Exendin-4通过抑制Toll样受体4/核因子κB信号通路对帕金森病小鼠保护作用的研究*
陶计委, 南亚强, 周杰, 乐利明, 关海滨, 蒋谷峰, 范斐阳
Exendin-4通过抑制Toll样受体4/核因子κB信号通路对帕金森病小鼠保护作用的研究*
陶计委1,2, 南亚强1,2, 周杰2△, 乐利明2, 关海滨2, 蒋谷峰2, 范斐阳2
(1兰州大学第二医院,甘肃 兰州 730030;2中国人民解放军联勤保障部队第九四〇医院神经外科,甘肃 兰州 730050)
探讨exendin-4对1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)诱导的帕金森病(PD)小鼠的保护作用及机制。将48只雄性C57BL/6小鼠随机分为空白对照(control)组、exendin-4组、MPTP组和MPTP+exendin-4组,每组12只。MPTP和MPTP+exendin-4组腹腔注射MPTP(30 mg/kg),每天1次,连续5 d建立亚急性PD小鼠模型,造模成功后,exendin-4组及MPTP+exendin-4组小鼠给予腹腔注射exendin-4(2.5 μg/kg),每天2次,连续7 d治疗;选择爬杆实验和挂线实验评估小鼠行为学变化。采用Western blot法检测各组小鼠中脑酪氨酸羟化酶(TH)、离子钙结合接头分子1(Iba-1)、Toll样受体4(TLR4)、髓样分化因子88(MyD88)和核因子κB(NF-κB)p65蛋白表达水平,免疫组化法测定各组小鼠中脑黑质TH及TLR4的表达水平;酶联免疫吸附法测定中脑肿瘤坏死因子α(TNF-α)和白细胞介素6(IL-6)水平。与对照组比较,MPTP组小鼠转头时间及爬杆时间延长(<0.01),悬挂实验评分降低(<0.01),中脑黑质TH阳性神经元数及中脑TH蛋白表达减少(<0.01),Iba-1及TLR4、MyD88、p-NF-κB p65、核NF-κB p65蛋白及炎症因子TNF-α和IL-6表达水平增加(<0.01);与MPTP组相比,MPTP+exendin-4组小鼠转头时间及爬杆时间缩短(<0.01),悬挂实验评分增加(<0.01),中脑黑质TH阳性神经元数及中脑TH蛋白表达增加(<0.01),Iba-1、TLR4、MyD88、p-NF-κB p65、核NF-κB p65、TNF-α和IL-6表达水平降低(<0.01)。在MPTP诱导的PD小鼠模型中,exendin-4可通过抑制小胶质细胞及TLR4/NF-κB炎症信号通路激活而发挥抗神经炎症作用,保护多巴胺能神经元,改善PD小鼠运动功能。
Exendin-4;帕金森病;TLR4/NF-κB信号通路;神经炎症
帕金森病(Parkinson disease, PD)是一种好发于中老年人群的常见神经退行性疾病,其患病率随着年龄增加而增加,在65岁以上人群中患病率达1%,80岁以上人群中患病率达3%[1]。其病理特点为中脑黑质多巴胺能神经元变性死亡,纹状体多巴胺含量下降及路易小体形成[2-3]。神经炎症在PD发病机制中扮演着重要角色[4-5],Toll样受体4(Toll-like receptor 4, TLR4)作为神经炎症重要组成部分参与了PD的发病[6-9]。2型糖尿病与帕金森病患病风险呈正相关,并且它们有许多潜在的共同病理机制[10]。胰高血糖素样肽1(glucagon-like peptide-1, GLP-1)是一种由肠道L细胞分泌的多肽激素,其可通过增加胰岛素合成、促进胰岛素分泌和抑制胰高血糖素分泌等调节血糖,但其在体内易被二肽基肽酶4降解,血浆半衰期较短;exendin-4是从巨蜥唾液中分离出的GLP-1受体激动剂,它与哺乳动物GLP-1大约有50%的氨基酸序列相同,与GLP-1具有相似的生理功能,可改善胰岛细胞功能,葡萄糖浓度依赖性地促进胰岛素分泌,且其可抵抗二肽基肽酶4降解,因此具有较长血浆半衰期,临床上主要用于2型糖尿病治疗[11];近年来研究显示,exendin-4易通过血脑屏障[12],在PD动物模型中,其可通过抑制肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)及白细胞介素1β(interleukin-1β, IL-1β)等促炎因子的表达[13],促进α-突触核蛋白降解[14]等多种途径保护多巴胺能神经元;临床试验显示,exendin-4可改善帕金森病患者运动功能[15],且可能具有疾病修饰作用。本研究基于TLR4、髓样分化因子88(myeloid differentiation factor 88, MyD88)及核因子κB(nuclear factor-κB, NF-κB)炎症信号通路,利用1-甲基-4-苯基-1,2,3,6-四氢吡啶(1-methy1-4-phenyI-1,2,3,6-tetrahydropyridine, MPTP)诱导PD小鼠模型,通过行为学、病理及分子生物学等实验探讨exendin-4在PD小鼠模型中具体作用机制,为exendin-4应用于临床提供实验依据。
材料和方法
1 动物
48只SPF级雄性C57BL/6小鼠,10~12周龄,体重20~25 g,由中国农业科学院兰州兽研所提供,许可证号为SCXK(甘)2015-0001;每笼饲养6只小鼠,自由进食和饮水,相对湿度40%~60%,室温20~25 ℃。研究严格遵守《实验动物的护理和使用指南》的指导原则,已通过中国人民解放军联勤保障部队第九四〇医院伦理委员会的批准(审批编号:2021KYLL239)。
2 主要试剂
exendin-4购于Sigma-Aldrich;MPTP购于北京索莱宝科技有限公司;离子钙结合接头分子1(ionized calcium-binding adapter moleoule 1, Iba-1)抗体购于Abcam;TLR4和酪氨酸羟化酶(tyrosine hydroxylase, TH)抗体购于Proteintech;β-actin、histone H3、MyD88和NF-κB p65抗体购于北京博奥森生物技术有限公司;p-NF-κB p65抗体购于Cell Signaling Technology;HRP标记的羊抗兔II抗购于北京博奥森生物技术有限公司;免疫显色试剂(UItraSensitive SP鼠/兔)购于福州迈新生物技术开发有限公司;TNF-α和IL-6测定试剂盒购于江苏酶标生物科技有限公司。BX51正置显微镜购自Olympus; SDS-PAGE装置及ChemiDoc MP Imaging System购于Bio-Rad;高速冷冻离心机购于Sigma。
3 主要方法
3.1模型制备及分组给药小鼠随机分为空白对照(control)组、exendin-4组、MPTP组和MPTP+exendin-4组,每组12只,共48只。给药前小鼠适应环境一周及行爬杆训练;根据文献,MPTP组及MPTP+exendin-4组给予MPTP(30 mg/kg)腹腔注射,每天1次,连续5 d复制亚急性PD模型[16-17]。同时control组、exendin-4组给予等量生理盐水腹腔注射,每次注射MPTP后小鼠出现震颤及竖毛等急性损伤症状,但能逐渐恢复,随着给药天数的增加,小鼠运动功能逐渐损害,出现运动迟缓及动作笨拙等异常表明造模成功。造模成功后,参考文献用药剂量[14],exendin-4组及MPTP+exendin-4组小鼠给予2.5 μg/kg exendin-4连续腹腔注射,每天2次,连续7 d治疗,control组和MPTP组同时给予等体积生理盐水腹腔注射,给药结束后行爬杆、悬挂实验及采集标本。
3.2行为学实验包括爬杆实验和悬挂实验两部分,exendin-4末次注射后的次日进行爬杆实验。(1)爬杆实验:根据我们课题组之前的研究方法,将小鼠头朝上,上肢放在杆顶端圆球上(高55 cm,直径1 cm,圆球直径2 cm)。小鼠从放置到全身朝下的时间为掉头时间,从放置顶端爬到后肢着陆的时间为爬杆总时间,每只小鼠测试3次,取平均值[18];(2)悬挂实验:将小鼠两前爪放于细线中间以悬挂小鼠,记录小鼠抓住细线的肢体数目。四个爪子抓住细线记4分,三个爪子抓住细线记3分,两个爪子抓住细线记2分,一个爪子抓住细线记1分,掉落记0分, 每只小鼠测试3次,取平均值[19]。
3.3取材行为学实验结束后用10%水合氯醛麻醉取材,每组取6只小鼠用4%多聚甲醛行心脏灌注固定取完整脑组织,行免疫组化实验;每组取6只小鼠麻醉后分离包括黑质部分的中脑组织,行Western blot实验及酶联免疫吸附实验。
3.4Western blot检测蛋白表达水平剥离小鼠包括黑质部分的中脑组织,加入含PMSF及磷酸酶抑制剂的RIPA裂解液匀浆后,4 ℃、13 040.4×离心20 min,取上清液,采用BCA法测定蛋白浓度;核蛋白提取参照核蛋白提取试剂盒说明书进行。SDS-PAGE分离蛋白,分离胶浓度根据蛋白分子量选择。将分离的蛋白质转移至0.22 μm的PVDF膜上,5%脱脂奶粉封闭120 min,相应I抗(TH、Iba1和histone H3, 1∶1 000; TLR4、MyD88、p-NF-κB p65和NF-κB p65, 1∶500; β-actin, 1∶2 000)4 ℃冰箱摇床孵育过夜。次日,TBST清洗3次后,将目标条带与对应种属的II抗室温孵育2 h,TBST清洗3次后曝光,ImageJ软件分析相应条带灰度值。
3.5免疫组织化学实验将固定好的脑组织进行石蜡包埋切片,切片进行二甲苯脱蜡、梯度乙醇水化后、抗原修复10 min,PBS洗3次×3 min,试剂1(内源性过氧化物酶阻断剂)孵育10 min,PBS洗3次×10 min,试剂2(非特异染色阻断剂)孵育10 min,然后滴加Ⅰ抗(TH, 1∶500; TLR4, 1∶200)4 ℃孵育过夜;第2天,PBS洗3次×3 min,试剂3(生物素标记的羊抗小鼠/兔IgG聚合物)孵育10 min,PBS洗3次×3 min,试剂4(链霉菌抗生物素蛋白-过氧化物酶)孵育10 min,显微镜下DAB显色,苏木素复染,返蓝,乙醇脱水、二甲苯透明,中性树胶封片,在光学显微镜下观察并采集图像,利用ImageJ软件分析统计TH染色阳性细胞数目及TLR4平均光密度。
3.6ELISA检测取小鼠中脑组织,分别按照TNF-α及IL-6测定试剂盒说明书进行操作。
4 统计学处理
使用SPSS 20.0软件和GraphPad Prism 8.0软件进行统计学分析。计量数据用均数±标准差(mean±SD)表示。多组间比较采用单因素方差分析,两两比较采用LSD法。以<0.05为差异有统计学意义。
结果
1 exendin-4对MPTP诱导的PD小鼠行为学影响
行为学结果显示,相比control组,MPTP组小鼠的转头时间及爬杆时间显著延长(<0.01),而MPTP+exendin-4组小鼠较MPTP组转头时间及爬杆时间显著缩短(<0.01);悬挂实验中MPTP组小鼠的评分比control组显著减少(<0.01),而MPTP+exendin-4组小鼠的评分较MPTP组显著增加(<0.01),见图1。
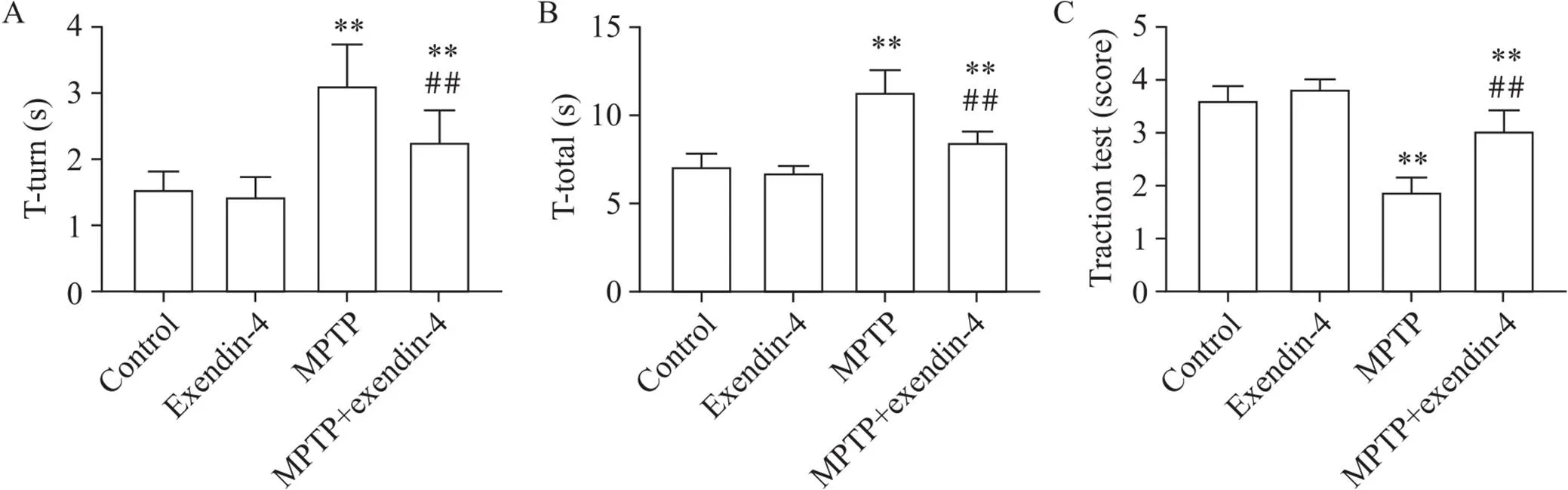
Figure 1.Results of behavioral tests of the mice in each group. A: comparison of turning time (T-turn) of the mice in each group; B: comparison of climbing total time (T-tatal) of the mice in each group; C: comparison of traction test score of the mice in each group. Mean±SD. n=12. **P<0.01 vs control group; ##P<0.01 vs MPTP group.
2 exendin-4对MPTP诱导的小鼠黑质TH阳性神经元及中脑TH蛋白水平的影响
免疫组化显示,TH主要表达于神经元胞质中,MPTP注射后小鼠中脑黑质TH阳性神经元较control组细胞数目显著减少(<0.01),胞质染色减少;MPTP+exendin-4组小鼠TH阳性神经元较MPTP组数量显著增多(<0.01),胞质染色增多,见图2A。Western blot结果显示,MPTP注射后小鼠中脑组织TH蛋白表达水平较control组显著减少(<0.01),MPTP+exendin-4组小鼠中脑TH蛋白表达水平较MPTP组显著增多(<0.01),见图2B。
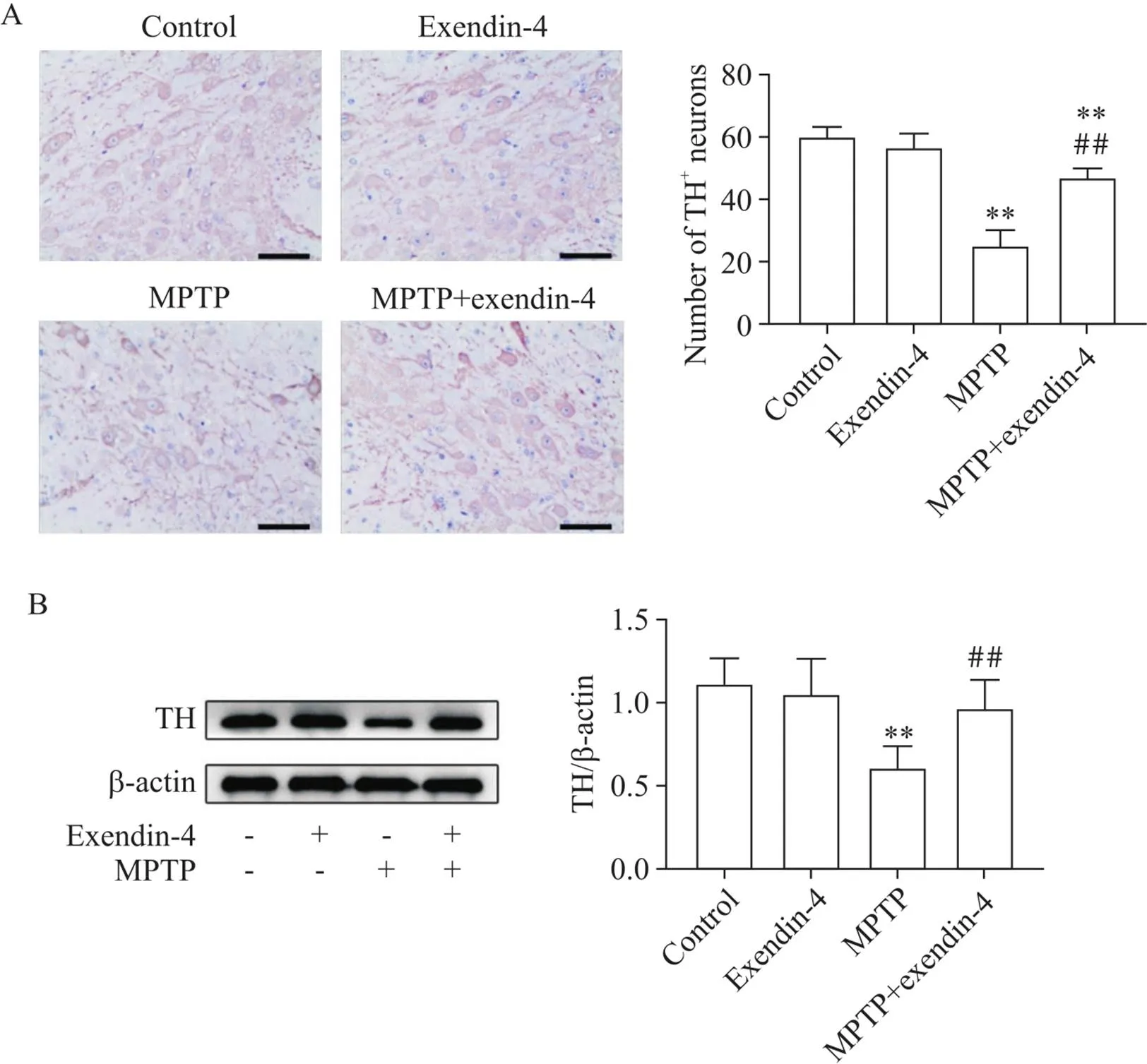
Figure 2.Comparison of TH expression of the mice in each group. A: immunohistochemical staining of TH+ neurons in the midbrain substantia nigra of the mice in each group (scale bar=50 μm); B: Western blot images of TH protein in midbrain tissues of mice in each group and the statistical results. Mean±SD. n=6. **P<0.01 vs control group; ##P<0.01 vs MPTP group.
3 exendin-4对MPTP诱导的小鼠中脑TLR4/MyD88/NF-κB信号通路及Iba-1表达水平的影响
免疫组化染色显示,TLR4蛋白主要表达于细胞膜上,MPTP模型组小鼠中脑黑质TLR4表达较control组显著增加(<0.01),MPTP+exendin-4组小鼠中脑黑质TLR4表达较MPTP组显著减少(<0.01);TLR4的Western blot结果与免疫组化结果一致,MPTP组小鼠中脑组织TLR4蛋白表达较control组显著增加(<0.01),MPTP+exendin-4组小鼠中脑组织TLR4蛋白表达较MPTP组显著减少(<0.01);相比control组,MPTP组小鼠中脑TLR4下游MyD88蛋白表达显著增加(<0.01),NF-κB p65磷酸化水平显著升高(<0.01),胞核NF-κB p65蛋白表达显著增加(<0.01);相比MPTP组,MPTP+exendin-4组TLR4下游MyD88蛋白表达显著减少(<0.01),NF-κB磷酸化显著减少(<0.01),胞核NF-κB蛋白表达显著减少(<0.01);Iba-1是小胶质细胞激活标志物,相比control组,MPTP组小鼠中脑Iba-1蛋白表达显著增加(<0.01),MPTP+exendin-4组小鼠中脑Iba-1蛋白表达水平较MPTP组显著降低(<0.01),见图3。
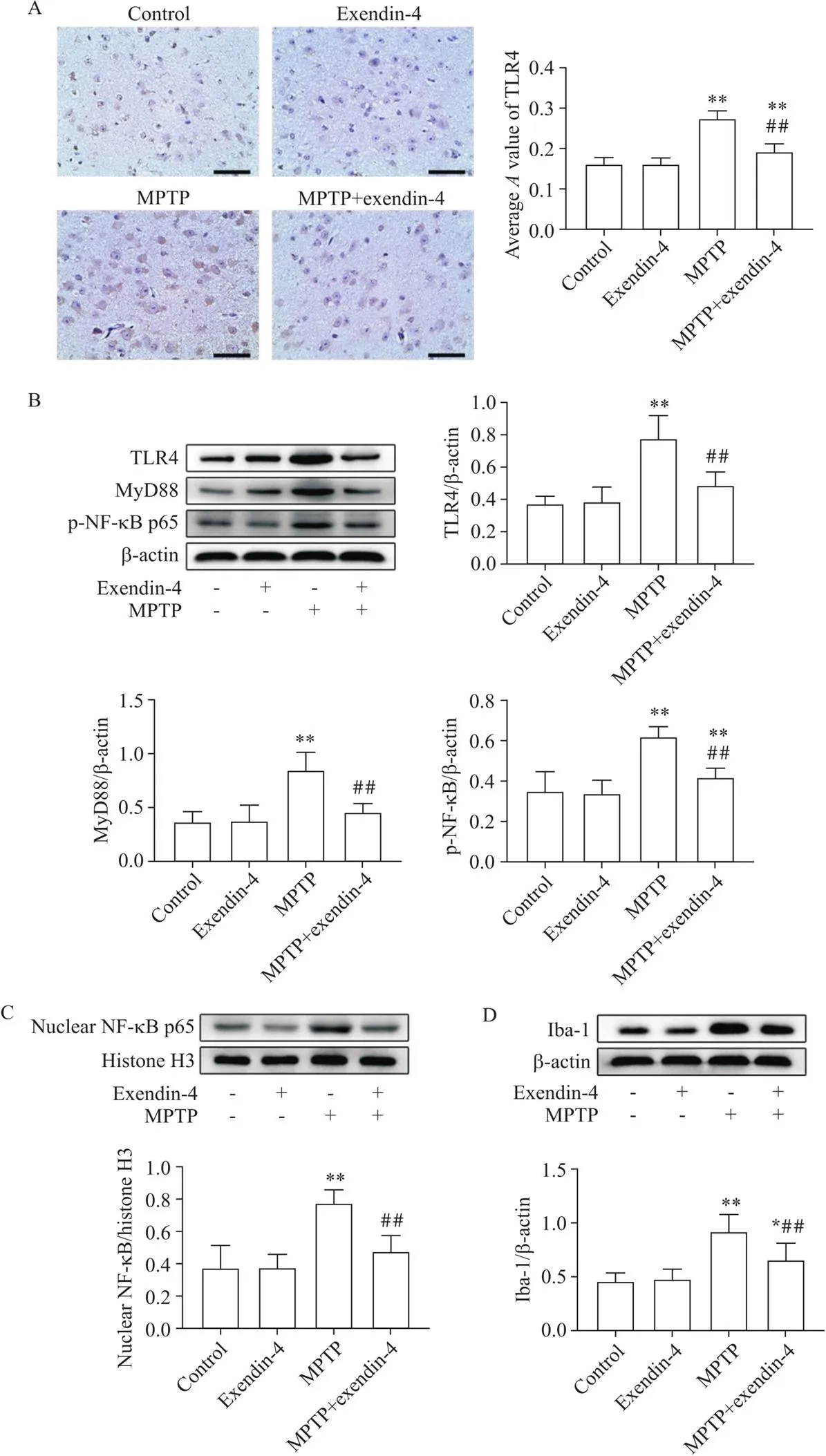
Figure 3.Comparison of TLR4/MyD88/NF-κB signaling pathway and expression level of Iba-1 in midbrain of the mice in each group. A: immunohistochemical staining of TLR4 in the midbrain substantia nigra of mice in each group (scale bar=50 μm); B: TLR4, MyD88 and p-NF-κB p65 detected by Western blot; C: nuclear NF-κB p65 detected by Western blot; D: Iba-1 detected by Western blot. Mean±SD. n=6. *P<0.05, **P<0.01 vs control group; ##P<0.01 vs MPTP group.
4 exendin-4对MPTP诱导的小鼠中脑炎症因子表达的影响
酶联免疫吸附实验显示,MPTP组小鼠中脑TNF-α及IL-6表达水平较control组显著增加(<0.01),而MPTP+exendin-4组小鼠中脑TNF-α及IL-6表达水平较MPTP组显著降低(<0.01),见图4。

Figure 4.Comparison of the expression levels of inflammatory factors TNF-α and IL-6 in midbrain of the mice in each group. Mean±SD. n=6. **P<0.01 vs control group; ##P<0.01 vs MPTP group.
讨论
PD病理特点为中脑黑质-纹状体多巴胺能神经元变性死亡,纹状体多巴胺含量下降及路易小体形成,其运动症状主要表现为静止性震颤、肌强直、运动迟缓和姿势步态异常等[20-21]。利用MPTP腹腔注射能够模拟帕金森病的病理及行为学改变,是目前建立PD模型较常用的方法[22];TH是多巴胺能神经元标志物[23],在本实验中,给予MPTP腹腔注射后小鼠出现黑质区域TH阳性神经元受损及中脑TH蛋白表达水平降低,并出现转头时间及爬杆时间延长、悬挂实验评分降低等运动功能障碍,较好地模拟了PD的病理学及行为学改变;给予exendin-4治疗后可减少MPTP对小鼠黑质区域TH阳性神经元损害,增加PD小鼠中脑TH蛋白表达水平,改善了MPTP引起的运动功能障碍,发挥了神经保护作用。
既往研究显示,exendin-4可通过促进α-突触核蛋白降解在PD动物模型中发挥神经保护作用[14];临床试验显示,exendin-4可改善帕金森病患者运动功能[15],且可能具有疾病修饰作用。神经炎症在PD发病机制中扮演着重要角色[4-5],然而,exendin-4在帕金森病中抗神经炎症具体机制仍不明确;小胶质细胞是中枢神经系统主要炎症细胞,TLR4/NF-κB信号通路是机体重要的炎症通路,TLR4激活后可通过MyD88依赖性通路或MyD88非依赖性通路激活下游NF-κB,促进NF-κB核转移,进而促进炎症因子TNF-α及IL-6等的释放[24-25]。在PD发病过程中,TLR4/NF-κB信号通路作为神经炎症重要组成部分参与了PD的发病[6-9]。我们的研究显示,给予MPTP腹腔注射后小鼠中脑小胶质细胞激活增加,TLR4/MyD88/NF-κB信号通路表达增加,炎症因子TNF-α及IL-6释放增加;给予exendin-4治疗后可抑制PD小鼠中脑小胶质细胞激活及TLR4/NF-κB信号通路表达,减少NF-κB磷酸化和核转移,减少炎症因子TNF-α及IL-6的释放,在MPTP诱导的PD小鼠模型中起到抗神经炎症作用。
综上,我们的研究表明,在MPTP诱导的PD小鼠模型中,exendin-4可通过抑制小胶质细胞及TLR4 /NF-κB炎症信号通路激活发挥抗神经炎症作用,保护多巴胺能神经元,改善PD小鼠运动功能。
[1] Homayoun H. Parkinson disease[J]. Ann Intern Med, 2018, 169(5):C33-C48.
[2] Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease[J]. Brain, 2013, 136(Pt 8):2419-2431.
[3] Kalia LV, Lang AE. Parkinson's disease[J]. Lancet, 2015, 386(9996):896-912.
[4] Poewe W, SeppiK, Tanner CM, et al. Parkinson disease[J]. Nat Rev Dis Primers, 2017, 3:17013.
[5] Gelders G, Baekelandt V, Van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson's disease[J]. J Immunol Res, 2018, 2018:4784268.
[6] Kouli A, Camacho M, Allinson K, et al. Neuroinflammation and protein pathology in Parkinson's disease dementia[J]. Acta Neuropathol Commun, 2020, 8(1):211.
[7] Shao QH, Chen Y, Li FF, et al. TLR4 deficiency has a protective effect in the MPTP/probenecid mouse model of Parkinson's disease[J]. Acta Pharmacol Sin, 2019, 40(12):1503-1512.
[8] Campolo M, Paterniti I, Siracusa R, et al. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson's diseasesmodel[J]. Brain Behav Immun, 2019, 76:236-247.
[9]李海霞, 李映霞, 程芸, 等. Toll样受体4在帕金森病中的研究进展[J]. 中国病理生理杂志, 2021, 37(4):744-751.
Li HX, Li YX, Cheng Y, et al. Progress in role of Toll-like receptor 4 in Parkinson disease[J]. Chin J Pathophysiol, 2021, 37(4):744-751.
[10] Athauda D, Foltynie T. Insulin resistance and Parkinson's disease: a new target for disease modification?[J]. Prog Neurobiol, 2016, 145/146:98-120.
[11] Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus[J]. Nat Rev Endocrinol, 2012, 8(12):728-742.
[12] Zhang L, Zhang L, Li Y, et al. The novel dual GLP-1/GIP receptor agonist DA-CH5 is superior to single GLP-1 receptor agonists in the MPTP model of Parkinson's disease[J]. J Parkinsons Dis, 2020, 10(2):523-542.
[13] Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson's disease[J]. J Endocrinol, 2009, 202(3):431-439.
[14] Bu LL, Liu YQ, Shen Y, et al. Neuroprotection of exendin-4 by enhanced autophagy in a parkinsonian rat model of α-synucleinopathy[J]. Neurotherapeutics, 2021, 18(2):962-978.
[15] Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial[J]. Lancet, 2017, 390(10103):1664-1675.
[16] Tatton NA,Kish SJ.In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining[J]. Neuroscience, 1997, 77(4):1037-1048.
[17] 任传忠, 肖猛, 李玮, 等. 硫辛酸对MPTP诱导的小鼠帕金森病模型的神经保护作用[J]. 中国病理生理杂志, 2020, 36(1):127-133.
Ren CZ, Xiao M, Li W, et al. Effects of alpha-lipoic acid on MPTP-induced Parkinson disease in mice[J]. Chin J Pathophysiol, 2020, 36(1):127-133.
[18] Zhou J, Qu XD, Li ZY, et al. Salvianolic acid B attenuates toxin-induced neuronal damage via Nrf2-dependent glial cells-mediated protective activity in Parkinson's disease models[J]. PLoS One, 2014, 9(7):e101668.
[19] Sun MF, Zhu YL, Zhou ZL, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota,glial reaction and TLR4/TNF-α signaling pathway[J]. Brain Behav Immun, 2018, 70:48-60.
[20] Erro R, Stamelou M. The motor syndrome of Parkinson's disease[J]. Int Rev Neurobiol, 2017, 132:25-32.
[21] Jankovic J, Tan EK. Parkinson's disease:etiopathogenesis and treatment[J]. J Neurol Neurosurg Psychiatry, 2020, 91(8):795-808.
[22] Vingill S, Connor-Robson N, Wade-Martins R. Are rodent models of Parkinson's disease behaving as they should?[J]. Behav Brain Res, 2018, 352:133-141.
[23] 刘丽, 罗广轩, 林健琳, 等. 虫草素对MPTP诱导的帕金森病小鼠运动与认知能力的影响[J]. 中国病理生理杂志, 2019, 35(2):326-331.
Liu L, Luo GX, Lin JL, et al. Effects of cordycepin on motor and cognition in Parkinson disease mice induced by MPTP[J]. Chin J Pathophysiol, 2019, 35(2):326-331.
[24] Kuzmich NN, Sivak KV, Chubarev VN, et al. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis[J]. Vaccines (Basel), 2017, 5(4):34.
[25] Rahimifard M, Maqbool F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation[J]. Ageing Res Rev, 2017, 36:11-19.
Protective effect of exendin-4 on Parkinson disease mice by inhibiting Toll-like receptor 4/nuclear factor-κB signaling pathway
TAO Ji-wei1,2, NAN Ya-qiang1,2, ZHOU Jie2△, LE Li-ming2, GUAN Hai-bin2, JIANG Gu-feng2, FAN Fei-yang2
(1,730030,;2,940,730050,)
To investigate the protective effect and mechanism of exendin-4 on Parkinson's disease (PD) mice induced by 1-methy1-4-phenyI-1, 2,3,6-tetrahydropyridine (MPTP).Forty-eight male C57BL/6 mice were randomly divided into control group, exendin-4 group, MPTP group and MPTP+exendin-4 group, with 12 mice in each group. The mice in MPTP and MPTP+exendin-4 groups were intraperitoneally injected with MPTP (30 mg/kg) once a day for 5 d to establish subacute PD model. Exendin-4 (2.5 μg/kg) was injected twice a day for 7 d in exendin-4 and MPTP+exendin-4 group. The pole test and traction test were used to evaluate the behavioral changes of the mice. Western blot was used to detect the protein levels of tyrosine hydroxylase (TH), ionized calcium-binding adapter molecule 1 (Iba-1), Toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88) and nuclear factor-κB (NF-κB) in the midbrain of the mice. Immunohistochemistry was used to detect the expression levels of TH and TLR4 in the midbrain substantia nigra of the mice. The levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-a) in midbrain were detected by ELISA.Compared with control group, the head turning time and pole climbing time of the mice in MPTP group were prolonged (<0.01), the traction test score decreased (<0.01), the number of TH-positive neurons in the substantia nigra and the expression of TH protein in midbrain decreased (<0.01), and the expression levels of Iba-1, TLR4, MyD88, p-NF-κB p65, nuclear NF-κB p65, TNF-α and IL-6 increased (<0.01). Compared with MPTP group, the head turning time and pole climbing time of the mice were reduced in MPTP+exendin-4 group (<0.01), the traction test score increased (<0.01), the number of TH-positive neurons in the substantia nigra and the expression of TH protein in midbrain increased (<0.01), the expression levels of Iba-1, TLR4, MyD88, p-NF-κB P65, nuclear NF-κB p65, TNF-α and IL-6 decreased (<0.01).In MPTP-induced PD mouse model, exendin-4 protects dopaminergic neurons and improves the motor function of PD mice by inhibiting the activation of microglia and TLR4/NF-κB inflammatory signaling pathway.
Exendin-4; Parkinson disease; TLR4/NFκB signaling pathway; Neuroinflammation
R742.5; R363.2
A
10.3969/j.issn.1000-4718.2022.03.015
1000-4718(2022)03-0502-07
2021-12-04
2022-01-12
[基金项目]甘肃省卫生行业科研计划项目(No. GSWSKY2018-43)
Tel: 13309313131; E-mail: zhoujie7089@163.com
(责任编辑:林白霜,罗森)

