UV/NaClO和UV/过碳酸钠工艺降解水杨酸的对比
马晓雁,杨 帆,李青松,杨庆云,陈国元,李国新
UV/NaClO和UV/过碳酸钠工艺降解水杨酸的对比
马晓雁1,杨 帆1,李青松2,3*,杨庆云1,陈国元2,李国新2
(1.浙江工业大学土木工程学院,浙江 杭州 310014;2.厦门理工学院水资源环境研究所,福建 厦门 361024;3.厦门理工学院厦门市水资源利用与保护重点实验室,福建 厦门 361024)
采用UV/NaClO和UV/过碳酸钠(SPC)工艺降解水中水杨酸(SA),对比考察了氧化剂种类和投加量对SA去除的影响,采用淬灭法和电子顺磁共振波谱仪(EPR)鉴定识别了2种工艺中的自由基,通过竞争动力学的方法计算了SA与•OH、CO3•-的二级反应速率常数及反应体系中不同组分的贡献,从环境水样模拟、急性毒性和经济效益等角度比较了SA的去除效果.结果表明,UV/NaClO工艺和UV/SPC工艺降解SA的拟一级动力学常数分别为0.4378,0.3794min-1.UV/NaClO和UV/SPC体系中分别存在•OH、Cl•和O2•-、•OH及CO3•-等自由基.SA与•OH、CO3•-的二级反应速率常数分别为3.97´109,8´107L/(mol×s).UV/NaClO工艺中活性氯自由基(RCS)(79.91%)对SA去除起主导作用;而UV/SPC工艺中O2•-(51.75%)与•OH(41.42%)起主导作用.环境水样中SA在UV/NaClO和UV/SPC工艺中的降解受到抑制,其反应速率分别平均降低了67%和74%.UV/SPC工艺反应溶液的抑制率(25%)较UV/NaClO工艺反应溶液(63%)低38%.SA降解率达到97.5%以上时UV/SPC工艺的成本[37.1$/(m3×order)]是UV/NaClO工艺成本[4.0$/(m3×order)]的9.3倍,UV/NaClO工艺较UV/SPC工艺具有较高的经济效益.
UV-AOPs;自由基鉴定;自由基贡献;急性毒性;经济效益
近年来药物和个人护理品(PPCPs)在水体中被频繁检出,由于其潜在的危害与风险,水环境中PPCPs的去除对于保障饮水安全具有重要的意义[1-3].目前,基于紫外的高级氧化工艺(UV-AOPs)已广泛应用于去除水中的PPCPs[4].UV-AOPs常见的氧化剂有双氧水(H2O2)、次氯酸钠(NaClO)、臭氧、过硫酸盐、过碳酸钠(SPC)等,实际应用中可以根据水质情况采用不同的氧化剂以达到更好的去除效果[4-6].
UV/NaClO工艺能产生将污染物高效去除的•OH和活性氯自由基(RCS,包括Cl•、ClO•、Cl2•-等),且去除富含电子的有机污染物时,RCS比•OH具有更高的二级反应速率常数[7-9].SPC溶于水后经UV照射可产生•OH、O2•-、CO3•-等自由基,且不同pH值条件下主导的自由基不同,这一特点适用于处理不同的目标污染物[10-11].相比于NaClO等液体氧化剂,固体氧化剂SPC具有更好的稳定性、抗爆性和可获得性[5,12].关于UV/NaClO和UV/SPC工艺去除水中 PPCPs 的研究已有诸多报道,然而2种工艺在相同条件下降解同一污染物时去除效果、机理与经济效益尚不明确.
水杨酸(SA)是地表水中检出频次较高的典型PPCP,地表水中其浓度高达2014.4ng/L,对水生生物构成威胁[2-3].本文采用UV/NaClO和UV/SPC工艺降解水中的SA,从氧化剂投加量、自由基种类与贡献率、环境水样应用、急性毒性和经济效益等角度对比考察2种工艺的差异,以期为去除水中PPCPs工艺选择提供参考.
1 材料与方法
1.1 实验材料与仪器
SA(纯度99.9%,德国Dr.Ehrenstorfer);硝基苯(NB,AR,阿法埃莎化学有限公司);N,N—二甲基苯胺(DMA,纯度³99%,上海麦克林生化科技有限公司);三氯甲烷(CF,HPLC,美国Anaqua).过碳酸钠(SPC, CP)、对氯苯甲酸(pCBA,GC)、5,5-二甲基-1-氧化吡咯啉(DMPO,AR)等购于上海阿拉丁生化科技股份有限公司;五水硫代硫酸钠(Na2S2O3·5H2O, AR)、叔丁醇(TBA,HPLC)、乙腈(C2H3N,HPLC)、苯酚(PhOH,AR)等购于安谱实验科技股份有限公司. NaClO、H2O2、NaOH、HCl、NaHCO3、CH3COOH等购于国药集团化学试剂有限公司,除NaClO为化学纯外其余均为分析纯.实验用水为Milli-Q超纯水.
高效液相色谱仪(HPLC)(LC-20A,日本Shimadzu)、电子顺磁共振波谱仪(EPR) (SN0253,德国Bruker)、便携式余氯计(CL200,上海三信仪表厂)、pH计(ST2100,常州奥豪斯仪器有限公司)、磁力搅拌器(HJ-6A,江苏金坛峥嵘仪器)、纯水机(Milli-Q,美国Milipore)、发光细菌毒性检测仪(LumiFox 6800,深圳朗石科学仪器有限公司).
1.2 降解实验方法
实验在一个置于磁力搅拌器上的圆柱形容器(容积500mL)中进行,光源为低压紫外汞灯(0.18mW/cm2,波长254nm),汞灯外套有石英套管.实验溶液为浓度500μg/L的SA溶液(300mL),实验前用0.1mol/L的NaOH溶液或0.1mol/L的HCl溶液调节pH值至7.实验开始时首先投加一定浓度的NaClO或SPC,同时开启磁力搅拌器与汞灯开始计时,在特定时间取样经0.22μm滤膜过膜后进行HPLC分析.进样瓶在实验开始前先加入10μL浓度为0.01mol/L的Na2S2O3·5H2O以确保完全淬灭剩余氧化剂.所有实验重复3次并取平均值.
1.3 分析方法
SA的浓度采用HPLC进行检测.HPLC方法:流动相A:0.1%乙酸溶液;流动相B:乙腈;A:B=65:35;流速为1mL/min;紫外检测器波长为292nm;柱温为40℃;进样体积20μL;
1.4 自由基捕获实验方法
鉴定UV/NaClO工艺中Cl•和•OH时,分别调节pH=7和pH=12.5,UV/SPC工艺中•OH和CO3•-鉴定时调节溶液pH=7.由于DMPO-O2•-在水溶液中的存活时间极短,鉴定O2•-在二甲基亚砜溶液中进行[13].降解实验开始后,在1min时抽取1mL反应液立即与0.1mL 100mmol/L的DMPO溶液混合,迅速用50μL毛细管吸取至一定高度后封口,立即进行EPR检测.
1.5 急性毒性实验方法
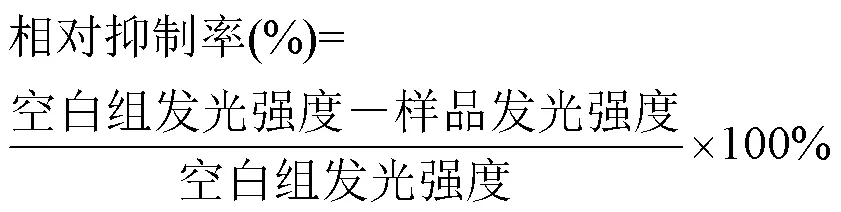
实验前混合复苏稀释液与发光细菌冻干粉形成细菌液,并活化15min,将空白样、待测样与渗透压调节液以900μL:100μL混合并摇匀.实验开始时空白样和待测样中分别加入50μL细菌液混匀,测量初始发光强度后送入仪器样本区,培养30min后再进行发光强度检测.急性毒性的结果可以通过相对抑制率来表示水样急性毒性强度.
2 结果与讨论
2.1 UV/NaClO和UV/SPC工艺对SA的降解
由图1可知,NaClO与SA的物质的量比从3.7增加至18.5,SA的拟一级动力学常数()由0.0842min-1增加至0.4378min-1,相同反应时间内相应SA的去除从57.3%增加至100%;SPC与SA的物质的量比由7增加至35时,SA的从0.1239min-1增加至0.3794min-1,对应的SA去除率从71.5%提高到97.8%;2种工艺处于同一量级,氧化剂与SA的物质的量比小于14时UV/SPC工艺较大,氧化剂与SA的物质的量比大于14时UV/NaClO工艺较大.SA的与去除率均随着氧化剂投加量的增大而增加,原因是NaClO的增加可以生成更多的•OH和RCS(式(2~7)),SPC的增加可以生成更多的•OH、O2•-和CO3•-等(式(8~12))[7,10,14].UV/NaClO和UV/SPC工艺氧化剂物质的量投加比增加至5倍时,分别增加至5.2和3.1倍,UV/NaClO工艺的增幅是UV/SPC工艺增幅的1.4倍;当SPC投加的物质的量比超过14时,的增幅变缓,可能原因是溶液中的CO32-和HCO3-会消耗•OH(式(10,13~14))[9],这与Yan等[11]采用Fe2+活化SPC降解磺胺甲恶唑得到的规律相似.
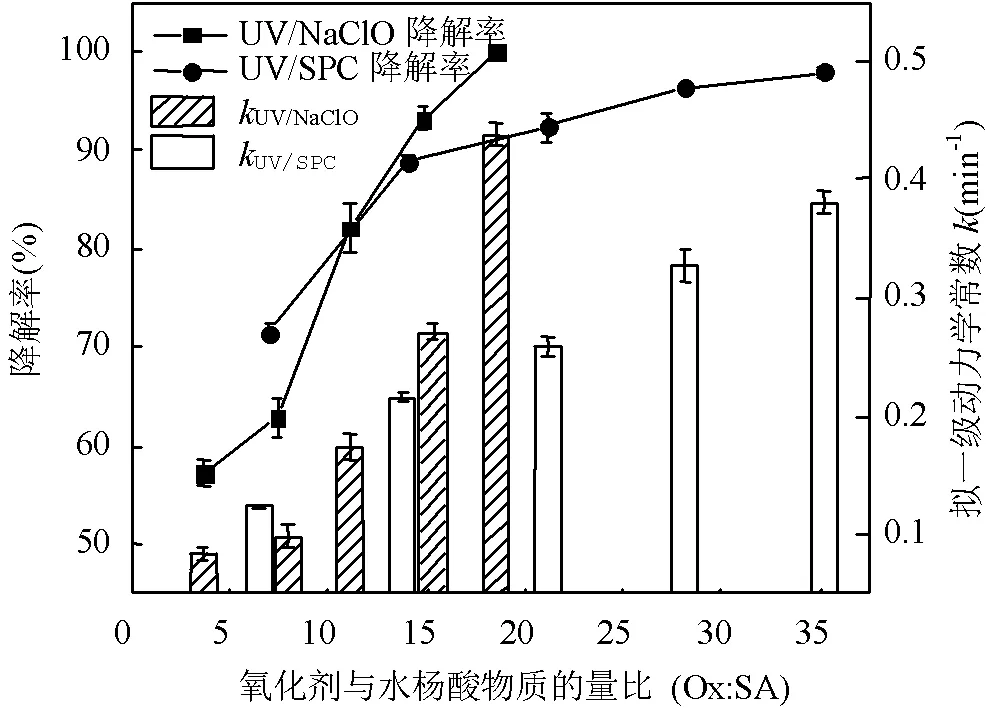
图1 UV/NaClO和UV/SPC对SA的去除
[SA]0=3.6μmol/L, [NaClO]: [SA]0=3.7、7.4、11.1、14.8、18.5,[SPC] :[SA]0=7、14、21、28、35, pH=(7.0±0.2)

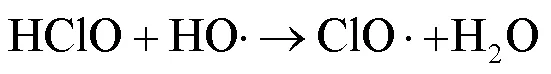
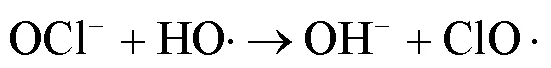
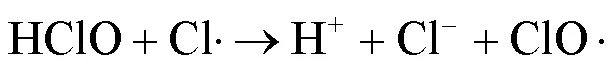
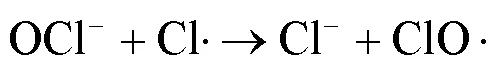
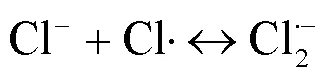
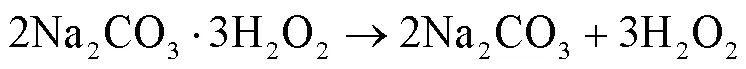
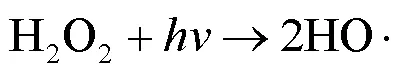
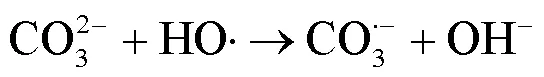
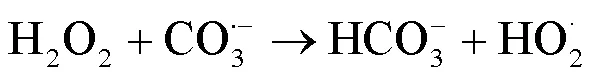
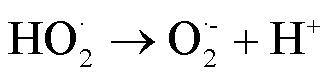
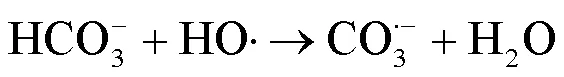
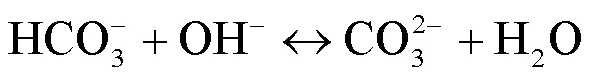
2.2 UV/NaClO和UV/SPC工艺中自由基的鉴定
TBA可用于淬灭•OH和Cl•[15];PhOH可用于淬灭•OH和CO3•-(PhOH-•OH=6×108L/(mol×s),PhOH-CO3•-= 2.2×107L/(mol×s))[12,16];CF可用于淬灭O2•-(CF-O2•-= 3.0×1010L/(mol×s))[10];加入自由基清扫剂TBA、PhOH和CF后2种工艺SA的去除见图2.
UV/NaClO工艺中SA的去除率随TBA的增加而减小,表明体系中含有•OH和Cl•.加入过量TBA (20mM)后SA的去除率仍大于单独NaClO(4.5%)和UV(13%)的去除率,说明体系中存在其他RCS的贡献.UV/SPC工艺中加入CF、TBA和PhOH后,SA的去除率均不同程度降低,表明体系中含有O2•-、•OH和CO3•-.Yue等[10]用UV/SPC工艺降解辣椒素同样证明了体系中存在O2•-、•OH和CO3•-.
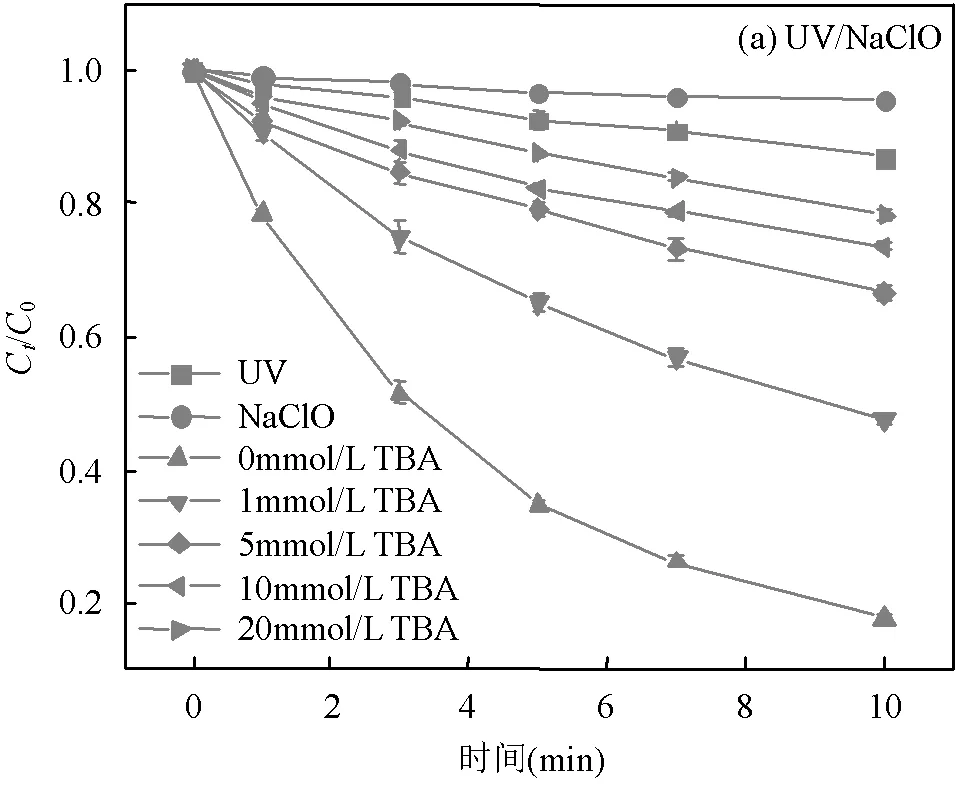
[SA]0=3.6μmol/L, [NaClO]=40μmol/L,[SPC]=75.6μmol/L,pH=(7.0±0.2)
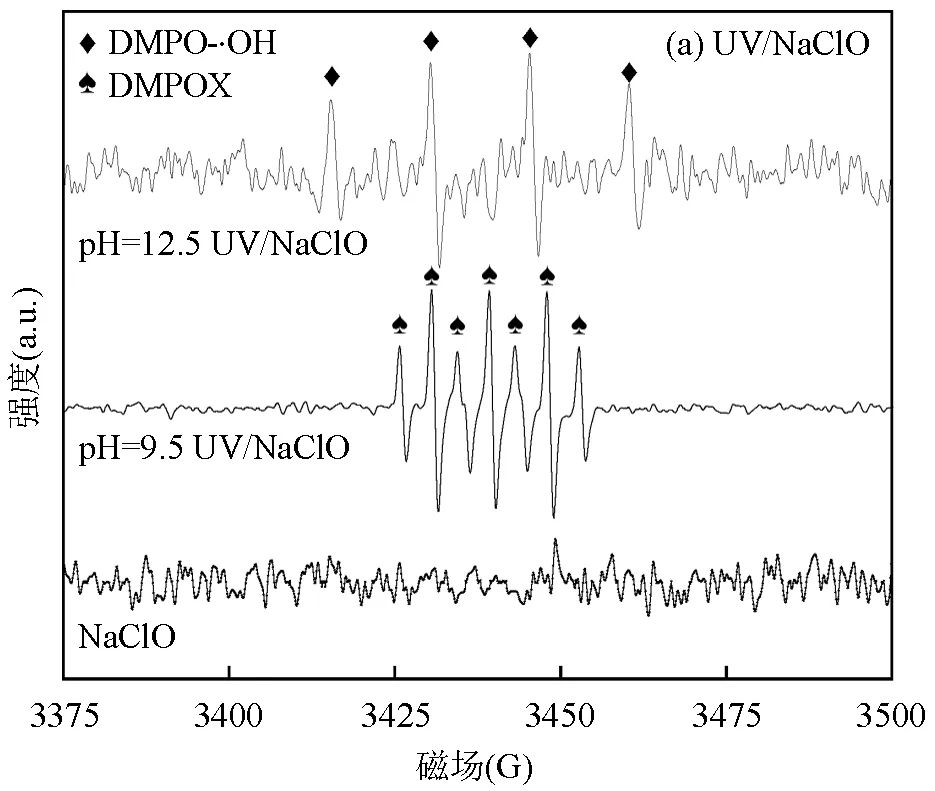
[NaClO]=40μmol/L, [SPC]=75.6μmol/L, [DMPO]=100mmol/L, 未特殊说明pH=(7.0±0.2)
采用DMPO作为自由基捕获剂检测UV/NaClO和UV/SPC工艺中产生的自由基,测定EPR图谱如图3.强碱性条件时UV/NaClO体系中•OH(EPR图谱四线强度比1:2:2:1)更明显,而弱碱性时DMPO将被氧化为DMPOX(EPR图谱七线强度比1:2:1:2:1:2:1),其为•OH和Cl•共同氧化的结果[17-18].而UV/SPC体系中检测到•OH和O2•-(EPR图谱四线强度比1:1: 1:1)[19].CO3•-作为UV/SPC体系中重要的自由基实验中未检测到,这可能是体系中产生的CO3•-量较少,以至EPR无法检出.
2.3 UV/NaClO和UV/SPC工艺降解SA过程中各组分贡献
2.3.1 SA与•OH、CO3•-的二级反应速率常数 二级反应速率常数用来描述自由基与化合物之间的反应快慢[16].通过UV/H2O2工艺降解SA与pCBA (pCBA-•OH=5´109L/(mol×s))进行竞争动力学实验计算SA与•OH的二级反应速率常数(式(15~16))[15](图4).联立式(15~16)得式(17),可求出SA与•OH的二级反应速率常数为3.94´109L/(mol×s).该结果略小于Peralta等[20]报道的SA与•OH的二级反应速率常数5´109L/(mol×s),但仍为同一数量级.
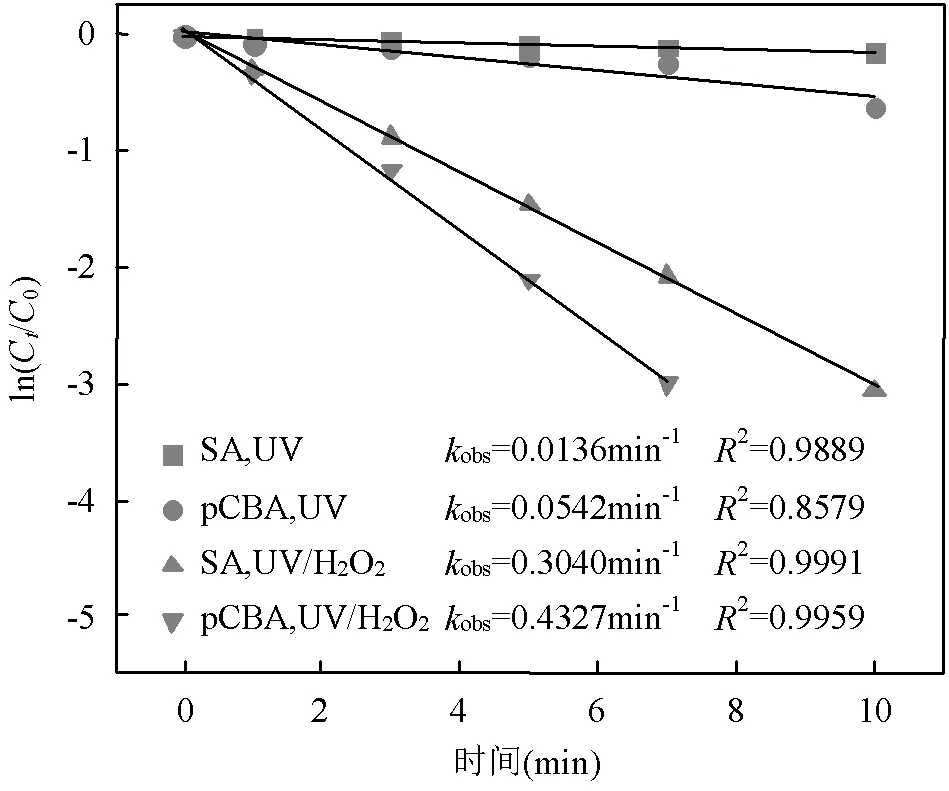
图4 SA和pCBA在UV/H2O2工艺中的降解
[SA]0=[pCBA]0=3.6μmol/L, [H2O2]=60μmol/L, pH=(7.0±0.2)
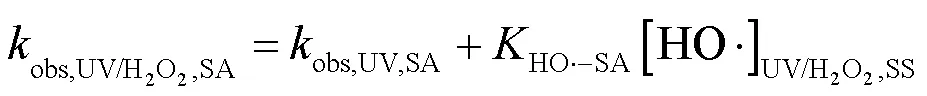
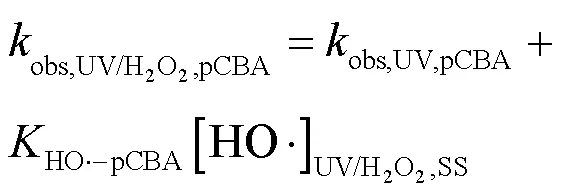
式中:obs,UV/H2O2,SA和obs,UV/H2O2,pCBA为降解SA、pCBA的拟一级动力学常数;obs,UV,SA和obs,UV,pCBA为单独UV降解SA、pCBA的拟一级动力学常数;HO•-SA和HO•-pCBA为•OH与SA、pCBA的二级反应速率常数;[HO•]UV/H2O2,SS表示•OH的稳态浓度.
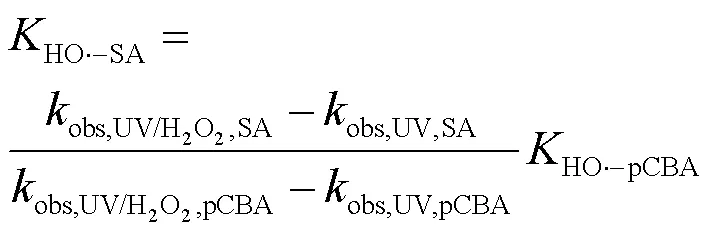
选用TBA与CF分别作为•OH与O2•-的清除剂[10,15],通过SA和PhOH在UV/SPC工艺中的降解求出SA与CO3•-的二级反应速率常数(式(18~ 19))[12],结果见图5.
据此简化式(18~19)为式(20~21),联立式(20~21)得到式(22),可求出SA与CO3•-的二级反应速率常数为1.66´107L/(mol×s).此前Wojnárovits等[16]报道CO3•-与有机分子的二级反应速率常数在102~109L/(mol×s)范围内,实验结果与该范围相符.
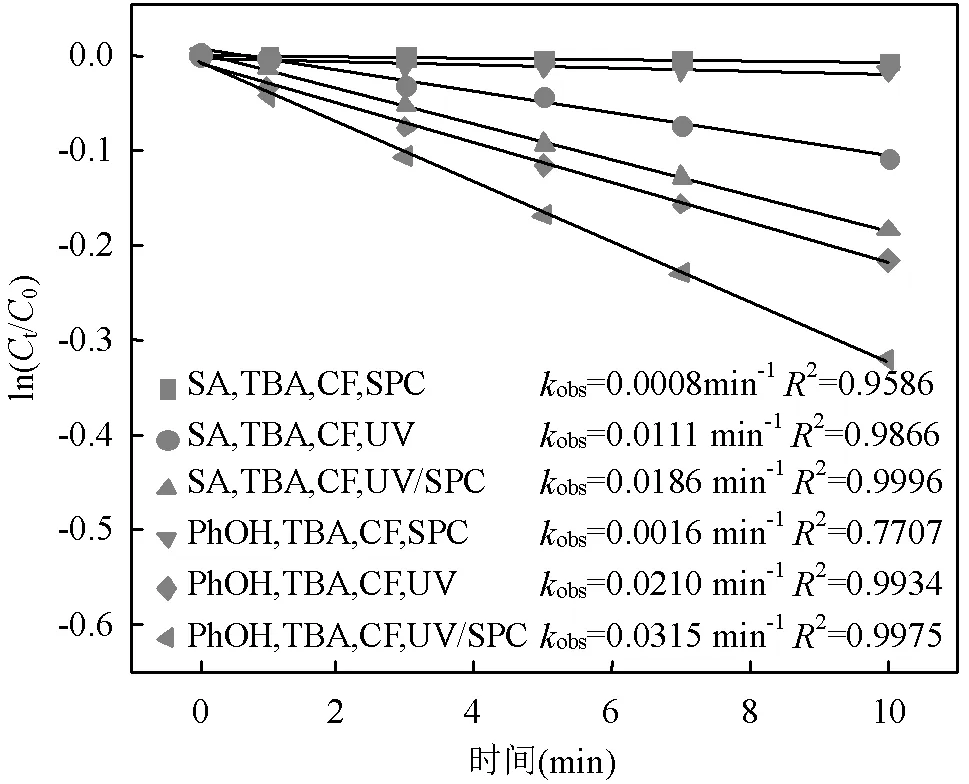
图5 SA和PhOH在UV/SPC工艺中的降解
[SA]0=[PhOH]0=3.6μmol/L, [SPC]=75.6μmol/L, [TBA]=10mmol/L,[CF]=10mmol/L, pH=(7.0±0.2)
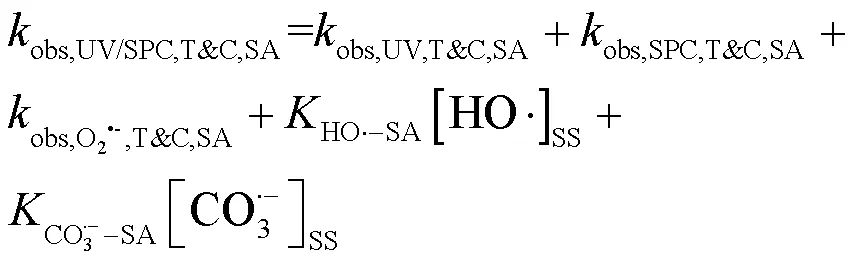
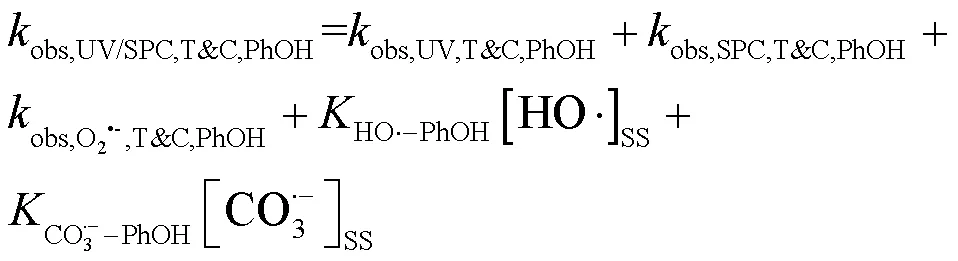
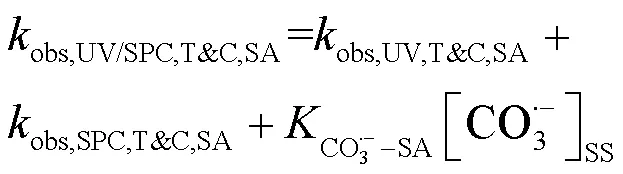
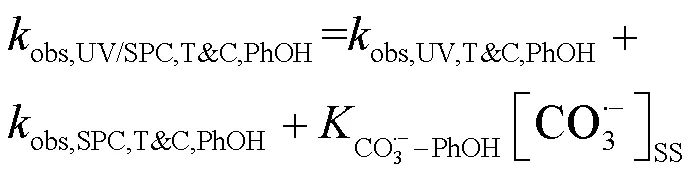
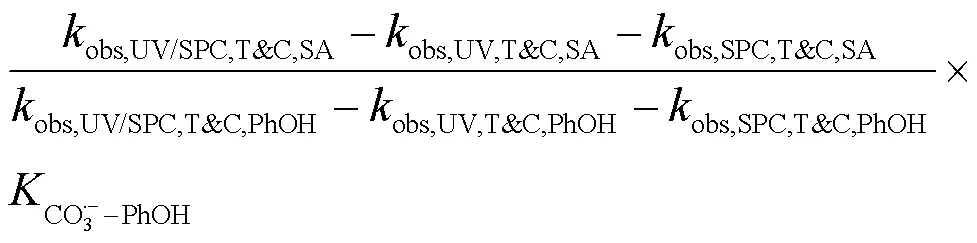
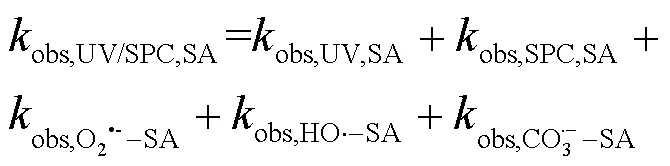
式中:T&C表示溶液中加入足量TBA与CF作为自由基淬灭剂;obs,UV/SPC,T&C,SA和obs,UV/SPC,T&C,PhOH为降解SA与PhOH的拟一级动力学常数;obs,UV,T&C,SA和obs,UV,T&C,PhOH为单独UV降解SA、PhOH的拟一级动力学常数;obs,SPC,T&C,SA和obs,SPC,T&C,PhOH为单独SPC降解SA、PhOH的拟一级动力学常数;obs,O2•-,T&C,SA和obs,O2•-,T&C,PhOH为O2•-降解SA、PhOH拟一级动力学常数,假定加入的CF完全淬灭O2•-,此处取0;HO•-PhOH为•OH与PhOH的二级反应速率常数;CO3•--SA和CO3•--PhOH为CO3•-与SA、PhOH的二级反应速率常数;[HO•]SS和[CO3•-]SS为该体系下•OH与CO3•-的稳态浓度,设定加入的TBA完全淬灭•OH,此处[HO•]SS取0.
2.3.2 UV/NaClO和UV/SPC不同组分对SA去除的贡献对比 选用NB、DMA分别作为•OH和CO3•-的探针(NB-•OH=3.9´109L/(mol×s),CO3•--DMA=1.8´109L/(mol×s)),采用探针法通过NB和DMA在UV/ NaClO和UV/SPC工艺中的降解(式(23~25))求出•OH和CO3•-的稳态浓度(图6)[21-22],进而求出不同组分对SA去除的贡献.将拟合所得到的拟一级动力学常数代入式(23~25)得出UV/NaClO工艺中•OH的稳态浓度为4.21´10-12mol/L,UV/SPC工艺中•OH和CO3•-的稳态浓度分别为2.72´10-11,8.20´10-11mol/L.
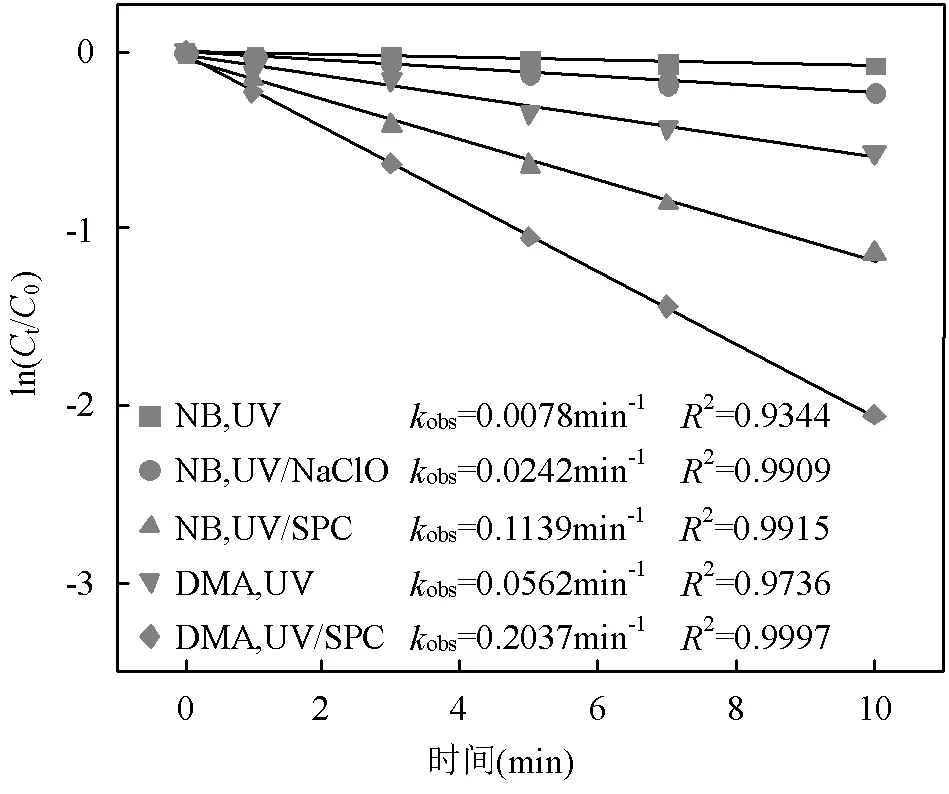
图6 NB和DMA在UV/NaClO和UV/SPC工艺中的降解
[NB]0=[DMA]0=3.6μmol/L, [NaClO]= 40μmol/L, [SPC]=75.6μmol/L, pH=(7.0±0.2)
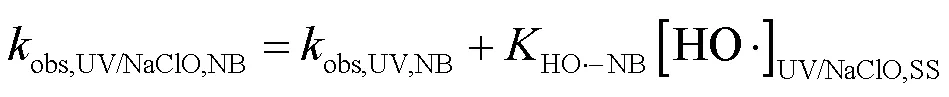
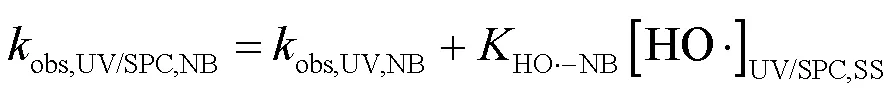
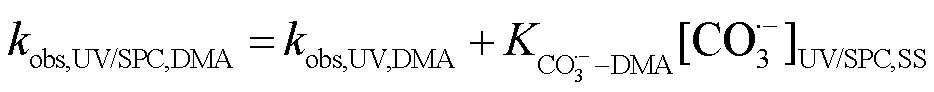
式中:obs,UV/NaClO,NB、obs,UV/SPC,NB和obs,UV/SPC,DMA为降解NB、DMA的拟一级动力学常数;obs,UV,NB和obs,UV,DMA分别为单独UV降解NB、DMA的拟一级动力学常数;HO•-NB和CO3•--DMA分别为NB与•OH、DMA与CO3•-的二级反应速率常数.
由SA与•OH、CO3•-的二级反应速率常数和•OH与CO3•-的稳态浓度,可求得2个工艺下•OH和CO3•-降解SA的拟一级动力学常数(式(26)~(28)).
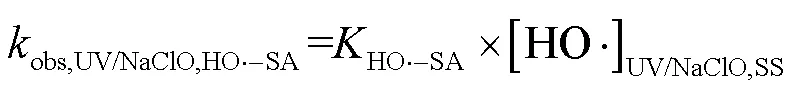
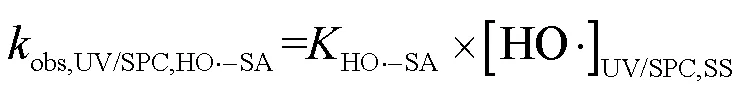
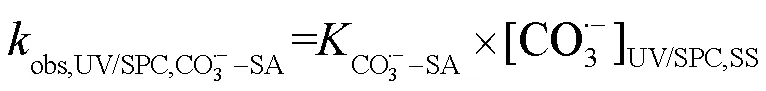
式中:obs,UV/NaClO,HO•-SA为UV/NaClO工艺下•OH降解SA的拟一级动力学常数;obs,UV/SPC,HO•-SA和obs,UV/SPC,CO3•--SA分别为UV/SPC工艺下•OH、CO3•-降解SA的拟一级动力学常数.
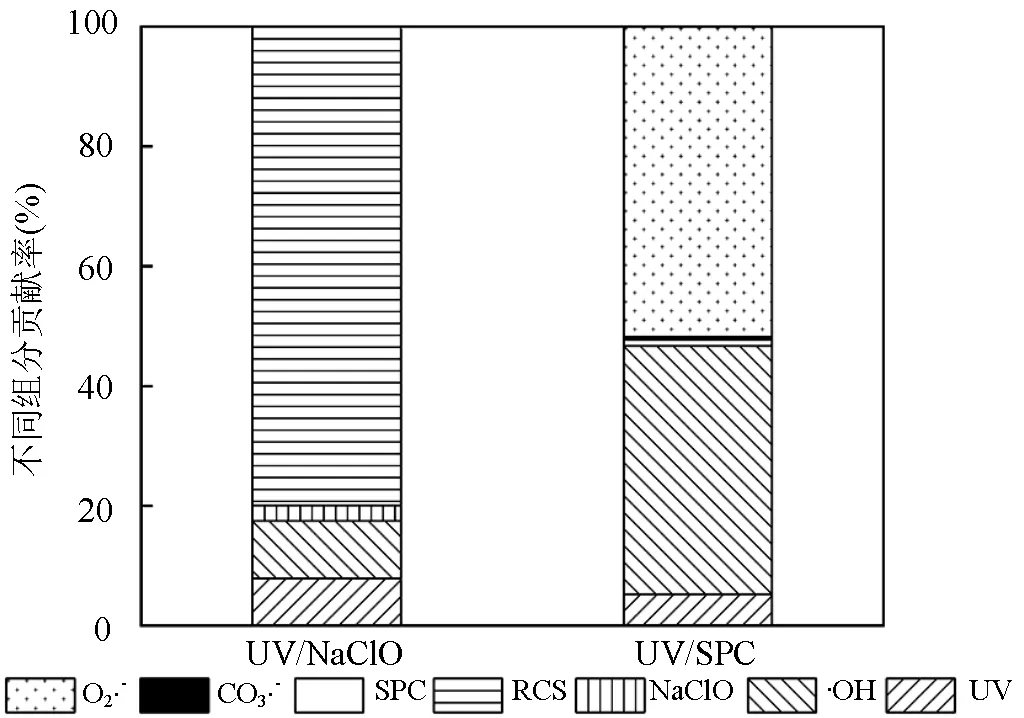
图7 不同物种在UV/NaClO和UV/SPC工艺中降解SA的贡献
[SA]0=3.6μmol/L, [NaClO]=40μmol/L,[SPC]=75.6μmol/L,pH=(7.0±0.2)
SA在2种工艺中的降解可表示为式(29~30),由式(29)可求得UV/NaClO工艺中RCS降解SA的拟一级动力学常数为0.1384min-1.由式(30)可求得UV/SPC工艺下O2•-降解SA的拟一级动力学常数为0.1339min-1.

由图7可知,UV/NaClO工艺中各组分贡献分别为:RCS(79.91%)>•OH(9.58%)>UV(7.85%)>NaClO(2.66%);UV/SPC工艺中各组分贡献分别为:O2•-(51.75%)>•OH(41.42%)>UV(5.25%)>SPC(1.04%)>CO3•-(0.54%).UV/NaClO工艺中占主导的自由基是RCS,其贡献是•OH的8.3倍,原因是生成的•OH转化为RCS(式(2~4)),该结果与李博强等[23]研究UV-LED/NaClO去除对乙酰氨基酚所得到的规律一致.而UV/SPC工艺中占主导的自由基是O2•-和•OH,两者贡献了SA去除的93.17%,O2•-的贡献超过50%,造成该结果的原因可能是CO32-的存在促进了O2•-的生成(式(10~12)).实际应用中可以加强自由基的生成与转化进而增强SA的降解效果.UV对两种工艺降解SA的贡献相差不大,这可能是因为2种工艺使用同一光源.•OH在2种工艺中的贡献率相差31.84%,这是因为UV/SPC工艺中•OH稳态浓度(2.72´10-11mol/L)是UV/NaClO工艺下•OH稳态浓度(4.21´10-12mol/L)的6.5倍.UV/SPC工艺中CO3•-在所有组分中的贡献最低,该结果为EPR无法检出CO3•-提供了佐证.原因是•OH与CO32-反应(式(10))的二级反应速率常数为3.9´108L/(mol×s),低于SA与•OH的二级反应速率常数3.94´109L/(mol×s)[9].
2.4 UV/NaClO和UV/SPC工艺环境水样中SA的降解
考察环境水样(厦漳地区北溪和江东泵站)、自来水及实验室超纯水(水质参数见表1)中对SA降解的影响,不同水体中SA的降解速率如图8.
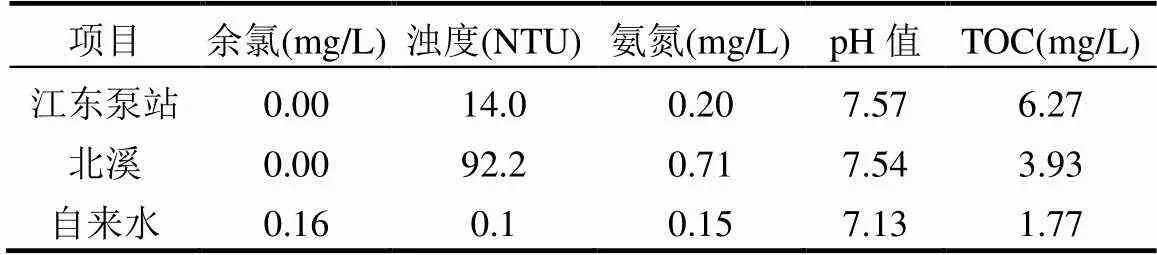
表1 环境水样的主要水质参数
UV/NaClO和UV/SPC工艺在江东泵站、北溪、超纯水和自来水水样中的反应速率分别为0.0517、0.0253、0.2174、0.1354min-1和0.0452、0.0213、0.2588、0.1383min-1.SA的降解速率为超纯水>自来水>江东泵站>北溪,这是因为一方面水样的浊度不同,影响UV的穿透;另一方面水体由有机物、无机物、浮游生物和微生物等复杂基质构成,这些物质在降解时也参与了反应并消耗了氧化剂及产生的自由基[24-25].Parastoo等[25]应用超声/臭氧工艺时得出浊度会消耗氧化剂.环境水样中UV/NaClO工艺降解SA的反应速率平均降低了67%,UV/SPC工艺中SA的反应速率平均降低了74%,UV/SPC工艺较UV/NaClO工艺易受自然水体的抑制,可能的原因是由于•OH的无选择性几乎可以与所有有机分子发生反应[6],而UV/SPC工艺中•OH的贡献率大于UV/NaClO工艺中•OH的贡献率,故其反应速率受自然水体的抑制更大.
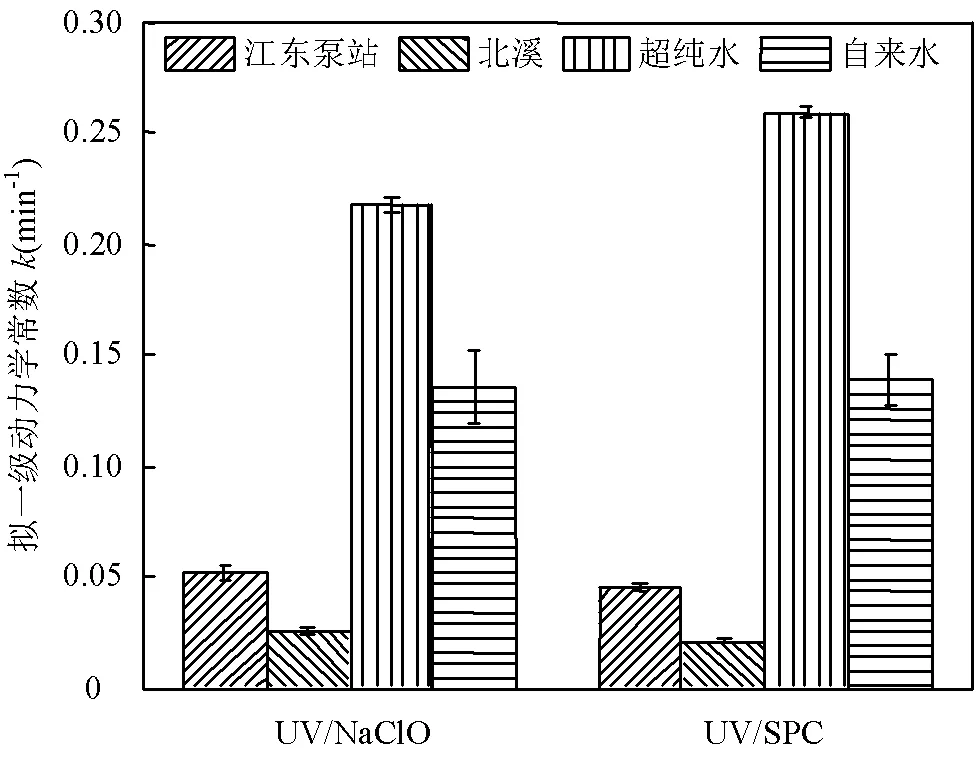
图8 环境水样对UV/NaClO和UV/SPC工艺降解SA的影响
[SA]0=3.6μmol/L, [NaClO]=40μmol/L,[SPC]=75.6μmol/L,pH=(7.0±0.2)
2.5 UV/NaClO和UV/SPC工艺降解SA过程中溶液急性毒性变化
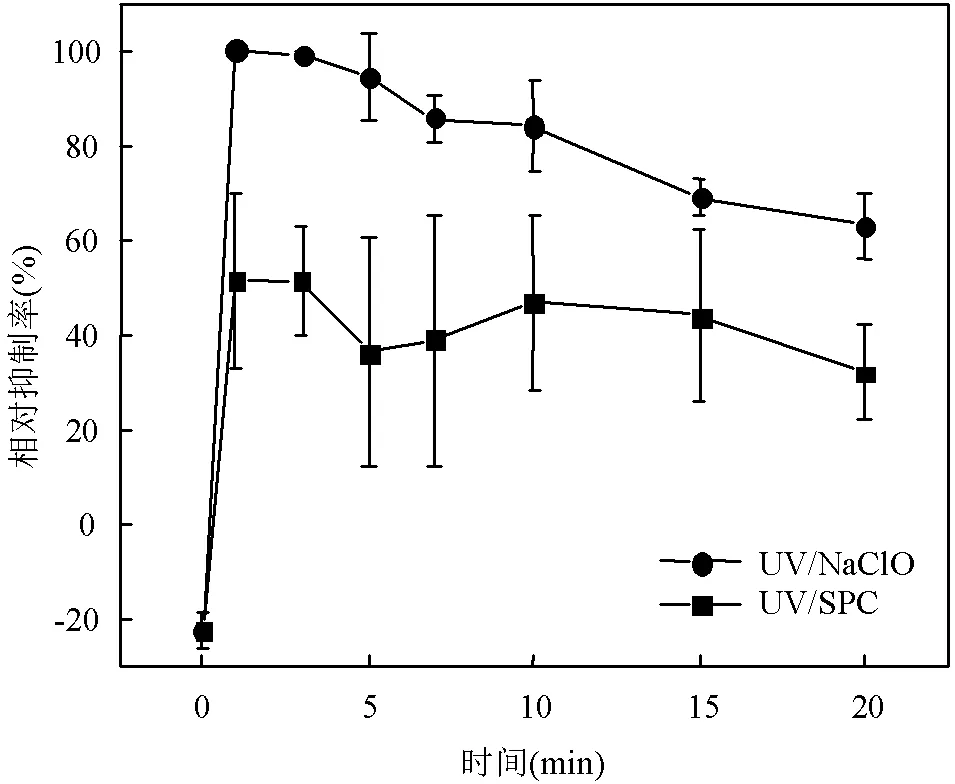
图9 UV/NaClO和UV/SPC工艺降解SA过程中急性毒性变化
[SA]0=3.6μmol/L, [NaClO]=40μmol/L,[SPC]=75.6μmol/L,pH=(7.0±0.2)
本文考察了2种工艺降解纯水中SA的溶液急性毒性变化,如图9,随着降解进行,UV/NaClO工艺中反应溶液的相对抑制率先增加到100%再降至63%,可能的原因是在产物中发现的典型消毒副产物三氯甲烷导致了反应初期毒性升高,随着反应的进行有毒产物进一步分解从而相对抑制率逐渐下降[26].而UV/SPC工艺中反应溶液相对抑制率保持在25%左右波动,UV/SPC工艺较UV/NaClO工艺低38%的抑制率、表现出较低的急性毒性.Zupanc等[27]人研究表明SA与•OH会生成PhOH和2,5-二羟基苯甲酸(2,5-DHBA)等产物.通过基于QSAR的ECOSAR软件模拟评估了这两种产物的急性毒性[28],结果表明产物急性毒性(PhOHEC50=2.40mg/L, 2,5-DHBAEC50=2.92mg/L,EC50数值越小代表毒性越大)均高于SA(SAEC50=11.35mg/L),故UV/SPC工艺降解SA过程中溶液相对抑制率升高可能是由于生成PhOH和2,5-DHBA造成.推测随着降解的进行产物将会与生成的自由基进一步发生反应,最终相对抑制率进一步降低.
2.6 UV/NaClO和UV/SPC工艺的经济效益比较
为从经济效益角度比较2个工艺,采用单位去除能耗(EE/OUV)表示m3水中降解1个数量级SA所需的电能(式(31)).单个工艺总成本(Cost/Ototal)可表示为电能成本(Cost/OUV)与氧化剂成本(Cost/ Ooxidant)之和(式(32~34)),其中工艺总成本单位为$/ (m3×order),表示m3水中降解1个数量级的SA所需要的成本[26].
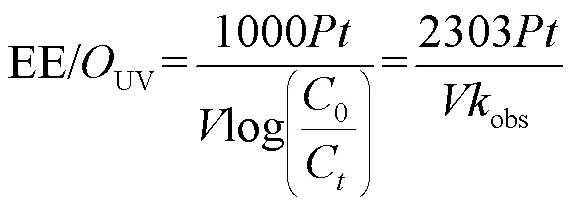
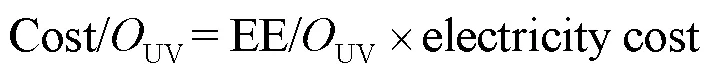
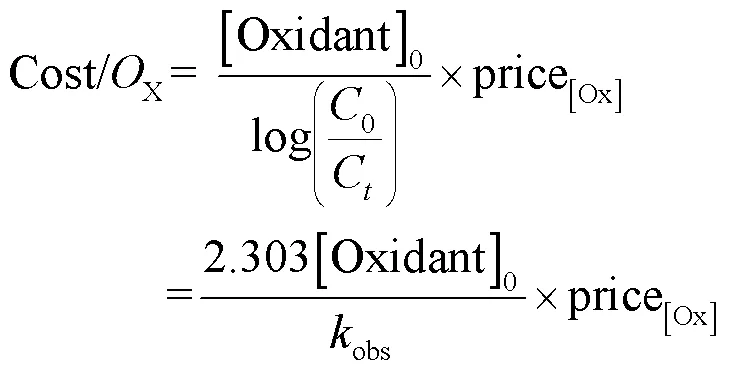
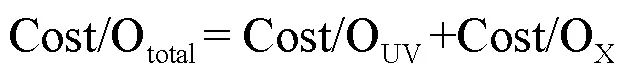
式中:表示UV汞灯输入功率,为2×10-3kW;表示反应时间,h;表示反应溶液的体积,L;obs表示拟一级动力学常数,min-1;0表示SA的初始浓度,为500μg/L;C表示SA在反应时刻的浓度,μg/L; [Oxidant]0表示氧化剂的投加量,mg/L; electricity cost表示电费,这里取0.1$/kWh; price[Ox]表示氧化剂的单价,NaClO为0.13$/kg; SPC为0.3$/kg[12,26].
如图10所示,单独UV降解SA的成本为18.9$/(m3×order),UV/NaClO工艺成本随着投加量的增加呈下降趋势,从最高8.7$/(m3×order)降至4.0$/ (m3×order).Ox/SA=3.7~7.4时UV/NaClO工艺效益曲线发生了转折,原因是NaClO投加比较低时整体降解效率随着投加比的增加提升不明显,但NaClO增加一倍导致氧化剂成本增加1倍,故表现为Ox/SA= 3.7的总体降解成本低于Ox/SA=7.4时. UV/SPC工艺成本随着投加量的增加从24.4$/ (m3×order)上升至37.1$/(m3×order),且高于单独UV降解SA的成本.SA降解率达到97.5%以上时, UV/SPC工艺成本是UV/NaClO工艺的9.3倍.从经济效益角度UV/NaClO工艺较UV/SPC工艺更节约成本,该结果与Lu等[26]比较UV、UV/NaClO和UV/过硫酸盐的经济效益得到的结果相似.
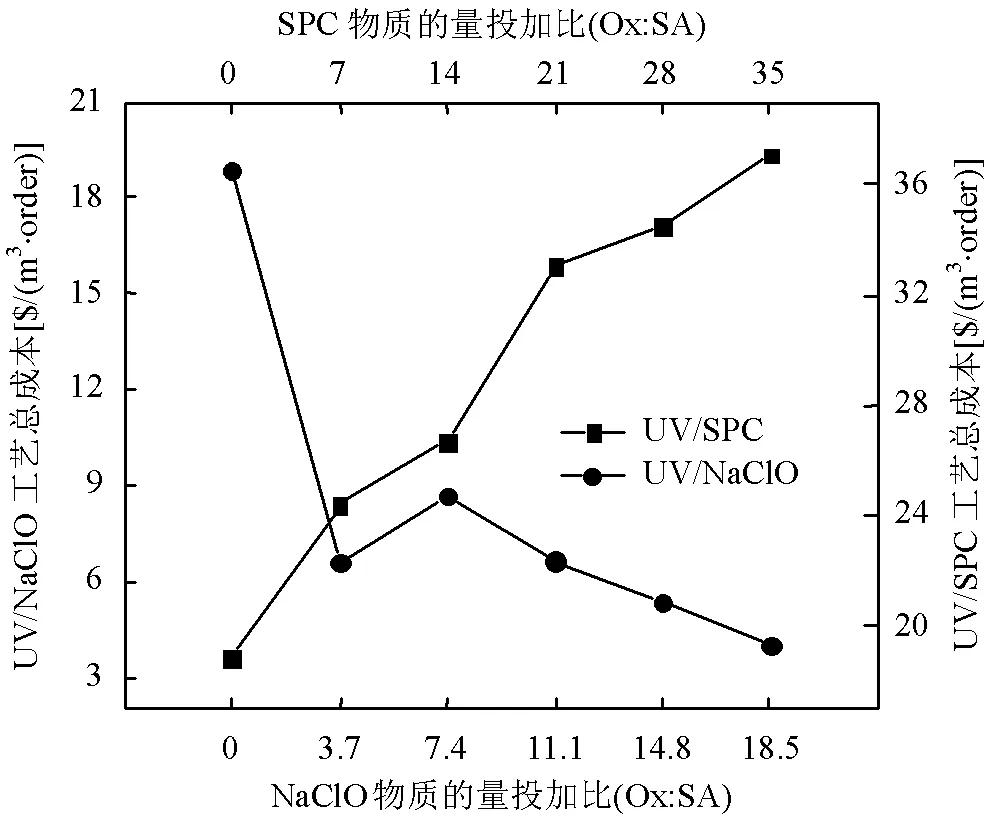
图10 UV/NaClO和UV/SPC工艺降解SA的经济效益评价
[SA]0=500μg/L, pH=(7.0±0.2)
3 结论
3.1 两种工艺对SA均能有效降解,反应速率均随着NaClO、SPC投加量的增加而增加,氧化剂摩尔投加比超过7.4后UV/NaClO工艺比UV/SPC工艺有更高的反应速率与降解率,UV/NaClO工艺和UV/SPC工艺反应速率最高分别达0.4378, 0.3794min-1.
3.2 UV/NaClO体系存在•OH和Cl•, UV/SPC体系中存在O2•-、•OH和CO3•-.SA与•OH、CO3•-的二级反应速率常数分别为3.97´109,8´107L/(mol×s). UV/ NaClO工艺下•OH的稳态浓度为4.21´10-12mol/ L,UV/SPC工艺下•OH、CO3•-的稳态浓度分别为2.72´10-11,8.20´10-11mol/L.自由基在SA的降解中起主导作用,UV/NaClO工艺中以RCS为主导的各组分贡献顺序为:RCS>•OH>UV>NaClO; UV/SPC工艺中以O2•-和•OH为主导的各组分贡献顺序为:O2•->•OH>UV>SPC>CO3•-.
3.3 两种工艺在环境水样中降解SA时反应速率均受到了抑制,UV/SPC工艺较UV/NaClO工艺受自然水体的抑制更明显.从急性毒性角度,UV/SPC工艺反应溶液较UV/NaClO工艺反应溶液低了38%的抑制率.从经济效益角度,UV/NaClO工艺较UV/SPC工艺具有较高的经济效益.
[1] Xiang Y, Wu H, Li L, et al. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China [J]. Ecotoxicology and Environmental Safety, 2021,213: 112044.
[2] Peng X, Ou W, Wang C, et al. Occurrence and ecological potential of pharmaceuticals and personal care products in groundwater and reservoirs in the vicinity of municipal landfills in China [J]. Science of the Total Environment, 2014,490:889-898.
[3] Yang Y, Ok Y S, Kim K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review [J]. Science of the Total Environment, 2017,596-597:303-320.
[4] Miklos D B, Wang W L, Linden K G, et al. Comparison of UV-AOPs (UV/H2O2, UV/PDS and UV/Chlorine) for TOrC removal from municipal wastewater effluent and optical surrogate model evaluation [J]. Chemical Engineering Journal, 2019,362:537-547.
[5] Yu X, Kamali M, Van Aken P, et al. Synergistic effects of the combined use of ozone and sodium percarbonate for the oxidative degradation of dichlorvos [J]. Journal of Water Process Engineering, 2021,39:101721.
[6] Ma D, Yi H, Lai C, et al. Critical review of advanced oxidation processes in organic wastewater treatment [J]. Chemosphere, 2021, 275:130104.
[7] Li S, Ao X, Li C, et al. Insight into PPCP degradation by UV/NH2Cl and compareson with UV/NaClO: Kinetics, reaction mechanism, and DBP formation [J]. Water Research, 2020,182:115967.
[8] Kong X, Wu Z, Ren Z, et al. Degradation of lipid regulators by the UV/chlorine process: Radical mechanisms, chlorine oxide radical (ClO•)-mediated transformation pathways and toxicity changes [J]. Water Research, 2018,137:242-250.
[9] Wang J, Wang S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants [J]. Chemical Engineering Journal, 2021,411:128392.
[10] Yue L, Cheng J, Hua J, et al. A sodium percarbonate/ultraviolet system generated free radicals for degrading capsaicin to alleviate inhibition of methane production during anaerobic digestion of lipids and food waste [J]. Science of the Total Environment, 2021,761:143269.
[11] Yan P, Sui Q, Lyu S, et al.Elucidation of the oxidation mechanisms and pathways of sulfamethoxazole degradation under Fe(II) activated percarbonate treatment [J]. Science of the Total Environment, 2018, 640-641:973-980.
[12] Gao J, Duan X, O'shea K, et al. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion [J]. Water Research, 2020,171:115394.
[13] Diaz-Uribe C E, Daza M C, Martínez F, et al. Visible light superoxide radical anion generation by tetra(4-carboxyphenyl)porphyrin/TiO2: EPR characterization [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2010,215(2/3):172-178.
[14] Eslami A, Mehdipour F, Lin K-Y A, et al.Sono-photo activation of percarbonate for the degradation of organic dye: The effect of water matrix and identification of by-products [J]. Journal of Water Process Engineering, 2020,33:100998.
[15] Ma J, Minakata D, O'shea K, et al.Determination and Environmental Implications of Aqueous-Phase Rate Constants in Radical Reactions [J]. Water Research, 2021,190:116746.
[16] Wojnarovits L, Toth T, Takacs E. Rate constants of carbonate radical anion reactions with molecules of environmental interest in aqueous solution: A review [J]. Science of the Total Environment, 2020,717: 137219.
[17] Huang X, Wang Y, Li X, et al. Autocatalytic decomplexation of Cu(II)-EDTA and simultaneous removal of aqueous Cu(II) by UV/chlorine [J]. Environmental Science & Technology, 2019,53(4): 2036-2044.
[18] Li T, Jiang Y, An X, et al.Transformation of humic acid and halogenated byproduct formation in UV-chlorine processes [J]. Water Research, 2016,102:421-427.
[19] Wang J, Wang S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism [J]. Chemical Engineering Journal, 2020,401:126158.
[20] Peralta E, Roa G, Hernandez-Servin J A, et al. Hydroxyl Radicals quantification by UV spectrophotometry [J]. Electrochimica Acta, 2014,129:137-141.
[21] Kwon M, Yoon Y, Kim S, et al. Removal of sulfamethoxazole, ibuprofen and nitrobenzene by UV and UV/chlorine processes: A comparative evaluation of 275nm LED-UV and 254nm LP-UV [J]. Science of the Total Environment, 2018,637-638:1351-1357.
[22] Zeng T, Arnold W A. Pesticide photolysis in prairie potholes: probing photosensitized processes [J]. Environmental Science & Technology, 2013,47(13):6735-6745.
[23] 李博强,马晓雁,李青松,等.UV-LED/NaClO工艺对水中对乙酰氨基酚的降解 [J]. 中国环境科学, 2019,39(11):4681-4688.
Li B Q, Ma X Y, Li Q S, et al. Degradation of acetaminophen in aqueous by UV-LED/NaClO process [J]. China Environmental Science, 2019,39(11):4681-4688.
[24] Zhang Y, Yao X, Wu Q, et al. Turbidity prediction of lake-type raw water using random forest model based on meteorological data: A case study of Tai lake, China [J]. Journal of Environmental Management, 2021,290:112657.
[25] Setareh P, Khezri S M, Hossaini H, et al.Coupling effect of ozone/ultrasound with coagulation for improving NOM and turbidity removal from surface water [J]. Journal of Water Process Engineering, 2020,37:101340.
[26] Lu X, Shao Y, Gao N, et al. Investigation of clofibric acid removal by UV/persulfate and UV/chlorine processes: Kinetics and formation of disinfection byproducts during subsequent chlor(am)ination [J]. Chemical Engineering Journal, 2018,331:364-371.
[27] Zupanc M, Petkovšek M, Zevnik J, et al. Anomalies detected during hydrodynamic cavitation when using salicylic acid dosimetry to measure radical production [J]. Chemical Engineering Journal, 2020, 396:125389.
[28] Wu Y, Deng L, Bu L, et al.Degradation of diethyl phthalate (DEP) by vacuum ultraviolet process: influencing factors, oxidation products, and toxicity assessment [J]. Environmental Science and Pollution Research, 2019,26(6):5435-5444.
Comparison of UV/NaClO and UV/ Sodium percarbonate processes for degradation of salicylic acid.
MA Xiao-yan1, YANG Fan1, LI Qing-song2,3*, YANG Qing-yun1, CHEN Guo-yuan2, LI Guo-xin2
(1.College of Civil Engineering, Zhejiang University of Technology, Hangzhou 310014, China;2.Water Resource and Environment Institute, Xiamen University of Technology, Xiamen 361024, China;3.Key Laboratory of Water Resources Utilization and Protectionof Xiamen, Xiamen University of Technology, Xiamen 361024, China)., 2022,42(3):1182~1190
The degradation of salicylic acid (SA) in aqueous solution by UV/NaClO and UV/SPC processes was investigated. The effects of oxidizer types and dosage on SA removal were compared. The free radicals in the two processes were identified by quenching method and electron paramagnetic resonance(EPR) spectroscopy. The second-order rate constants of •OH and CO3•-with SA and the contributions of different components in the reaction system were determined by competitive kinetics. The removal of SA was compared in terms of environmental water samples simulation, acute toxicity and economic benefit. The pseudo-first-order kinetic rate constants of UV/NaClO and UV/SPC processes were 0.4378 and 0.3794min-1, respectively. •OH and Cl• were detected in UV/NaClO process, while O2•-, •OH and CO3•-were detected in UV/SPC process. The second-order rate constants of •OH and CO3•-with SA were calculated to be 3.97´109and 8´107L/(mol×s), respectively. Reactive chlorine species (RCS) (79.91%) functioned a dominant role in the removal of SA in UV/NaClO process, while O2•-(51.75%) and •OH (41.42%) functioned a dominant role in UV/SPC process. The degradation of SA in environmental water samples by UV/NaClO and UV/SPC processes was inhibited, and the removal rates were reduced by 67% and 74%, respectively. The inhibition rate of UV/SPC process (25%) was 38% lower than that of UV/NaClO process(63%). The cost of UV/SPC process[37.1$/(m3×order)] was 9.3 times higher than that of UV/NaClO process[4.0$/(m3×order)] when SA degradation rate was above 97.5%. UV/NaClO process had higher economic benefit than UV/SPC process.
UV-AOPs;radical identify;contribution of free radicals;acute toxicity;economic benefit
X703
A
1000-6923(2022)03-1182-09
马晓雁(1978-),女,山东莱州人,教授,博士,主要研究方向为饮用水微量有机污染物控制.发表论文30余篇.
2021-08-18
国家自然科学基金资助项目(51878582,51978618);福建省科技计划引导性资助项目(2021Y0041);福建省自然科学基金资助项目(2020J01256);福建省高校新世纪优秀人才支持计划项目(JA14227);厦门理工学院科研攀登计划项目(XPDKT19026)
*责任作者, 研究员, leetsingsong@sina.com

