Synthesis, characterization, and antioxidant activity of carboxymethyl chitosan derivatives containing sulfonium salt*
Xueqi SUN , Jingjing ZHANG , Yingqi MI , Qin MIAO , Wenqiang TAN ,Qing LI , Zhanyong GUO ,**
1 Key Laboratory of Coastal Biology and Bioresource Utilization, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China
2 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract To improve the solubility and bioactivity of chitosan, a new class of carboxymethyl chitosan derivatives possessing sulfonium salts was successfully designed and synthesized, including Methyl sulf ide carboxymethyl chitosan (MCMCS), Ethyl sulf ide carboxymethyl chitosan (ECMCS), Propyl sulf ide carboxymethyl chitosan (PCMCS), and Butyl sulf ide carboxymethyl chitosan (BCMCS). To determine the structure of the new class of the derivatives, methods of the Fourier transform infrared spectroscopy (FT-IR),1 H nuclear magnetic resonance spectrometer ( 1 H NMR), and 13 C nuclear magnetic resonance spectrometer( 13 C NMR) were used. Moreover, the antioxidant activity of the derivatives for three types of free radicals,i.e., hydroxyl radical, superoxide radical, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was evaluated in vitro. In addition, the L929 cells were adopted to test the cytotoxicity of chitosan and its derivatives by CCK-8 assay. The class of the carboxymethyl chitosan derivatives showed a strong scavenging ability against the three free radicals at 1.6 mg/mL, with scavenging rate of over 70% and some up to 100%. At this high rate, the overall cell viability in the toxicity test reached more than 80%, indicating that the synthetic derivative had a little cytotoxicity. The results show that the introduction of carboxymethyl group to chitosan increased the water-solubility of chitosan, and the combination of sulfonate ions with diff erent chain lengths further enhanced the antioxidant activity of chitosan. Therefore, the sulfonium-containing carboxymethyl chitosan derivatives had excellent bioactivity with good application prospects in food, biomedicine, and medical f ields.
Keyword: antioxidant activity; carboxymethyl chitosan derivative; sulfonium salts; structural characteristics/synthesis
1 INTRODUCTION
Chitin, a major component of cell walls of fungi and the shells of crustaceans such as crab, shrimp, and crawf ish, is a white, hard, and inelastic structural polysaccharide (Xia et al., 2011). It is deacetylated to produce a natural nontoxic biopolymer-chitosan that is a cationic polysaccharide made from alkaline N-deacetylation (Jayakumar et al., 2010). Since the chitosan was discovered in late 1850s, many of the biological activities of chitosan have been reported,such as antifungal activity, antibacterial activity,antitumor activity, immunoenhancing eff ects,protective eff ects against infection, and accelerating calcium and iron absorption, which depends on its fundamental physico-chemical properties (Kim and Rajapakse, 2005). Therefore, due to its excellent biological properties, chitosan and its derivatives have become potential competitors in the f ields of agriculture, food, cosmetics, textiles, environmental protection, biomedicine, and new energy development(Rinaudo, 2006).
However, chitosan can only be dissolved in aqueous solutions with a pH<6.5, which limits the potential application of chitosan. To break the limitation of chitosan’s insolubility, various reports were conducted to make water-soluble derivatives of chitosan by chemical modif ication techniques,including PEG-grafting, sulfonation, quaternarization,N- and O-hydroxylation, and carboxymethylation(Rinaudo, 2006). Of them, carboxymethylation is one of the most important chemical modif ication methods(Chen and Park, 2003). A polyelectrolyte containing anionic f ixed charges was prepared, when chitosan was converted toN,O-carboxymethyl chitosan (N,OCMCS) by introducing -CH2COOH groups onto -OH and -NH2along chitosan molecular chain (Chen et al.,2004b). Carboxymethyl chitosan derivatives are widely used for antioxidants in pharmaceutical,veterinary medicine, biomedical, and environmental f ields because it can act as electron donor to convert free radicals into more stable products to terminate the free radical chain reactions (Xie et al., 2001; Kim and Rajapakse, 2005).
Recently, quaternary phosphonium salts and quaternary ammonium salts are reported of exhibiting higher antioxidant activity. However, to date there are surprisingly very few reports about the preparation of the chitosan derivatives bearing diff erent types of sulfonium salts. Sulfur compounds are known for exhibiting a diverse spectrum of biological activities such as antimicrobial, antioxidant, anticancer, and radical-scavenging properties (Brurits et al., 2000).Sulfur has been used as a component of various skin disorders and a pesticide or a fungicide for the treatment of a wide range of plant diseases (Kim et al., 2020; Saedi et al., 2020; Priyadarshi et al., 2021).Published literature shows, thioether compounds could cause apoptosis or autophagy in several types of cancer cell in special pathway. By contrast, it also prevents normal apoptosis by regulating expression of some genes. Accordingly, thioether had favorable antioxidant activity and could be used as potential antioxidant (Ankri and Mirelman, 1999). It is reasonable to assume that the introduction of quaternary sulfonium salts into chitosan can help to enhance biological activity and increase the application values of chitosan. This study provides novel insights into the potential of this chitosan derivative as drug adjuvants that possess a stronger antioxidant activity.
Chitosan showed unsatisfactory biological activity,and due to the limitation of functional groups, it cannot directly react with thioether compounds.Carboxymethyl chitosan is a chitosan derivative that is introduced the carboxyl groups onto the amino groups of chitosan. Carboxymethyl chitosan contains anionic f ixed charges; it can be used as a reaction intermediate product to form new compounds by combining anions and cations with compounds that contains cations(Mourya and Inamdar, 2008). Based on the above works, we attempted to synthesize chitosan derivatives that have both excellent bioactivity of chitosan itself and antioxidant activity of thioether compounds. It was reported that the halohydrocarbon could easily attack thioethers to give sulfonium salts (Gensch et al.,1968). Thus, we f irst alkylate sulf ide to form positively charged sulfonium salt derivatives. Subsequently, the sulfonium groups were introduced into carboxymethyl chitosan. Eventually, a series of chitosan derivatives,which was expected of having high antioxidant activity, was synthesized. The antioxidant activity of the chitosan derivatives and the eff ect of cell survival were further explored. This study provides novel insights into the potential of this chitosan derivative as drug adjuvants that possess the stronger antioxidant activity.
2 MATERIAL AND METHOD
2.1 Material
Chitosan was purchased from Golden-Shell Pharmaceutical Co. Ltd. (Zhejiang, China), and the degree of deacetylation is 0.735 and molecular weight is 5-8 kDa. Dimethyl sulf ide (product code C10144858), ethyl sulf ide (product code C10364421),propyl sulf ide (product code C10317521), butyl sulf ide (product code C10213834), and chloroacetic acid (product code E111977) were purchased from Sigma-Aldrich Chemical Corp (Shanghai, China).Ethanol (product code 80031707), dimethyl carbinol(product code 80109218), and sodium hydroxide(product code 10019762) were all in analytical grade that purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Method
2.2.1 Analytical method
2.2.1.1 Fourier transform infrared (FT-IR) spectroscopy
During the test, samples were grounded and mixed with analytical grade KBr at a weight ratio of 1/100 and recorded all spectra on a Nicolet IS50 Fourier transform infrared Spectrometer (United States,provided by Thermo Fisher Scientif ic) at 25 °C using the transmittance mode. Using potassium bromide tableting method, all spectra were scanned in the range of 4 000-400 cm-1. The resolution was 4.0 cm-1,and the number of scans was 32.
2.2.1.2 Nuclear magnetic resonance (NMR) spectroscopy
With tetramethyl silane (TMS) as internal standard on mg/Lscale, the chitosan and its derivatives (50 mg)were dissolved in 1 mL of 99.9% deuterium oxide(D2O).1H nuclear magnetic resonance (1H NMR) and13C nuclear magnetic resonance (13C NMR) spectra were detected on a Bruker AVIII-500 Spectrometer(Switzerland, provided by Bruker Tech. and Serv. Co.Ltd., Beijing, China) at 25 °C.
2.2.1.3 Microplate reader (MR)
The microplate reader absorbance of the tested mixture was measured with a DNM-9602G microplate reader (China, Pulang New Technology Co. Ltd.,Beijing, China). The data were processed with the Microsoft Excel and the Origin 8, and expressed in mean±SD.
2.2.2 Synthesis of chitosan derivatives
2.2.2.1 Synthesis ofN,O-carboxymethyl chitosan(N,O-CMCS)
Chitosan powder (10 g) was dispersed in 80 mL of isopropyl alcohol and stirred in a 500-mL f lask at 50 °C. Then, 20-mL aqueous NaOH solution was dropped into the solution. After stirring for 2 h at 50 °C, monochloroacetic acid (11.7 g) was dissolved in 20-mL isopropyl alcohol, and then monochloroacetic acid solution was added into the stirred slurry in 30 min. The solid-liquid reaction system was stirred at the temperature for 4 h. After that, the reaction mixture was f iltered, the solid product was f iltered,and thoroughly washed with methanol. The resultantN,O-CMCS was freeze-dried in a lyophilizer (Chen et al., 2004a; Tan et al., 2016).
2.2.2.2 Synthesis of Methyl sulf ide carboxymethyl chitosan (MCMCS), Ethyl sulf ide carboxymethyl chitosan (ECMCS), Propyl sulf ide carboxymethyl chitosan (PCMCS), and Butyl sulf ide carboxymethyl chitosan (BCMCS)
A certain amount of diff erent thioethers (10 mmol)was dissolved in 30-mL acetone; 10-mmol iodomethane was added into the solution, and stirred for 72 h at room temperature. Acetone was evaporated on a rotary evaporator at 50 °C. We got the sulfonium salts that we need by alkylation. 3.5-mmolN,Ocarboxymethyl chitosan was dissolved in 20-mL deionized water solution, and then mixed with the above-mentioned sulfonium salt. The mixture reaction system was stirred for 12 h at room temperature. The reaction solution was poured into 50 mL of absolute ethanol, and the precipitate was f iltered with suction.The solids obtained were rinsed three times with absolute ethanol, and then the unreacted organic sulfonium was extracted in a Soxhlet apparatus with ethanol for 48 h (Chen et al., 2004a; Zhang et al.,2018a). The products were dialyzed against distilled water for 48 h in dialysis bags with a molecular weight cutoff of 100 g/mol, then they were obtained by freeze-drying.
2.2.3 Antioxidant activity assay
2.2.3.1 The ability of DPPH radical scavenging assay
The ability of DPPH radical scavenging by chitosan,N,O-CMCS, MCMCS, ECMCS, PCMCS,and BCMCS was assessed as per previous methods(Chen and Ho, 1997; Burits and Bucar, 2000; Mensor et al., 2001; Hu et al., 2014; Almada et al., 2017). The procedure was as follows: dissolving 35.49-mg DPPH with 500-mL ethyl alcohol to prepare the stock solution and then stored in dark. The experimental group: 1-mL testing sample (0.1, 0.2, 0.4, 0.8, and 1.6 mg/mL) and 2-mL ethanol solution of DPPH(180 μmol/L) were incubated for 30 min at 25 °C in dark, ascorbic acid was used as positive controls. The control group: 1-mL testing sample (0.1, 0.2, 0.4, 0.8,and 1.6 mg/mL) and 2-mL ethanol solution were incubated for 30 min at 25 °C in dark. The blank group: 1-mL deionized water and 2-mL ethanol solution of DPPH (180 μmol/L) were incubated for 30 min at 25 °C in dark. Then, the absorbance of the remained DPPH radical was taken at 517 nm. Each sample concentration was tested in replication and the scavenging rate was calculated by Eq.1:
2.2.3.2 Hydroxyl-radical scavenging activity assay
The hydroxyl radical scavenging activity was measured as per Guo et al. (2005, 2006). The method procedures are briefed as: the phosphate buff er was prepared by dissolving 20.79-g Na2HPO4·12H2O and 2.64-g NaH2PO4·12H2O with 500-mL deionized water. Saff ron solution was prepared by dissolving 36-mg Saff ron with 100-mL phosphate buff er. The EDTA-Fe2+solution was prepared by dissolving 55.6-mg FeSO4·7H2O and 148.9-mg EDTA-Na with 100-mL deionized water. Hydrogen peroxide solution was prepared by dissolving 10-mL 30% hydrogen peroxide solution with 100-mL phosphate buff er.These prepared reagents were prepared on the spot and stored away from light. The experimental group:1-mL testing sample (0.1, 0.2, 0.4, 0.8, and 1.6 mg/mL)and 500-μL EDTA-Fe2+solution, 1-mL phosphate buff er, 1-mL saff ron solution, 1-mL hydrogen peroxide solution were incubated for 30 min at 37 °C in dark, ascorbic acid was used as positive controls.The control group: 1-mL deionized water and 500-μL EDTA-Fe2+solution, 1-mL phosphate buff er, 1-mL testing sample (0.1, 0.2, 0.4, 0.8, and 1.6 mg/mL), and 1-mL hydrogen peroxide solution were incubated for 30 min at 37 °C in a dark place. The blank group:1-mL deionized water, 500-μL EDTA-Fe2+solution,1-mL phosphate buff er, 1-mL saff ron solution, and 1-mL hydrogen peroxide solution were incubated for 30 min at 37 °C in dark. The resultant mixture was brought to 37 °C and the absorbance measured at 517 nm. Each sample concentration was tested in triplication and the scavenging rate was calculated by Eq.2:

2.2.3.3 Superoxide-radical scavenging activity assay
The superoxide radical scavenging activity was measured as per Xing et al. (2006). The procedures were: the Tris-HCl buff er (16 mmol/L, pH 8.0) was prepared by dissolving 969.1-mg Tris and 0.4-mL concentrated hydrochloric acid with 500-mL deionized water. Nicotinamide adenine dinucleotide(reduced form) (NADH) solution was prepared by dissolving 36.57-mg NADH in 100-mL Tris-HCl buff er. The nitro blue tetrazolium (NBT) solution was prepared by dissolving 24.53-mg NBT with 100-mL Tris-HCl buff er. Phenazine methosulfate (PMS)solution was prepared by dissolving 1.838-mg PMS with 100-mL Tris-HCl buff er. These prepared reagents were prepared on spot and stored in dark. The experimental group: 1-mL testing sample (0.1, 0.2,0.4, 0.8, and 1.6 mg/mL), 500-μL NADH solution,500-μL NBT solution, and 500-μL PMS solution were incubated for 30 min in the room temperature in dark,ascorbic acid was used as positive controls. The control group: 1-mL testing sample (0.1, 0.2, 0.4, 0.8,and 1.6 mg/mL), 500-μL Tris-HCl buff er, 500-μL NBT solution, and 500-μL PMS solution were incubated for 30 min in the room temperature in a dark place. The blank group: 1.5-mL deionized water,500-μL NADH solution, 500-μL NBT solution,500-μL PMS solution were incubated for 30 min in the room temperature in dark. The absorbance of resultant mixture was measured in triplication at 560 nm and the scavenging rate was calculated by Eq.3:
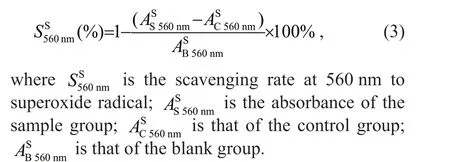
2.2.4 Cytotoxicity assay
The cells were mouse f ibroblasts called “mouse connective tissue L cell line 929 clone” that purchased from the Cell Bank of the Chinese Academy of Sciences, and the number of catalog was “GNM28”.A parent cell line L was established from the normal subcutaneous loose connective tissue of a 100-dayold male C3H/An mouse into adipose tissue. L929 cells were established by capillary separation of single cells from the 95thgeneration cell line L. They were grown in RPMI medium that contains 1%penicillin, 1% streptomycin, 10% fetal bovine serum at 37 °C and maintained under 5% CO2atmosphere.The cytotoxicity of all samples on L929 cells in diff erent concentrations (62.5, 125.0, 250.0, 500.0,and 1 000.0 μg/mL) was measured by CCK-8 assay in vitro with minor modif ication (sample concentration).The L929 cells were cultured in RPMI medium(mixture containing 1% penicillin, 1% streptomycin,and 10% fetal bovine serum) at 37 °C. These cells were seeded in density of 1.0×105cells/hole on a 96-well f lat-bottomed plate and incubated (37 °C, 5%CO2). Twenty-four hours after cell attachment,samples that had diff erent f inal concentrations were introduced into the cells. Next, the cells were cultivated for 24 h. Then, 10-μL CCK-8 solution was mixed in every well, and further cultured at 37 °C for 4 h. The absorbance at 450 nm was recorded using a microplate reader (Mi et al., 2018; Zhang et al., 2019).Record the cell viability using the Eq.4:
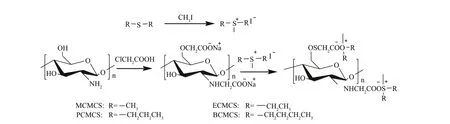
Fig.1 Synthesis routes for CS, N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
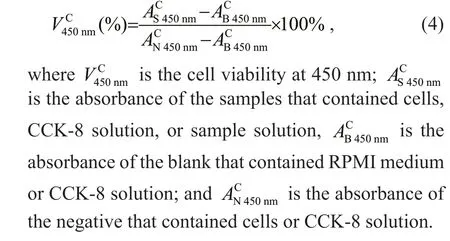
2.2.5 Statistical analysis
All the experiments were processed in triplicate and the data were reported as means ± the standard deviation (SD,n=3). The analysis with signif icant diff erence was determined by Scheff e’s multiple range test. The results were considered statistically signif icant atP<0.05.
3 RESULT AND DISCUSSION
3.1 Chemical synthesis and characterization
The synthetic strategy of the carboxymethyl chitosan derivatives containing sulfonium salt is shown in Fig.1.First, sulfonium salts were formed by sulf ide compounds and methyl iodide through alkylation. The synthesis ofN,O-carboxymethyl chitosan was carried out in a solution containing monochloroacetic acid and isopropyl alcohol. Then, the carboxymethyl chitosan derivatives containing sulfonium salt were synthesized through the reaction ofN,O-carboxymethyl chitosan and the sulfonium salts at room temperature. The sample’s structure after each step of the synthesis was characterized by FT-IR,1H NMR, and13C NMR, and shown in Figs.1-3, respectively.
3.1.1 FT-IR spectra

Fig.2 FTIR spectra of chitosan (CS), N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
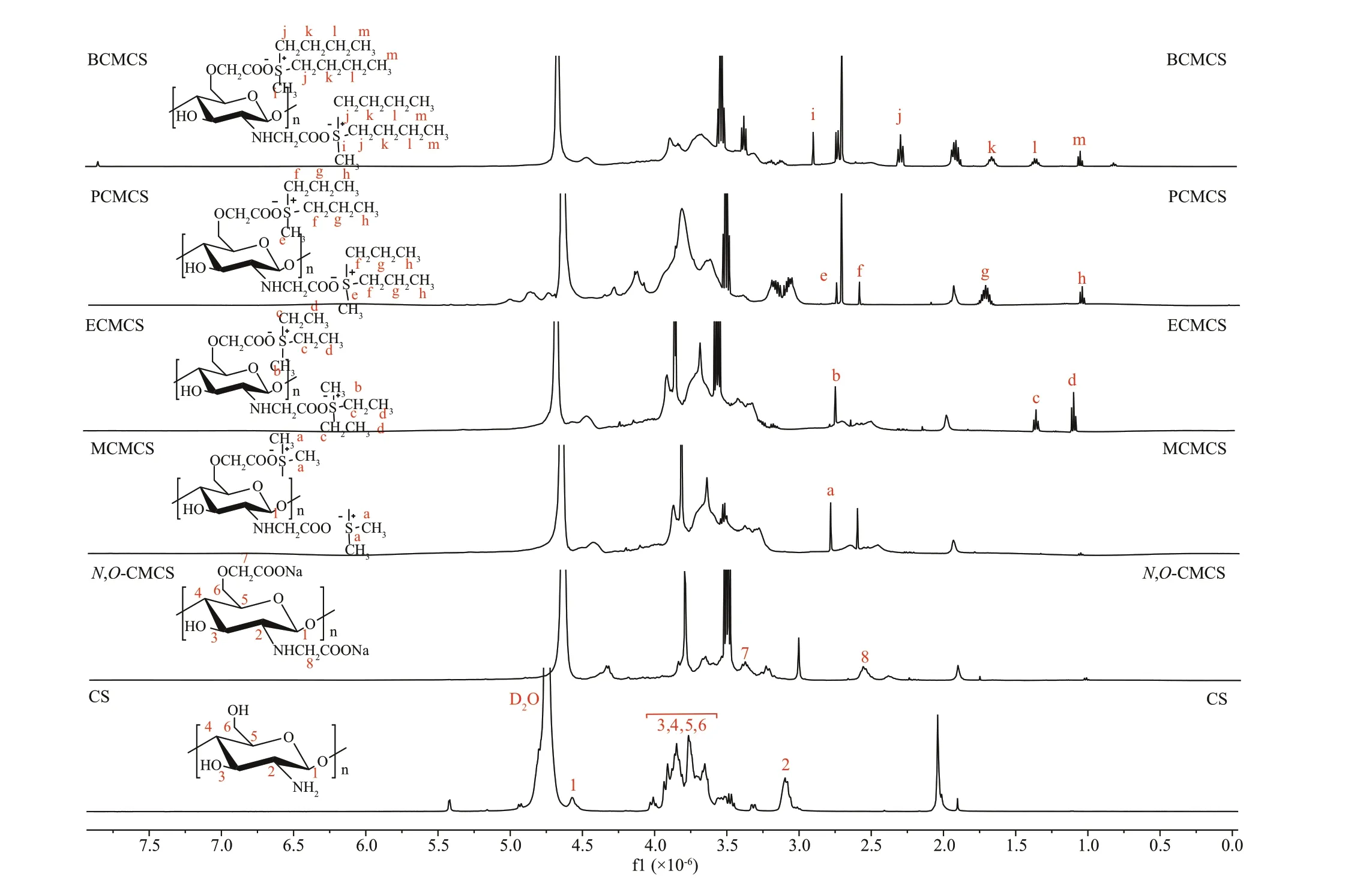
Fig.3 1 H NMR spectra of chitosan (CS), N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
The FT-IR spectra for chitosan,N,O-CMCS,MCMCS, ECMCS, PCMCS, and BCMCS were recorded. For chitosan, the spikes appear at 3 374.77,2 881.69, 1 605.69, and 1 069.19 cm-1(Mi et al.,2018). It exhibits distinct strong broad absorption band at 3 374.77 cm-1, which presents the characteristic absorbance of -OH and -NH2. The characteristic spike at 2 877 cm-1is concluded as stretching vibration of C-H. The band at 1 600 cm-1is attributed to deformation vibration of N-H in the amino group (Tan et al., 2016). In addition, the characteristic spike at 1 072 cm-1is assigned the stretching vibrations of the C-O (Zhang et al., 2018b).Compared with chitosan, due to the carbonyl of carboxymethyl chitosan existed in the form of sodium salt, the infrared spectrum has not obvious characteristic spike. InN,O-CMCS, owing to carboxymethylation, the spikes at 1 613 cm-1were contributed to -COONa of carbonyl groups (Xu et al., 2010). If a small amount of acid is added, the carbonyl group (-O-C=O) in the sodium carboxymethyl salt will become a carboxyl group(-COOH), and an absorption spike appears at 1 720 cm-1, which indirectly proves the successful synthesis of carboxymethyl chitosan (Lu et al., 2007).For MCMCS, ECMCS, PCMCS, and BCMCS, new spikes appear at 941.75, 937.95, 934.15, and 933.91 cm-1, respectively, can be attributed to the S-C bending (Oh et al., 2019). New spikes appearing at 713.92, 710.90, 709.43, and 708.19 cm-1in the spectra of chitosan derivatives can be caused by the stretching vibration of -C-S-C-. These results prove that the structure ofN,O-CMCS and carboxymethyl chitosan containing sulfonium salt were determined correctly, but further conf irmation is needed.
3.1.2 NMR spectra
The1H NMR spectra of chitosan,N,O-CMCS,MCMCS, ECMCS, PCMCS, and BCMCS are shown in Fig.3. In the f igure, the signal at 4.47×10-6was the signal spike of H1, the signals that ranged from 3.1×10-6to 4.0×10-6were attributed to H2, H6, H5,H4, and H3 protons of chitosan backbone, the signal at 2.1×10-6was the signal of -NCOCH3residue (Sun et al., 2006). In1H NMR spectrum ofN,O-CMCS, the signal at 3.4×10-6was attributed to the CH2at carboxymethyl group (-O-CH2-COOD), the signal at 2.68×10-6was attributed to the CH2at carboxymethyl group (-N-CH2-COOD) (Chen et al., 2004b; Lu et al.,2007). Therefore, the amino groups were partly carboxymethylated along with the hydroxyl groups.In1H NMR spectrum of MCMCS, the signal at 2.9×10-6was attributed to the methyl in MCMCS;those at (2.9-1.25)×10-6were caused by the methyl and methylene in ECMCS; those at (2.8-1.2)×10-6were resulted from the methyl and methylene in PCMCS; those at (3.0-1.4)×10-6were due to the methyl and methylene in BCMCS. The results show that the target functional group was successfully incorporated into carboxymethyl chitosan (Wang et al., 2019).
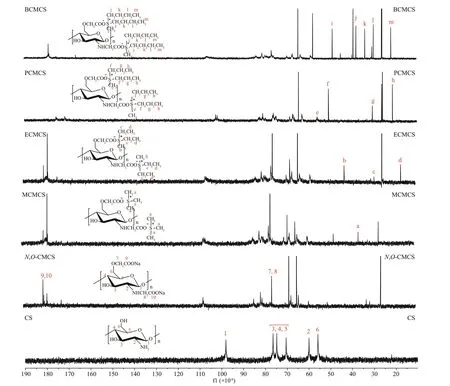
Fig.4 13 C NMR spectra of chitosan (CS), N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
Figure 4 describes the13C NMR spectrum of chitosan,N,O-CMCS, MCMCS, ECMCS, PCMCS,and BCMCS. The spikes at (56-78)×10-6were attributed to the chemical shift of carbons of chitosan.The spike for -COOH that took the place of -OH was obvious at 180×10-6, which was due to the carboxyl group in carboxymethyl chitosan. The spike at 69.9×10-6was attributed to the methylene in carboxymethyl chitosan (Chen and Park, 2003). The signal at 17×10-6was the spike of -NCOCH3residue.In13C NMR spectrum of MCMCS, the spike at 27×10-6belongs to the methyl in MCMCS, spikes at(37-11)×10-6belong to the methyl and methylene in ECMCS; spikes at (46.5-12)×10-6belong to the methyl and methylene in PCMCS; and those at (41-12)×10-6belong to the methyl and methylene in BCMCS (Li and Wan, 2018). Based on these data, the presence of sulfonium salt groups could be determined,and carboxymethyl chitosan containing sulfonium salt was successfully synthesized.
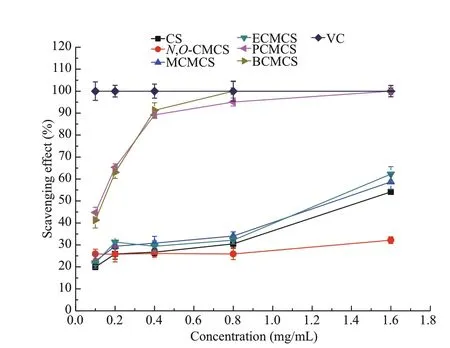
Fig.5 Scavenging ability of DPPH radicals by chitosan (CS),N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
3.2 Antioxidant activity
3.2.1 Assay on scavenging ability to DPPH radicals
The scavenging abilities of chitosan,N,O-CMCS,MCMCS, ECMCS, PCMCS, and BCMCS against DPPH radical are shown in Fig.5. The result of the scavenging abilities against DPPH radical was similar to those of scavenging superoxide radical and hydroxyl radical too. At the concentration of 1.6 mg/mL, the scavenging indices are listed as followed: vitamin C 100%, chitosan 34.15%,N,OCMCS 32.16%, MCMCS 58.72%, ECMCS 62.21%,PCMCS 100%, and BCMCS 100%. All carboxymethyl chitosan derivatives could improve the ability of scavenging DPPH radical signif icantly. For all the tested samples, the longer alkyl molecular chain sulfonium salts the carboxymethyl chitosan possessed,the stronger their scavenging ability against DPPH free radical was. The alcoholic DPPH solution is dark purple. When the odd electron of DPPH is paired off ,the absorption of the purple disappears, and the resulting decolorization is stoichiometric with respect to the number of electrons taken up. The results inferred that the antioxidant potential of carboxymethyl chitosan derivatives were associated with the electron supplying capacity of substituted groups of sulfonium salts of diff erent chain lengths.
3.2.2 Assay of scavenging activity to hydroxyl radicals
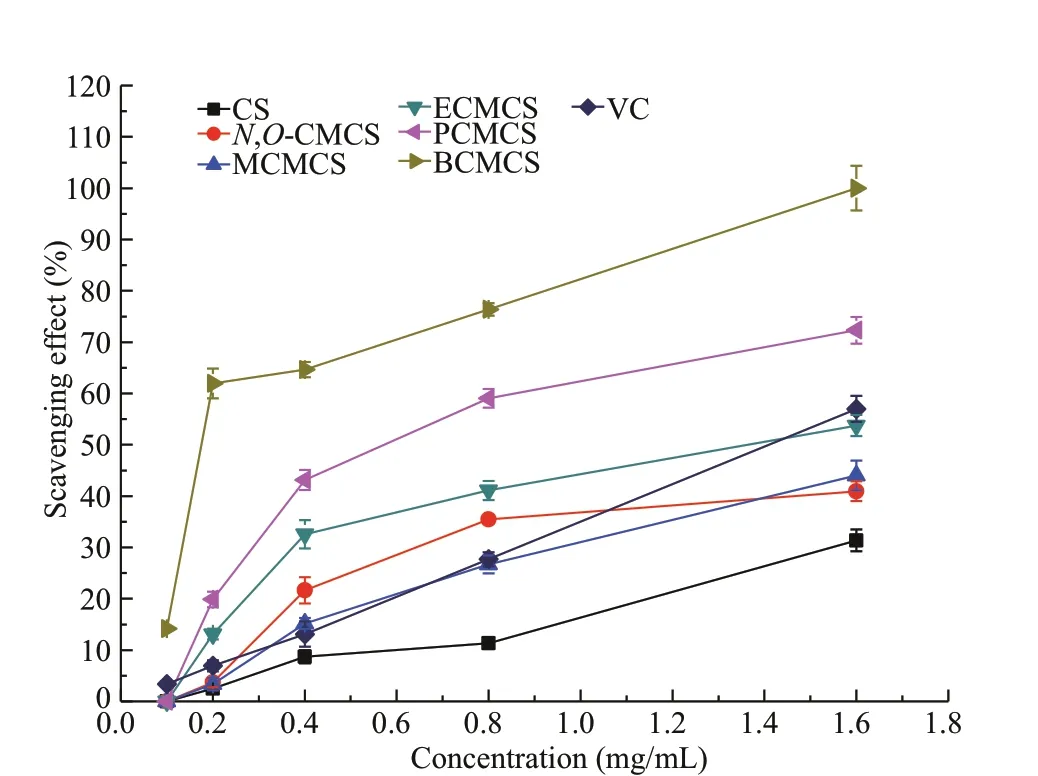
Fig.6 Scavenging activity to hydroxyl radicals by chitosan(CS), N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
Hydrogen peroxide is able to undergo a set of reaction to release the hydroxyl radical in the presence of iron in phosphate buff er, which is harmful to the body by reacting with biological molecule such as amino acid or DNA (Adjimani and Asare, 2015).Figure 6 shows the chart of the scavenging ability to hydroxyl radicals by chitosan and the synthesized carboxymethyl chitosan derivatives. The concentration of it ranged from 0.1 to 1.6 mg/mL. The results of the scavenging ability were similar to above results on the superoxide radicals. First, all of the synthesized products had better scavenging ability of hydroxyl radical than that of chitosan, indicating that the sulfonium salt groups grafted into carboxymethyl chitosan contribute largely to the ·OH radicalscavenging action. Secondly, the scavenging rates of chitosan and the synthesized carboxymethyl chitosan derivatives, such as BCMCS,were enhanced with the increasing of the concentration. At the concentrations of 0.1, 0.2, 0.4, 0.8, and 1.6 mg/mL, the scavenging rates to hydroxyl radical were 14.18%, 61.96%,64.66%, 76.38%, and 100%, respectively. Thirdly, of all the tested samples, the scavenging ability of the synthesized products to hydroxyl radicals became stronger and stronger as the alkyl molecular chain sulfonium salts increased. The results infer that the antioxidant potential of carboxymethyl chitosan derivatives were associated with the electron supplying capacity of substituted groups of sulfonium salts of diff erent chain lengths.
3.2.3 Assay on scavenging ability to superoxide radicals
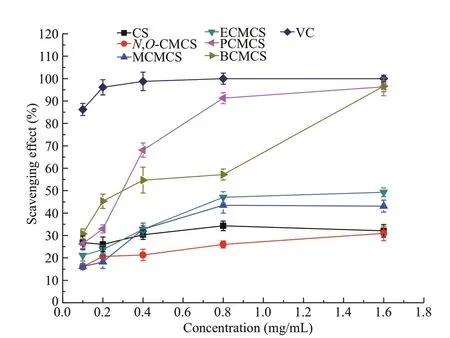
Fig.7 Scavenging ability to superoxide radicals by chitosan(CS), N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt
Superoxide can be decomposed to single oxygen and hydroxyl radicals, which initiate the peroxidation of lipids, and is believed to be a primary factor in various degenerative diseases. Figure 7 shows the scavenging ability to superoxide radicals by chitosan and all carboxymethyl chitosan derivatives; the concentration of it ranged from 0.1 to 1.6 mg/mL. At pH 7.4, the PMS-NADH mixture generated O2•ˉ anions, which can reduce NBT. The scavenging eff ect can be detected and measured by the increase in absorbance. First, the scavenging ability of superoxide radicals by all carboxymethyl chitosan was concentration-dependent. Secondly, MCMCS,ECMCS, PCMCS, and BCMCS had better scavenging ability of superoxide radicals than chitosan andN,OCMCS. Moreover, of all the tested samples, the longer alkyl molecular chain sulfonium salts that carboxymethyl chitosan possessed, the stronger their scavenging ability against superoxide radicals was. In other words, the ability to scavenge superoxide radicals decreased in the order of BCMCS > PCMCS> ECMCS > MCMCS >N,O-CMCS > CS, which agrees with the sort order of electron supplying ability(CH3(CH2)3S(CH2)3CH3> CH3(CH2)2S(CH2)2CH3>CH3CH2SCH2CH3> CH3SCH3) of carboxymethyl chitosan (Hu et al., 2014). Therefore, the antioxidant potential of carboxymethyl chitosan derivatives were associated with the electron supplying capacity of substituted groups of sulfonium salts with diff erent chain lengths.
In this study, the three classic free radicalscavenging tests were carried out, and our results clearly demonstrate that the synthesized carboxymethyl chitosan derivatives containing sulfonium salts at the periphery exhibited potent free radical-scavenging activity. The experimental data show that for the substituted groups, the enhanced antioxidant eff ect is mainly attributed to the sulfonium salt group. The sulfonium salt group has an excellent abilities of electron donation and electron delocalization for having a lone pair of electrons between the large dipole and the sulfur atom.Carboxymethyl chitosan containing sulfonium salt can quench free radicals by donating electrons, or attack free radicals by electrons, helping to stabilize the form of free radicals, so that more reactive-free radicals can be quenched by stronger electrondonating groups (El Ashry et al., 2014). These can convert free radicals into more stable products,thereby terminating the free radical chain reaction(Saundane and Manjunatha, 2016).
3.3 Cytotoxicity analysis
The cytotoxicity test was carried out by CCK-8 assay to explore the biocompatibility of chitosan and all samples derivatives at diff erent concentrations.The exact mechanism of the antimicrobial action of chitosan and their derivatives is still unknown, but diff erent mechanisms have been proposed. There are diff erences between microbial cells and L929 cells.Microbial cells have cell walls, while L929 cells do not, and their ribosomes are diff erent. Interaction between positively charged chitosan molecules and negatively charged microbial cell walls or membranes leads to the leakage of proteinaceous and other intracellular constituents. Chitosan and its derivatives act mainly on the outer surface of the microbial cells and interact with the walls and membrane of the cell to alter cell permeability, so it can kill microbial cells without harming the growth of L929 cells (Rabea et al., 2003). Figure 8 shows the result that the growth of L929 cells that had been treated with chitosan and all sample derivatives for 24 h demonstrated diff erent degrees of inhibition. Figure 9 shows the cell growth pictures in 500.0 μg/mL. When the morphology of the cell was spindle or oval, the cytotoxicity was less.When the test concentration of the samples was 1 000.0 μg/mL, the survival rate of the cells treated with chitosan was about 76%, meaning that pristine chitosan had low toxicity at high concentrations, but when the tested concentration was less than 500 μg/mL, the survival rate of the cells treated with chitosan was greater than 83.38%. At 1 000.0 μg/mL, the survival rate of the cells treated withN,O-CMCS was greater than 90%. After sulfonium salts groups of diff erent chain lengths were grafted ontoN,O-CMCS,the cytotoxicity of the obtained products had no signif icant change. Among them, even at the highest test concentration (1 000.0 μg/mL), some cell viabilities could reach over 80%, even 100%. The results indicate that these derivatives had no cytotoxicity at this concentration.
4 CONCLUSION
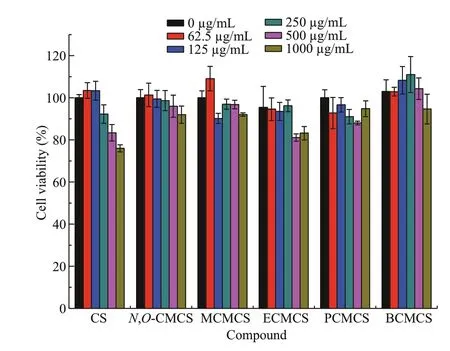
Fig.8 The cytotoxicity of chitosan (CS), N, O-CMCS, and carboxymethyl chitosan containing sulfonium salt on L929 cells
In this work, a new class carboxymethyl chitosan derivatives possessing sulfonium salts was successfully designed and synthesized through a simple three-step reaction. The antioxidant assay indicated that the introduction of sulfonium salts increased def initely the antioxidant activity of carboxymethyl chitosan derivatives. This is mainly because the sulfonium salt groups acted as an excellent electron donor to convert free radicals into more stable products, thereby showing an anti-oxidant eff ect. In the cytotoxicity assay, the cytotoxic eff ect of carboxymethyl chitosan derivatives containing sulfonium salts was low. Therefore, as the study suggested, these designed chitosan derivatives could promote the application of chitosan in antioxidant activity. Further comprehensive studies will be conducted to conf irm the theory on antioxidant activity and advance the applications in healthy food and medicine industries.
5 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Fig.9 The cell growth at concentration of 500.0 μg/mL of each sample
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
