and 10 mmol L-115NO3-solutions at pH 5.5 and 7.5, thus confirming a previous report [26] that CHL1 is a dual-affinity and pH-dependent nitrate transporter.Oocytes expressing OsNPF5.16 incubated with 10 mmol L-115NO3-at pH 5.5 took up15NO3-at 1.317 nmol per oocyte,whereas those in the water-injected control took up15NO3-at 0.208 nmol per oocyte. Uptake was higher at pH 5.5 than at pH 7.5, indicating that OsNPF5.16 is also a pH-dependent nitrate transporter. However, when oocytes expressing OsNPF5.16 were incubated with 0.25 mmol L-115NO3-at pH 5.5 or with 0.25 mmol L-115NO3-at pH 7.5, they showed no15NO3-uptake activity difference from water-injected controls (Fig. 7A and B). To determine the uptake affinity of OsNPF5.16, the uptake activity of oocytes expressing OsNPF5.16 at pH 5.5 was measured using several concentrations of15NO3-as substrates. A Michaelis–Menten plot (Fig. 7C) showed that the apparent mean Kmvalue for nitrate transport was 3.977 mmol L-1. Thus, OsNPF5.16 is a pH-dependent, low-affinity nitrate transporter.
3.6. Effect of OsNPF5.16 altered expression on CKs levels
It has been reported [27–30] that plant hormones, including CKs, auxins, and SLs, influence rice branching. To investigate the relationship between OsNPF5.16 expression and plant hormones,we first measured the expression of key genes that respond to CK, auxin, and SL pathways in the tiller basal part among ZH11 and the OE and Ri lines. For CK, we detected the expression of 8 OsRR and 6 OsORR genes. In comparison with ZH11, expression levels of OsRR1, OsRR5, OsRR8 and OsORR5 were significantly increased in OE lines but significantly decreased in Ri lines.Although the expression of OsRR3,OsRR4 and OsORR4 in some lines also differed significantly from that in ZH11, no trend in the expression of these genes was found among ZH11 and the OE and Ri lines (Fig. 8A). For auxin, seven YUCCA members and 10 IAA family genes were detected. Interestingly, the expression of YUCCA5 and IAA12 was significantly decreased in the OE lines but increased in the Ri lines compared to those in ZH11. However,the expression trends of YUCCA6 and YUCCA20 were opposite to those of YUCCA5 and IAA12 (Fig. S5A). For SL, the expression of D3, D27, D17, D10, D14, D53, Os900, and Os1400 was measured.Compared with ZH11, the expression of D53 was down-regulated in the OE lines but did not differ significantly in the Ri lines.Expression of the other SL response genes did not differ among ZH11 and the OE and Ri lines (Fig. S5C).

Fig.5. Altered expression of OsNPF5.16 influenced rice seedling growth.(A)Phenotypes of seedlings at 34 DAG among ZH11 and OE and Ri lines under 1 mmol L-1 NH4NO3 supply.Scale bar,20 cm.Biomass per plant(B),root number(C),root length(D),NO3-concentration(E),and total N content(F)among ZH11 and OE and Ri lines.Values in(B–F) are mean ± SD (n = 20) and three replications were assayed in each analysis; *, **, and *** indicate significant difference at P <0.05, P <0.01, and P <0.001, respectively.
To further determine whether changing the expression of OsNPF5.16 would affect the level of phytohormones, we measured the CKs, auxins, and SLs contents in tiller basal parts in ZH11 and the OE and Ri lines.Compared to ZH11,the OE lines showed a significant increase in CKs levels, including dihydrozeatin-Oglucoside riboside (DHZROG), cis-zeatin-O-glucoside riboside(cZROG), dihydrozeatin-7-glucoside (DHZ7G) and trans-zeatin(tZ), while the Ri lines showed a significant decrease in these CKs levels (Fig. 8B). Although other CKs such as cis-zeatin (CZ) and N6-isopentenyladenine (IP), were also significantly enriched in the OE lines, no significant difference in CZ and IP content was found in the Ri lines compared with those in ZH11(Fig.8B).However, the auxins and SLs levels were not significantly different among ZH11, OE lines, and Ri lines (Fig. S5B and D). These results indicated that elevated expression of OsNPF5.16 is capable of increasing rice CKs accumulation by transporting nitrate.

Fig. 7. Functional analysis of OsNPF5.16 in oocytes. Nitrate uptake activity of OsNPF5.16-injected and CHL1-injected (as positive control) oocytes. (A) High-affinity nitrate uptake activity was investigated by incubation of oocytes at pH 5.5 or pH 7.5 for 3 h.(B)Low-affinity nitrate uptake activity was investigated by incubation of oocytes at pH 5.5 or pH 7.5 for 3 h. (C) Nitrate uptake kinetics of OsNPF5.16. OsNPF5.16-injected oocytes were incubated with different concentrations of 15N-NaNO3 at pH 5.5 for 1.5 h.Values are means ± SD (n = 6 oocytes for each concentration). *** indicates significant difference at P <0.001. All experiments were repeated three times.
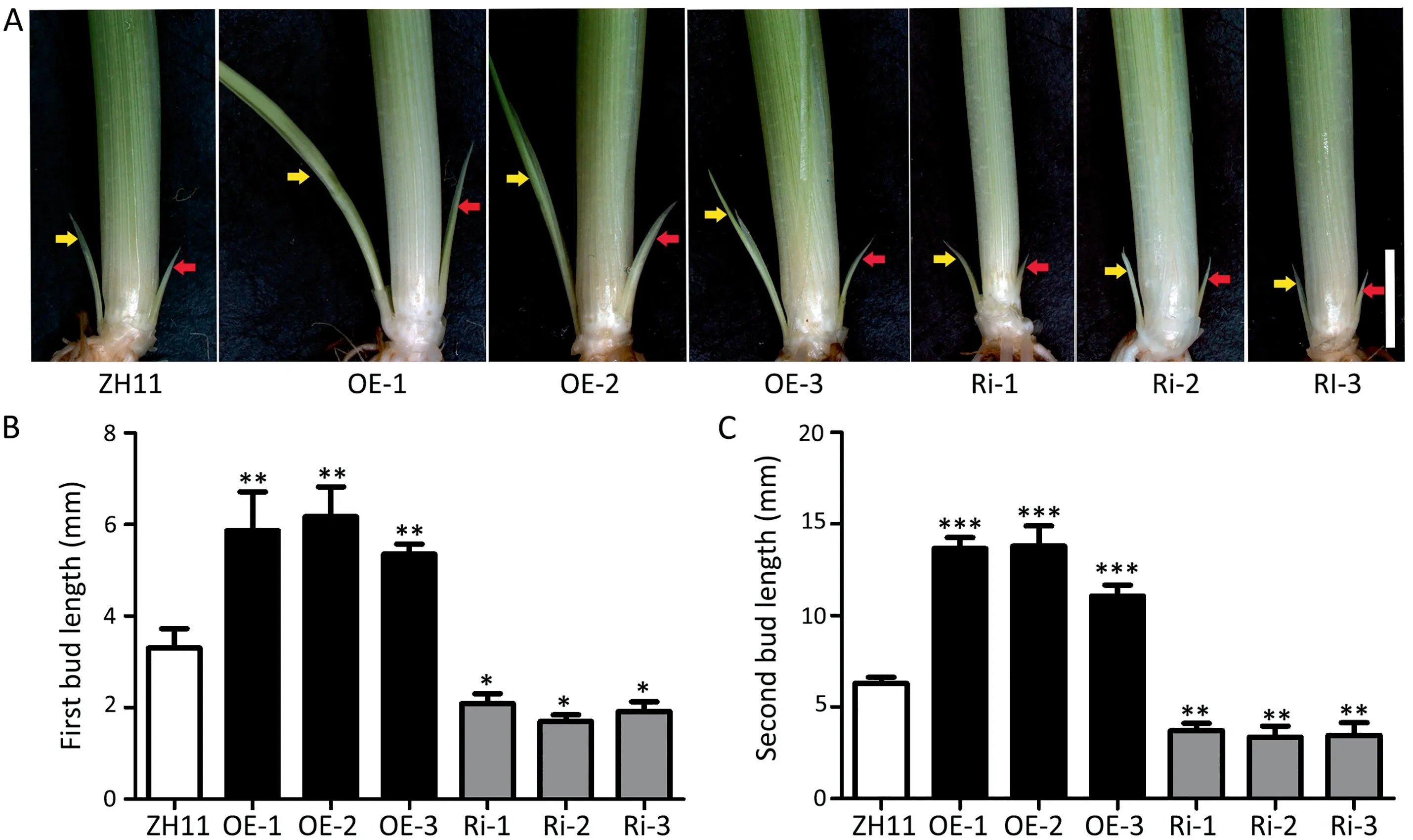
Fig.6. Elevated OsNPF5.16 expression promoted tiller bud growth.(A) Comparison of tiller buds among ZH11, OE lines, and Ri lines under 1 mmol L-1 NH4NO3 supply. Red arrows indicate first tiller buds and yellow arrows indicate second tiller buds,Scale bar,5 mm.Statistical analysis of the first tiller bud length(B)and the second tiller buds(C)among ZH11,OE lines,and Ri lines.Values are mean±SD(n >20)and three replications were measured in each analysis.*,**,and***indicate significant difference at P <0.05,P <0.01, and P <0.001, respectively.
4. Discussion
4.1.Haplotype difference of the OsNPF5.16 gene was closely associated with the differentiation of indica and japonica rice
Indica and japonica are two subspecies of Asian cultivated rice that differ in various developmental and physiological traits [9].Indica shows a higher uptake of both nitrate and ammonium than japonica [9]. In the present study, a new nitrate transporter gene,OsNPF5.16, which was more highly expressed in indica than in japonica, differed between indica and japonica. Multiple indels in the promoter of OsNPF5.16 differentiating indica(Hap 2)and japonica(Hap 1)could be responsible for the variation in the expression of the two haplotypes. Recently, other genes involved in the N metabolism and indica/japonica differentiation in rice have been reported, such as OsNPF6.5 [9], OsAAP3 [31], OsAAP4 [32], OsAAP5[20] and OsAAP6 [33]. In addition to OsAAP6 affecting rice grain quality,other genes are reported to regulate rice tillering and yield.OsAAP3, OsAAP4, and OsAAP5 affected N use and grain yield in rice by selective amino acid transport, while the OsNPF6.5 allele in indica increased NUE and grain yield in comparison with japonica by increasing nitrate uptake[9].The phosphorylation of the nitrate transporter protein AtNRT1.1 at Thr-101 (T101) was crucial for its transport and sensing functions in Arabidopsis[26,34].A SNP in the CDS of OsNPF6.5 that results in a Thr327 Met substitution in NRT1.1B-Nipponbare/IR24 was crucial for the transport function[9]. Our results suggest that OsNPF5.16 may contribute to differences between indica and japonica rice by regulating nitrate uptake and utilization by means of promoter variations that influence nitrate gene expression.
4.2.OsNPF5.16 is a nitrate-induced and low affinity nitrate transporter

Fig.8. Altered expression of OsNPF5.16 regulated CKs concentrations.(A)Comparison of CK-response genes in tiller basal part of seedlings at 34 DAG among ZH11,OE lines,and Ri lines.(B)Detection of CKs concentrations in tiller basal part.DHZR,dihydrozeatin ribonucleoside;cZR,cis-zeatin riboside;iP9G,N6-isopentenyl-adenine-9-glucoside;DHZROG, dihydrozeatin-O-glucoside riboside; cZROG, cis-zeatin-O-glucoside riboside; 2MeScZR, 2-methylthio-cis-zeatin riboside; cZ, cis-zeatin; DHZ7G, dihydrozeatin-7-glucoside;IP,N6-isopentenyladenine;tZ,trans-zeatin.Values are means±SD(n=3)of three replicates.*,**and***indicate significant differences at P <0.05,P <0.01,and P <0.001, respectively.
To effectively use N sources in their environment, plants have developed two NO3-uptake systems:a high-affinity NO3-transport system for root acquisition of NO3-at high uptake (>1 mmol L-1)and a low-affinity NO3-transport system for root acquisition of NO3-at low uptake[35,36].In Arabidopsis,the NRT1 family consists predominantly of low-affinity NO3-transporters and the NRT2 family consists of high-affinity NO3-transporters[26,37,38].In the present study, assays of15N-nitrate uptake using Xenopus oocytes showed that a new member of the NPF5 subfamily in rice,OsNPF5.16, is also a low-affinity nitrate transporter (Fig. 6) that influences grain yield by regulating tillering.For the NPF5 subfamily in Arabidopsis thaliana, AtNPF5.5 has been reported as a low affinity nitrate transporter that functions in nitrate transport in embryos [39]. For other NPF subfamilies in rice, members OsNPF2.2 [40], OsNPF2.4 [5], OsNPF4.5 [7], OsNPF7.2 [12], and OsNPF8.9 [17] were reported to be low-affinity nitrate transporters. OsNPF6.5 (NRT1.1B) showed nitrate-transport activity under both low and high nitrate conditions because it is a dualaffinity nitrate transporter[9].The role of rice OsNPF5.16 in nitrate transport revealed in this study improves our understanding of the rice NPF5 subfamily. The expression of OsNPF5.16 in the nutrient solution of NaNO3or NH4NO3was higher than that in (NH4)2SO4and upregulated in roots with increasing nitrate concentration(Figs. 3, S2). Thus, OsNPF5.16 as well as OsNPF2.2 [40], OsNPF7.2[12] and OsNPF6.5 [9] were induced by nitrate. Most of the NPF genes,including OsNPF6.5,were upregulated in OsNPF5.16 OE lines but downregulated in Ri lines in comparison with those in ZH11(Fig. S4). Thus, OsNPF5.16 may cooperate with OsNPF6.5 and other NPF genes to promote rice nitrate uptake from the environment.
4.3.Modified expression of OsNPF5.16 regulates rice tillering and grain yield mainly by influencing CKs levels
Phytohormones function in regulating the tillering of rice. SLs and auxins reduce tiller number by inhibiting tiller bud growth,whereas CKs promote tiller bud growth and increase rice tiller number[27,28,41].N concentrations also further influence rice tiller bud growth by affecting plant hormones content, and a low N concentration enhanced auxin and SL pathways,thus hindering tiller bud growth[42].A previous study[13]showed that changes in OsNPF7.2 expression influenced the contents of SLs and CKs in rice,affecting cell division and tiller bud elongation. In the present study, the OsNPF5.16 gene influenced mainly CKs contents rather than auxins and SLs contents (Figs. 8, S5), thus regulating tiller bud growth, indicating that nitrate transporters of different subfamilies differed with respect to their regulation of rice growth and development by means of plant hormones. The amino acid permease OsAAP5 regulated rice tiller number and grain yield by affecting CKs contents [20], while OsAAP1 influenced auxin, CK,and SL pathway in regulating rice tillering [43]. Different types of N transporters exerted different effects on rice growth and development by regulating plant hormones. The signaling molecule NO produced by nitrogen metabolism directly interacts with transzeatin (TZ) to produce nitrifying CKs, to regulate the CK signaling pathway [44]. The mechanism of nitrate accumulation in plants to increase CKs levels remains to be studied.
Declaration of competing interest
The authors declare that they have no competing interests.
CRediT authorship contribution statement
Jie Wang:Conceptualization, Funding acquisition, Project administration, Investigation, Data curation, Validation, Writing –original draft.Renjing Wan:Investigation, Data curation, Validation.Haipeng Nie:Investigation, Data curation, Validation.Shaowu Xue:Investigation,Data curation,Validation.Zhongming Fang:Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing - review & editing.
Acknowledgments
This research was supported by the National Key Research and Development Program(2016YFD0100700),the Wuhan Science and Technology Project (2020020601012259), Hubei Natural Science Foundation (2020CFB117), the National Natural Science Foundation of China (31301250), the Talent Project from Guizhou Education Department (Qian jiao he KY zi (2021) 024), the Key Cultivation Project of Guizhou University(201903),and the Talent Project from Thousands of Innovative and Entrepreneurial in Guizhou Province.
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.08.005.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Cob color, an indicator of grain dehydration and agronomic traits in maize hybrids
- Effects of sgRNA length and number on gene editing efficiency and predicted mutations generated in rice
- Imbalance between nitrogen and potassium fertilization influences potassium deficiency symptoms in winter oilseed rape(Brassica napus L.)leaves
- ZmWRKY104 positively regulates salt tolerance by modulating ZmSOD4 expression in maize
- A novel genomic prediction method combining randomized Haseman-Elston regression with a modified algorithm for Proven and Young for large genomic data

