Characterization, functional properties, and antioxidant activities of macromolecular extracts isolated from Pyropia yezoensis*
Xiaoqing WANG , Lihua GENG , Yang YUE , Ning WU , Quanbin ZHANG ,Yongdong ZHOU , Jing WANG ,
1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Jiangsu Dongshang Eslite Co. Ltd., Yancheng 224145, China
Abstract Pyropia yezoensis is one of the most economical seaweed species in China. Determining how to make full use of mature P. yezoensis and exploring new ways to increase the value of its resources are important subjects of research for the development of the laver breeding industry. In this study, we provide a simple method to comprehensively extract the bioactive substances from P. yezoensis. The characteristics,functional properties, and antioxidant activities of two types of biopolymer extract from P. yezoensis were studied and analyzed relatively. Based on the characterization of water-soluble concentrate (WSC) and alkali-soluble concentrate (ASC), obtained via chemical analysis, including Fourier Transform Infrared Spectrometer (FT-IR) and Thermal Gravimetric Analyzer (TGA), and diff erential scanning calorimetry(DSC), they both had typical polysaccharide and protein characteristics and steady composition. ASC showed higher nitrogen solubility, water holding capacity, and foaming ability. ASC could not only be used as a protein supplement, but also performed well in improving the properties of foods in terms of water holding and fat-absorption. The emulsifying activity and oil-holding capacity of WSC were observed to be higher than those of ASC. Thus, WSC has the potential to be used as an emulsif ier. Surprisingly, WSC and ASC have radical scavenging capacity in vitro, which broadens the direction of their application.
Keyword: Pyropia yezoensis; water-soluble concentrate; alkali-soluble concentrate; chemical composition;functional properties
1 INTRO D UCTION
The world population is predicted to reach 9.5 billion by 2050 (Leridon, 2020). With the increasing pursuit of vegetarian and healthy food, the conventional food industry faces huge challenges.Plant protein applications can enhance the nutritional and functional properties of products, such as texture,emulsif ication properties, solubility, stability, and adhesion. The demands of consumers have pushed food researchers to turn to the exploration of new edible resources that can contribute to human health and well-being.
Red algae are important marine crop with a long evolutionary history as a nutritional supplement. Laver(Pyropiasp.) is one of the most economical seaweed varieties, worth $1.3 billion a year (Blouin et al.,2011), and is mainly planted in China, Japan, and South Korea (Bito et al., 2017).P.yezoensisis the main species in northern China. According to theCompendiumofChineseMateriaMedica,Pyropiasp.can “soften and strengthen phlegm, clear heat and nourish the heart, and nourish the kidney and diuretic”.Compared with other plants and seaweed,Pyropiasp.has high nutritional value. DriedP.yezoensiscontains a large amount of bioactive compounds, including proteins, polysaccharides, dietary f iber, and vitamin B12 (Bito et al., 2017). The protein content ofPyropiasp. is 30%-40%, which is close to typical high-protein plants like soybeans (Fleurence, 1999; Bleakley and Hayes, 2017). The content of total amino acids in 100 g (dry weight) ofP.latifoliaandP.yezoensisare equivalent, being 35.5% and 33.5%, respectively(Bedoux et al., 2014). Compared to animal protein,protein in most seaweed also has all essential amino acids and the fact that it is vegan-friendly suggests better applicable prospects (Fleurence, 1999).Pyropiasp. contains 18 amino acids, including 7 of the 8 amino acids essential for humans. The lysine content inPyropiais about 5 times that of grains; thus, eatingPyropiacan help the human body supplement lysine.The sulphated polysaccharide which exists inPyropiasp. is called porphyran. It makes up of 20% of the plant’s total dry weight and displays diversif ied bioactivities, including antioxidant, anti-tumor, antiinf lammatory, immunomodulation, and anticardiovascular functions (Geng et al., 2019). Based the abovementioned activities, we can positively deduce that porphyran can be used for functional food(Mohamed et al., 2012).
At present, the application ofP.yezoensisis still very limited. The main use ofP.yezoensisis to simply dry it to make leisure products for direct consumption,and there are few ref ined products (Hudek et al.,2014).P.yezoensisin its later period has a tougher structure, more attachments and is not edible. It is currently used as a raw material for producing agar,and its value is low due to its high production cost and pollution to the environment. Determining how to make full use of matureP.yezoensisand exploring new ways to increase the value ofP.yezoensisresources are important subjects for the development of the laver breeding industry.
The current study of porphyran focuses on the extraction, characterization, and bioactivities (Isaka et al., 2015; Xu et al., 2020). Concerning proteins from laver, most of the research has focused on the purif ication and isolation of phycoerythrin, while other proteins have been neglected (Niu et al., 2010,Chen et al., 2017). The functional properties and comprehensive extraction of polysaccharides and proteins fromP.yezoensis for food processing purposes have not been reported. It is particularly urgent and important to carry out deep processing and comprehensive utilization of low-valueP.yezoensis.
The aim of our study was to achieve the high-value utilization of the low-value seaweed by constructing a deep-processing method for step extracting polysaccharide concentrate and protein concentrate fromP.yezoensis. Meanwhile, we have studied the physicochemical characterization and functional properties of the extracts in order to pave the way for future applications in the f ield of food, and even further applications as functional foods.
2 MATERIAL AND METHOD
2.1 Material
Pyropiayezoensis, cultured on the coast of Dafeng,China, was collected in April 2019, authenticated by Professor Lanping DING and stored as a voucher specimen (No. 128) in the Institute of Oceanology,Chinese Academy of Sciences. The fresh algae were promptly washed, sun dried, and kept in plastic bags at room temperature for use.P.yezoensiswas powdered using a grinder. A dialysis bag was purchased from Sigma-Aldrich. All the chemicals used were of analytical grade.
2.2 Extraction of algae biomacromolecule concentrate
The method used for the extraction of the diff erent biopolymer concentrates was based on methods described by Fleurence et al. (1995) with some modif ications (Fig.1). In brief, 200-g algae powder was mixed with deionized water (1꞉30 w/v), stirred overnight at room temperature. After overnight treatment, the supernatant was collected by centrifugation at 4 000 r/min for 15 min then f iltered with bolting cloth. The pellet was collected for further extraction. Solid ammonium sulfate was gently added to the supernatant until the f inal concentration was 70%. The mixture needed to stand overnight before centrifugation (4 000 r/min, 15 min). After centrifugation, the pellet was re-suspended in deionized water and then dialyzed with deionized water with a 3-kDa dialysis bag to obtain seaweed water-soluble concentrate (WSC). Conductivity values for the water in the dialysis beaker were measured with a conductivity meter until the value was the same as the conductivity of the fresh deionized water. Dialyzed extracts were freeze-dried, powered,and stored in sealed bags in a desiccator at room temperature. The seaweed residue collected from the f irst extraction was re-suspended in 0.1-mol/L NaOH solution (1꞉20 w/v); this suspension was stirred for 2 h before centrifugation (4 000 r/min, 15 min). The second supernatant was also precipitated by ammonium sulfate (Final concentration was 70%)after adjusting its pH to 7.0. The precipitation (alkalisoluble concentrate; ASC) was treated in a similar manner as above.
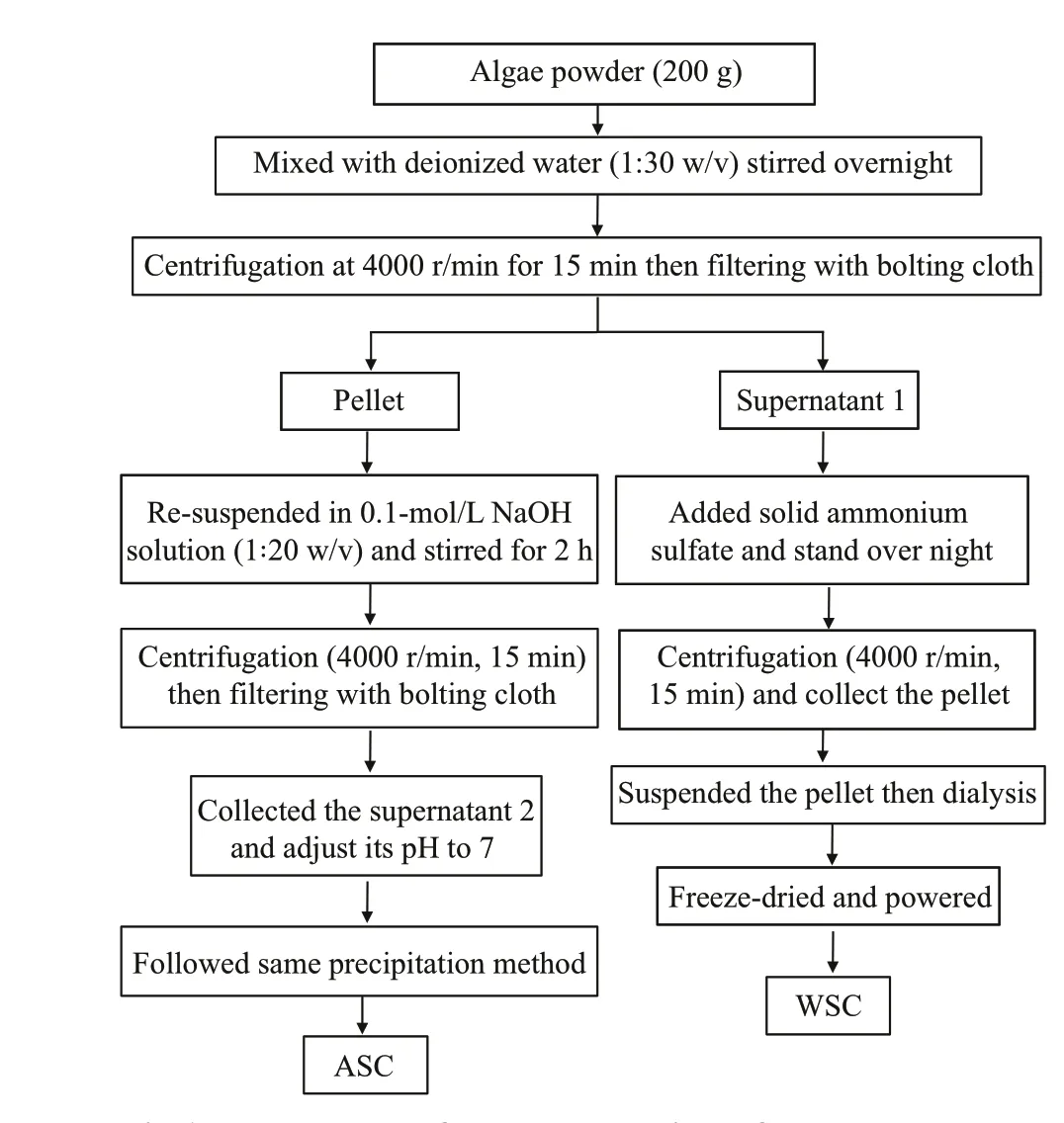
Fig.1 Flow-chart of the extraction for water-soluble concentrate (WSC) and alkali-soluble concentrate(ASC)
2.3 Chemical composition analytical method
The protein content was determined by the Kjeldahl method (Wathelet, 1999). The crude protein content was calculated by multiplying its nitrogen content by a nitrogen-to-protein conversion factor of 6.25. The polysaccharide content was determined by the phenolsulphuric acid method using galactose as a standard(Dubois et al., 1956). Neutral sugar composition was determined with 1-phenyl-3-methyl-5-pyrazolone precolumn derivatization high performance liquid chromatography (PMP-HPLC), using ribose as an internal standard (Honda et al., 1989). The weightaverage molecular weight (Mw) was assayed by highperformance gel-f iltration chromatography (HP-GPC),using a series of dextrans with diff erent molecular weights as standards. The molecular weight of sample was calculated by the calibration curve obtained by using various standard dextran. The composition of neutral monosaccharides in the WSC and ASC were analyzed by using the Alliance HPLC system(Shimadizu, LC-40, Japan) equipped with an Agilent C18 column (4.6 mm×250 mm, 5 μm). The molar ratio of monosaccharides was calculated by 1-phenyl-3-methyl-5-pyrazolone precolumn derivation HPLC as described previously. 3,6-anhydro-galactose was determined by resorcinol method (Yaphe, 1960).Sulfate content was analyzed by ion chromatograph with potassium sulfate as the standard. The Fourier Transform Infrared spectrum (FT-IR) was measured on a Nicolet iS10 FT-IR spectrometer (Thermo Fisher,Waltham, MA, USA) in the range of 500-4 000 cm-1. A series of dextrans was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (China). Other standard reagents were purchased from Sigma-Aldrich. The content of amino acids in the protein concentration was determined using an automatic amino acid analyzer (L-8500A,Hitachi, Japan).
2.4 Thermal property
The thermal properties were determined by diff erential scanning calorimetric (DSC) analysis and thermal gravimetric (TGA) analysis. DSC and TGA were carried out using a diff erential thermogravimeter(SDT Q600, TA Instruments, USA); Ar was used as a carrier.
2.5 Determination of functional property
2.5.1 Nitrogen solubility
Nitrogen solubility was determined according to the method of Bera and Mukherjee (1989). Samples(0.1 g) were dispersed in 10 mL of deionized water maintained at diff erent pH values (2.0 to 12.0), as well as in 10 mL of NaCl solutions (0.1 and 0.5 mol/L).Samples were shaken for 15 min at 145 r/min at room temperature before centrifugation (4 500 r/min,30 min). The supernatant was collected for nitrogen solubility measurement through the Kjeldahl method,and the percent nitrogen solubility was calculated using Eq.1:

2.5.2 Water-holding capacity (WHC) and fat absorption capacity (FAC)
Water-holding capacity (WHC) and fat absorption capacity (FAC) of WSC and ASC were determined following the methods of Rodríguez-Ambriz et al.(2005) and expressed as weight of water (for WHC)or soybean oil (for FAC) absorbed per gram of the sample. Samples (0.5 g) were put into a weighed centrifuge tube then 10-mL water or soybean oil was added. The mixture was vortexed for 2 min and left to stand for 30 min at room temperature. Then, the mixture was centrifuged (3 000 r/min, 30 min).Immediately after centrifugation, the supernatant was scrupulously removed and the tubes were weighed.
2.5.3 Emulsifying and surface-active property
The emulsifying activity index (EAI) and emulsion stability index (ESI) of WSC and ASC from diff erent lines were determined following the method of Suresh Kumar et al. (2014).
To explore the surface-active properties, the surface tension of 0.1% WSC and ASC were measured using a Dataphysics dynamic contact angle meter(DCAT 11, Micromeritics, USA).
2.5.4 Foaming capacity and stability
Foaming capacity (FC) was studied following the method of Timilsena et al. (2016). Brief ly, for 25 mL of 0.2% sample solution, the pH was adjusted to 2, 4,6, 8, and 10. The sample solution was whipped using a homogenizer at 15 000 r/min for 2 min, and then poured into a 50-mL measuring cylinder. The volume increment was evaluated as a percentage indicating foaming capacity, and it was calculated using Eq.2:
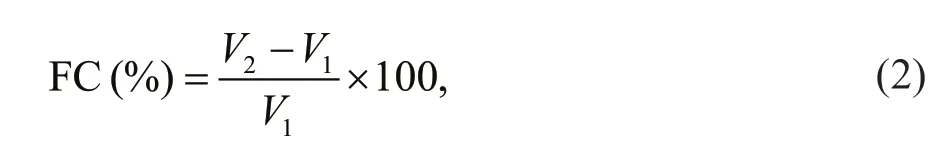
whereV2(mL) is the volume of sample solution after whipping andV1(mL) is the initial volume of sample dispersion.
2.6 Antioxidant activity
2.6.1 Hydroxyl radical scavenging activity
The potential of WSC and ASC in scavenging hydroxyl radicals was analyzed using a hydroxyl radical assay kit (Nanjing Jiancheng, China),following the manufacturer’s instructions. The absorbance was measured at 550 nm and ascorbic acid was used as the positive control. Hydroxyl radical scavenging activity (%) was calculated as previously reported (Sun et al., 2009).
2.6.2 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) radical scavenging activity
1,1-Diphenyl-2-Picrylhydrazyl radical scavenging activity was synthesized using a DPPH radical scavenging ability kit (Nanjing Jiancheng, China),following the manufacturer’s instructions. The absorbance was measured at 517 nm and ascorbic acid was used as the positive control. DPPH radical scavenging activity (%) was calculated using the equation previously published (Sun et al., 2009).
2.7 Statistical analysis
The data from three independent experiments are presented as mean±standard deviation (SD).
3 RESULT
3.1 Biopolymer content, recovery, and yield of extraction
The yield of protein and polysaccharide is shown in Table 1. The protein yield of water extract was 17.28%±0.12%, which is lower than alkali extract(23.06%±0.54%). The yield of polysaccharide acquired through water (10.10%±0.21%) is double than alkali extraction (5.00%±0.08%).
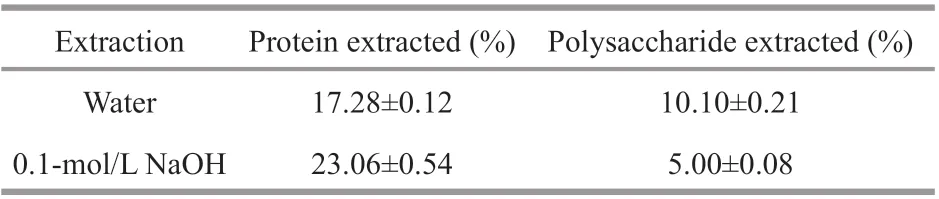
Table 1 Protein and polysaccharide extraction yield after diff erent extraction processes
3.2 The physicochemical characterization of WSC and ASC
3.2.1 Chemical composition analysis of WSC and ASC
The chemical composition of WSC and ASC are shown in Table 2. The total protein and polysaccharide content of the WSC was 28.37%±0.09% and 54.61%±0.86% based on dry weight, respectively.The ASC was mainly comprised of 69.68%±0.08%protein and 20.03%±0.77% polysaccharide. Galactose was the main monosaccharide in both WSC and ASC,and other neutral sugars such as xylose, glucose, and rhamnose were also detected at a low level (Table 2).The 3,6-anhydro-galactose levels of WSC and ASC were 1.05%±0.05% and 0.42%±0.04%. The weightaverage molecular weight (Mw) of WSC and ASC was estimated by HP-GPC to be 145.5 and 220.1 kDa respectively. The sulfate content of WSC and ASC were 29.308% and 11.416%, respectively, which is quite high. The amino acid prof ile of protein obtained from two extraction methods are shown in Table 3.ASC (76.77%) was much higher than WSC (23.60%)in terms of the total amino acid content. The most abundant essential amino acid in WSC and ASC was leucine, followed by valine, lysine, and threonine.The sample of WSC and ASC contained all the essential amino acids, with essential amino acids accounting for 38.21% and 37.45% of the total amino acid content. Furthermore, for non-essential amino acids in WSC and ASC, a large part of the amino acid fraction was composed of aspartic, alanine and glutamic acids.

Table 2 Chemical compositions (in dry weight) of WSC and ASC
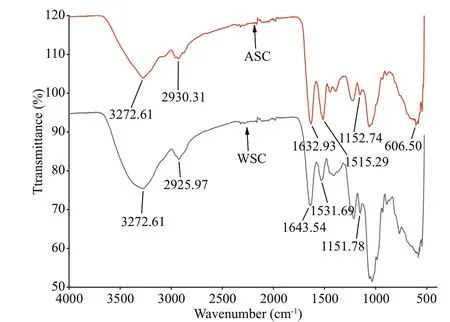
Fig.2 FT-IR Spectrum of WSC and ASC
3.2.2 FT-IR spectrum analysis
The FT-IR spectrum (Fig.2) of WSC and ASC had notable similarities, with diff erences in the absorption intensity of some characteristic peaks. The strong band detected at 3 272.61 cm-1appeared in both WSC and ASC spectra, which apparently displays typical polysaccharide characteristics and could belong to O-H stretching vibrations of the hydroxyl groups(Mohamed et al., 2012). In addition, there was anabsorption peak at around 1 150 cm-1in both WSC and ASC for the C-O-C band, suggesting the presence of polysaccharides (Kac̆uráková et al., 2000).Furthermore, in the IR spectrum of WSC and ASC,there were obvious protein bands: amide I (1 600-1 700 cm-1), amide II (1 500-1 580 cm-1) and amide III (1 200-1 400 cm-1) (Kong and Yu, 2007). The most signif icant spectral region showing the secondary structure components of the protein is the amide I band. The strong absorption peaks at 1 632 cm-1and 1 643 cm-1are essentially assigned to C=O stretching (amide I) and correspond to the β-sheet structure (Du et al., 2018).
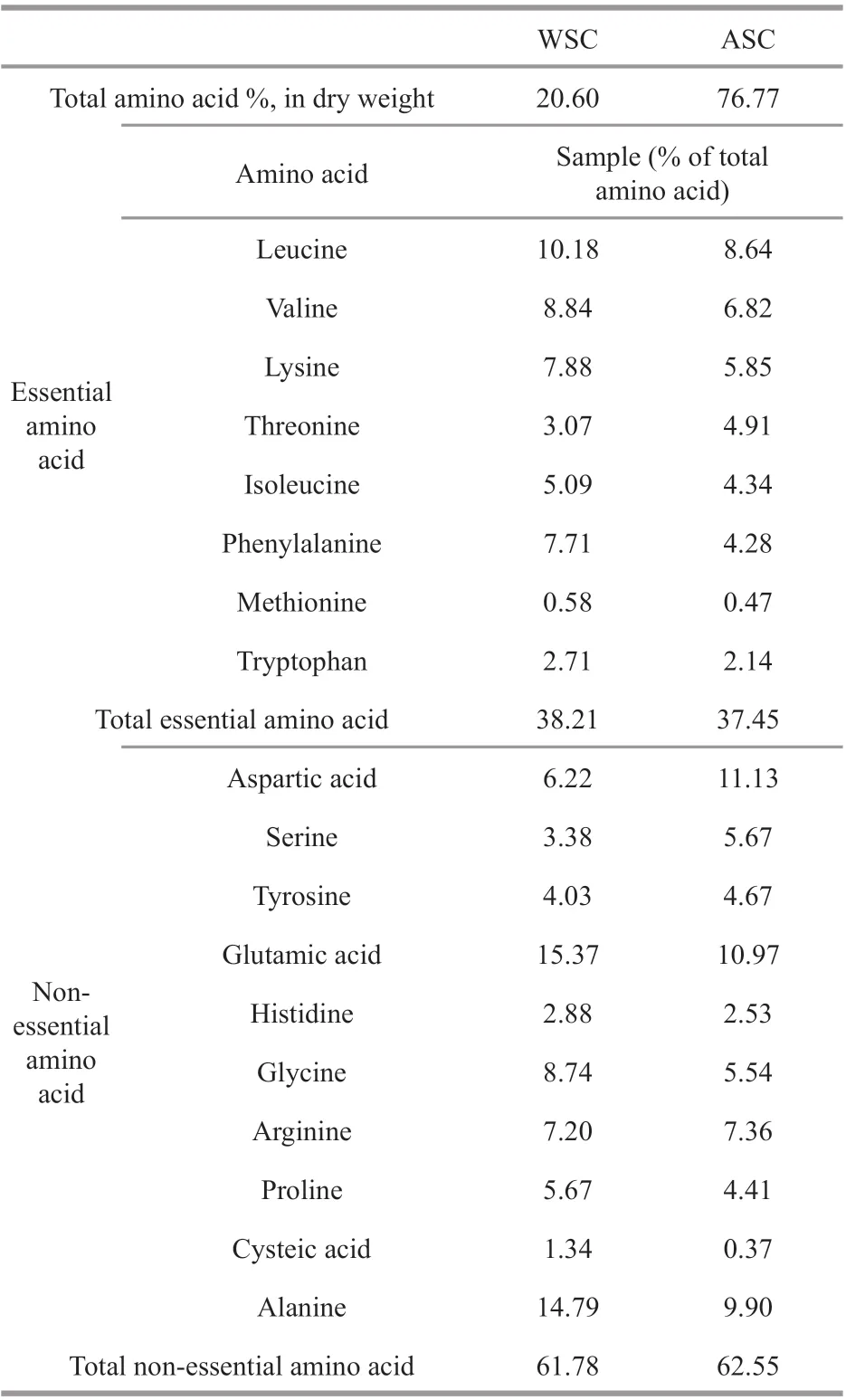
Table 3 Amino acid prof ile of proteins extracted from P. yezoensis
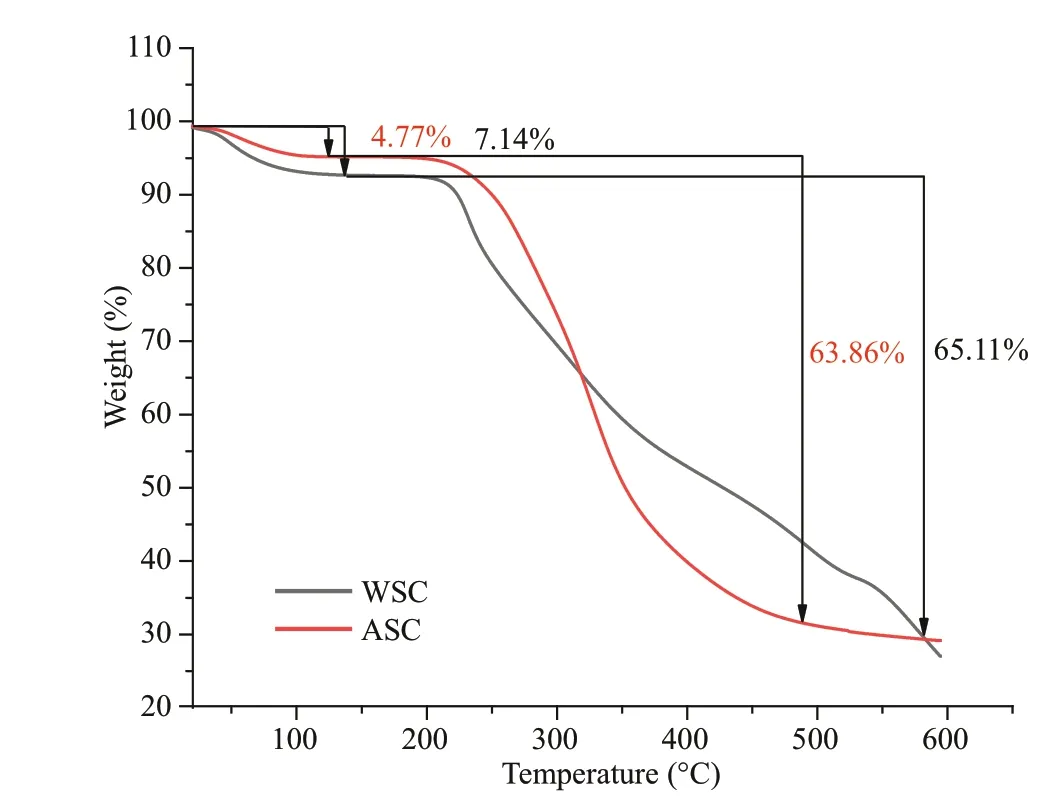
Fig.3 Typical TGA thermogram of WSC and ASC
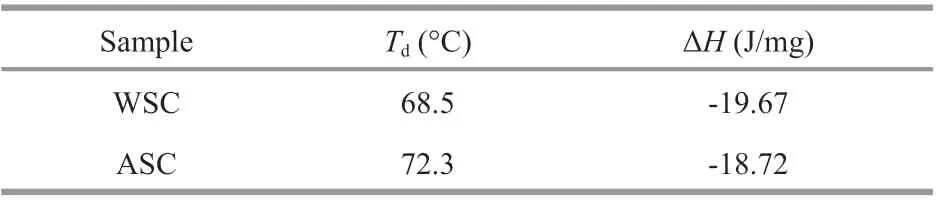
Table 4 Thermal properties of WSC and ASC
3.2.3 Thermal property
Figure 3 demonstrates the TGA curve of WSC and ASC which showed two processes of weight loss. The TGA curve of WSC, displayed in Fig.3, showing two typical processes, dehydration and degradation. The dehydration process continues to 130 °C, which constitutes the f irst phase of weightlessness. After reaching approximately 200 °C, the degradation of polysaccharides in the heating process becomes the main factor of weight loss. The relationship between weight loss and temperature of ASC is also observed mainly in two stages: the f irst 4.77% mass reduction occurs between 30 °C and 125 °C, which could be due to the loss of moisture and followed protein denaturation in combination with DSC results. The next immediate step is the degradation of ASC, with 63.86% of weight loss. It has been speculated that the decomposition of the principal component protein of ASC is the main cause of weight loss.
The results of DSC indicate the thermal denaturation temperatures (Td) and ΔHwhich were showed in Table 4. According to result of DSC, the denaturation temperatures of the protein in WSC and ASC were 68.5 °C and 72.3 °C, respectively, which is similar to previously observed microalgal protein denaturation temperatures (Chen et al., 2019). The enthalpy of the thermal denaturation (ΔH) of WSC and ASC were-19.67 and -18.72 J/mg, respectively.
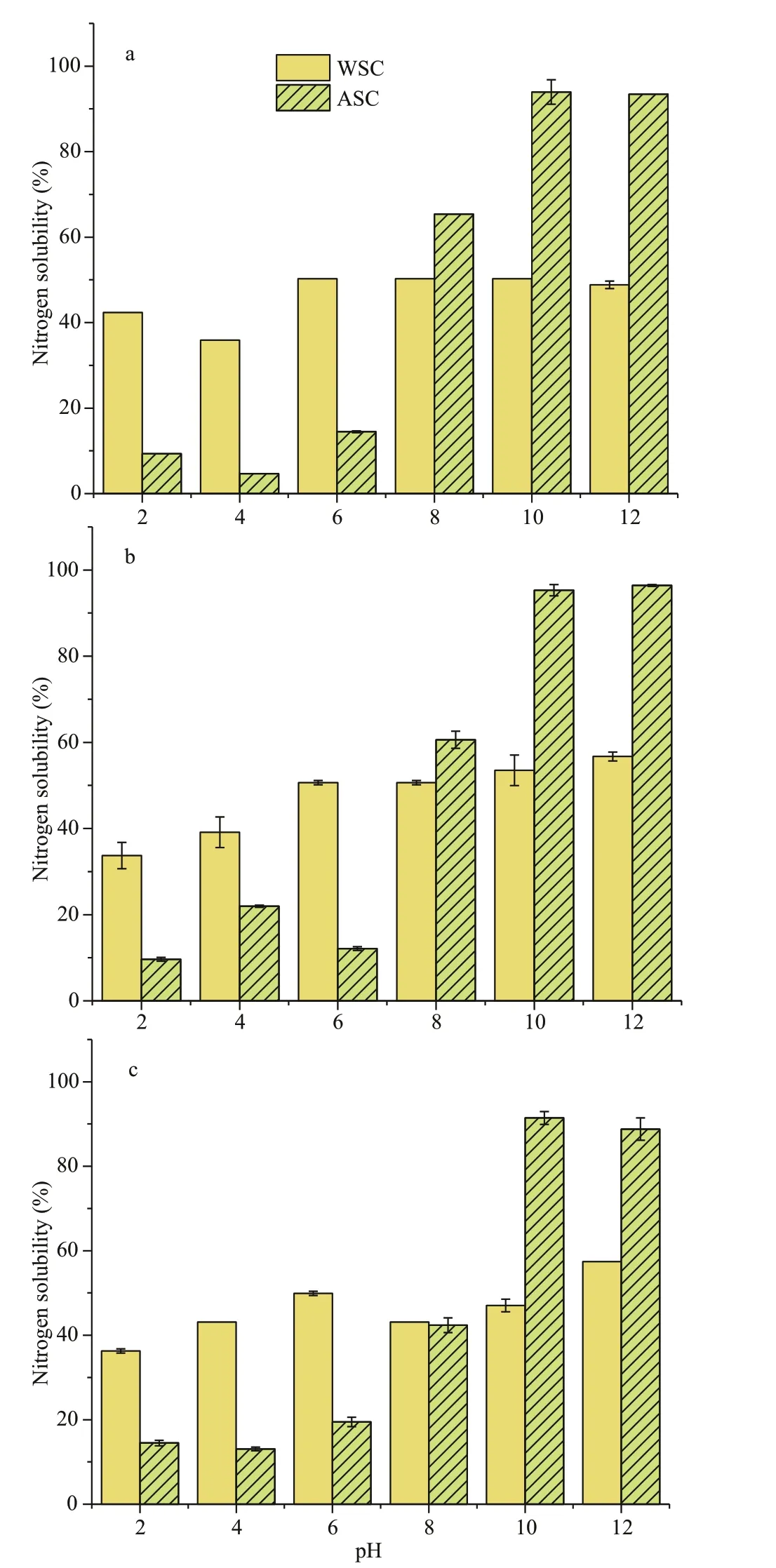
Fig.4 Nitrogen solubility of WSC and ASC in water (a),0.1-mol/L NaCl (b), and 0.5-mol/L NaCl (c) at diff erence pH
3.3 Functional property
3.3.1 Nitrogen solubility
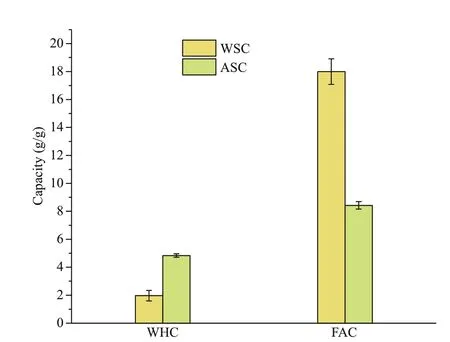
Fig.5 Water-holding capacity (WHC) and fat-absorption capacity (FAC) of samples
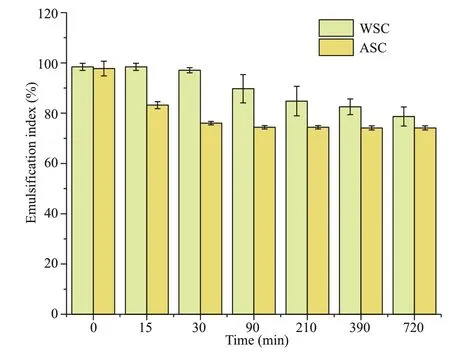
Fig.6 Emulsif ication index of WSC and ASC
Water, 0.1-mol/L NaCl and 0.5-mol/L NaCl were used as solvents for the measurement of nitrogen solubility of WSC and ASC (Fig.4). The nitrogen solubility of WSC did not show many diff erences in three solvents in the range of pH 2-12. For ASC, the minimum nitrogen solubility value was close to pH 4(4.7%), increasing with the pH until reaching its highest value (93.9%) around 10 when water is used as a solvent. In addition, in the presence of salt(0.5 mol/L NaCl) ASC still has excellent solubility,and its highest solubility (91.4%) is close to its solubility in aqueous solution.
3.3.2 Water-holding capacity (WHC) and fatabsorption capacity (FAC)
The WHC of WSC was 1.97±0.37 g/g, whereas the WHC of ASC was more competent, at 4.84±0.12 g/g(Fig.5). The FAC of WSC was 17.99±0.91 g/g (Fig.5),which is signif icantly higher than ASC (8.42±0.26 g/g).
3.3.3 Emulsifying and surface-active property
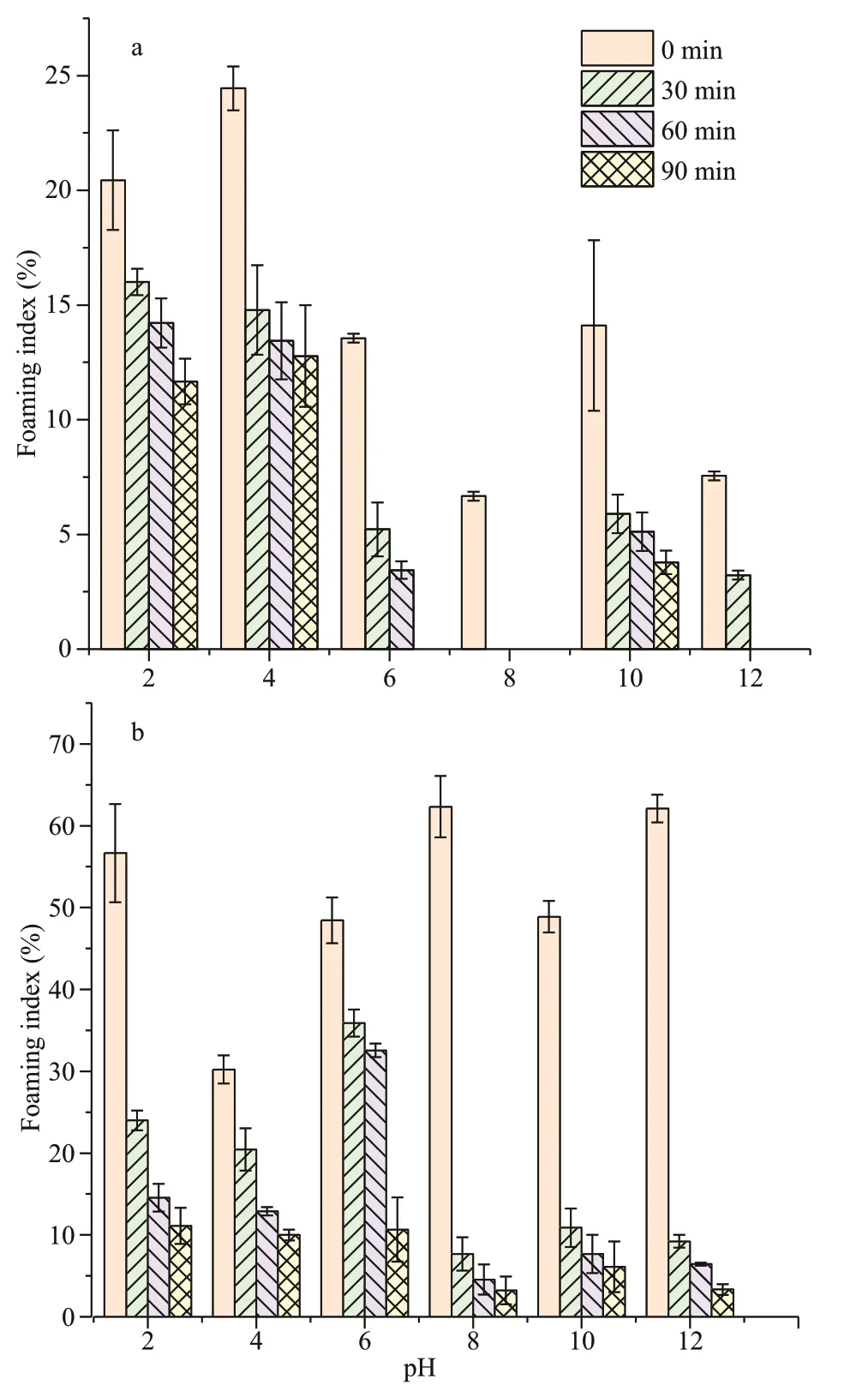
Fig.7 Foaming index of WSC (a) and ASC (b)
As is depicted in Fig.6, the emulsif ication index of WSC is relatively higher than that of ASC. The emulsion stability of WSC is also noteworthy. After standing for 720 min, the emulsif ication index decreased by only 19.7%. The highest emulsif ication index of ASC was slightly lower than that of WSC,but after 720 min standing it dropped by 23.6%. The surface tension of 0.1% of the WSC and ASC solution was determined to be 24.42±0.37 and 44.94±0.09 mN/m, respectively, which are signif icantly lower than the surface tension of the water.
3.3.4 Foaming capacity and stability
The foaming properties of WSC were def icient, as is shown in Fig.7a. Unlike WSC, the foaming capacity of ASC (Fig.7b) was obviously better, and was aff ected by pH, tending to decrease at pH 4 (30.22%±2.00%). With a slight decrease at alkaline pHs, the foaming capacity of ASC reached a maximum at pH 8 (62.33%±4.00%).
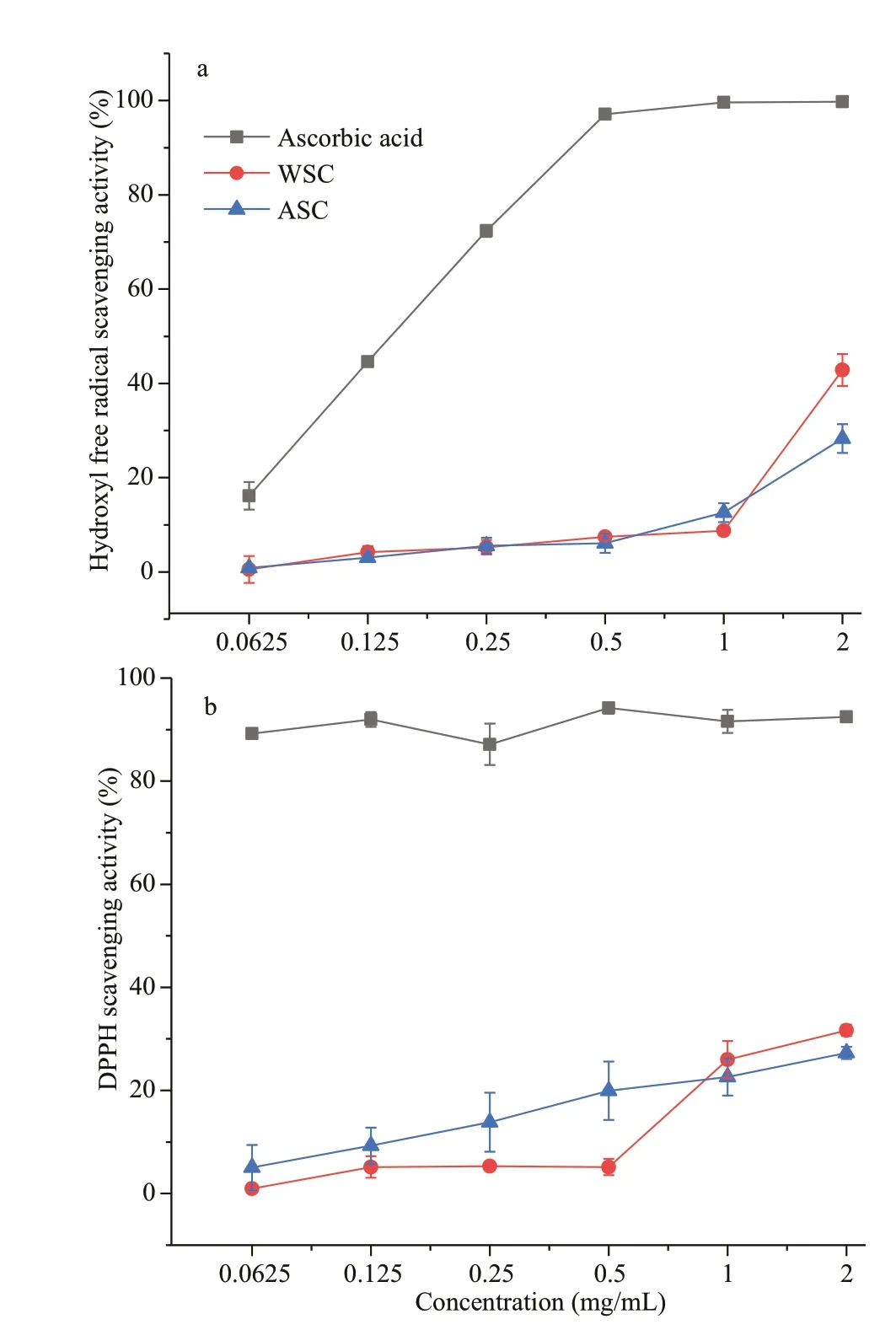
Fig.8 Scavenging activity of WSC and ASC on hydroxyl radicals (a) and DPPH (b)
3.4 Antioxidant activities in vitro
As illustrated in Fig.8, the scavenging activity of the DPPH and hydroxyl radicals of WSC and ASC were assessed at diff erent concentrations. For the DPPH and hydroxyl radicals, both WSC and ASC exhibited certain radical scavenging abilities. The hydroxyl radical scavenging activity of WSC increased from 0.55% to the maximum detection activity of 42.82%, with the sample concentration ranging from 0.062 5 to 2 mg/mL. ASC showed a similar hydroxyl radical scavenging ability, with the sample concentration ranging from 0.062 5 to 2 mg/mL, although the maximum detection activity(28.22%) was lower than that of WSC. The DPPH scavenging activity of WSC and ASC displayed almost identical abilities. As the sample concentration increased to 2 mg/mL, the DPPH radical scavenging ability increased to 31.67% for WSC and 27.31% for ASC.
4 DISCUSSION
To f ind out the most convenient and economical strategy of extracting biopolymers, the ultrasoundassisted extraction method was compared with the room temperature water extraction method. We found that, for both yield and purity, the concentration obtained by ultrasound-assisted extraction was only slightly higher than that obtained by room temperature water extraction (Supplementary Table S1). Therefore,room temperature water extraction was used, as it is a more accessible and production-friendly method.Compared to previous studies, this extraction method(water extraction followed by alkali solution extraction) obviously increased the protein content in the extract of theP.yezoensis. The total is approximately equal to the protein content ofP.yezoensis. Although the total polysaccharide yield(54.61%±0.86% for WSC, 20.03%±0.77% for ASC)is lower than that obtained using the previously published method of extracting polysaccharides(74.5%±4.5%) (Khan et al., 2020), the simplif ied procedure without drastic experimental conditions is still competitive. The results of chemical composition showed alkali treatment could eff ectively destroy plant cell walls by changing the chemical composition and structure of cellulose to increase protein production(Perović et al., 2020). In the study by Qu et al. (2013),the protein content in the extract was 45.61%±0.33%,which is lower than the ASC observed in this study.The 3,6-anhydro-galactose levels of WSC and ASC(1.05%±0.05% for WSC, 0.42%±0.04% for ASC) were lower than previously reported (5.1%)(Rees and Conway, 1962). Amino acid composition is an important index to evaluate the nutritional value of protein, and the content of essential amino acids is closely related to nutritional value (Nollet and Toldrá,2012). The amino acid composition of WSC and ASC were similar, and the amino acid content of them shows the same pattern. The high rate of aspartic and glutamic acids were also found in most seaweed(Fleurence, 2004). Notably, some amino acids were observed to have higher levels than those found in egg ovalbumin (Poole et al., 1984); these included aspartic acid, threonine, glycine, alanine, and threonine. The high content of aspartic and glutamic acids could add special f lavor and taste during food processing, and this also suggests a potential to be used as a nutritional.
The FT-IR results indicated that alkali treatment did not change the basic polysaccharide structure and the secondary protein structure, which also proves that the method of alkali-extracting laver biopolymers is feasible. As for thermal properties of WSC and ASC, this study used DSC analysis and TGA to characterize its thermal parameters. The thermal stability of WSC is relatively lower than that reported for porphyran (Xu et al., 2020), in part because watersoluble polysaccharide extract contains part of the protein. The similarity ofTdand ΔHin WSC and ASC exhibited similar potency in industrial processing.
Nitrogen solubility is an important indicator to assess food protein, because it has an eff ect on other functional properties such as foamability and emulsibility. The nitrogen solubility prof ile (Fig.4) of ASC presented typical protein characteristics, whereas the nitrogen solubility prof ile of WSC showed no apparent regularity. The change of pH seems to have less inf luence on the nitrogen solubility of WSC,although the lowest solubility was observed under acid pH conditions. This feature of WSC may be the reason that the nitrogen level is relatively low in WSC,though it may not exist completely in the protein. Salt concentration also did not show a signif icant eff ect on WSC nitrogen solubility, which could be an advantage for its application in food. This water solubility pattern of ASC is similar to many previously reported algaederived proteins (Suresh Kumar et al., 2014; Garcia-Vaquero et al., 2017; Abdollahi et al., 2019). Combined with the result of amino acid prof iling, the reason for this high solubility in the alkaline solution environment could be due to the presence of a large number of acidic amino acids in the algal protein concentrate. It can be stated that ASC would have a good application in various food products as well.
WHC is the ability of water to bind the polar side chains of the protein. It is related to the charge of the amino acids. The WHC of ASC is higher than the water-holding capacity of most algae protein extracts that have been studied. The current results indicate that ASC had a higher WHC than that ofKappaphycusalvarezii(2.22±0.04 g/g) and some microalgae, such asChlorellapyrenoidosa(2.02±0.05 g/g),Arthospiraplatensis(2.81±0.04 g/g), andNannochloropsisoceanica(2.87±0.07 g/g), but lower WHC than that ofHimanthaliaelongate(10.27±0.09 g/g) (Suresh Kumar et al., 2014; Chen et al., 2019; Zhu et al., 2019). High WHC is an ideal condition for baked foods, which is important for maintaining the taste and texture (Chandi and Sogi, 2007) of foods such as bread, cake, and custards. Oil absorption is an important functional property in food processing, especially for formulation foods (Chandi and Sogi, 2007). The loose structure of dry WSC products can hold more oil droplets, and its powder is too light to be compacted by centrifugation,which leads to its high FAC. The FAC of ASC was higher than microalgaeHaematococcuspluvialis(3.29±0.2 g/g),Arthrospira(Spirulina)platensisprotein (2.52±0.09 g/g), and rhodophtyic seaweedKappaphycusalvareziiprotein (1.29±0.20 g/g), and is similar to microalgaeArthospiraplatensis(8.37±1.45 g/g) andNannochloropsisoceanica(8.25±0.44 g/g). The presence of side chains of nonpolar amino acids could be the reason for this great oilholding capacity (Benelhadj et al., 2016).
The emulsifying ability is a considerable parameter among the functional properties evaluated in food industry, which can be inf luenced by diff erent kinds of oil. In this study, soybean oil was chosen because it is a widely used edible oil in the food industry. The emulsifying ability of WSC was undertaken by its main component polysaccharide. Recently, the application of polysaccharides as emulsif iers in the food industry has attracted wide attention (Chivero et al., 2014; Olawuyi et al., 2020). When evaluating the potential of WSC and ASC to be used as an emulsif ier,it is also necessary to consider their ability to reduce surface tension. The ability to lower the surface tension of the phases plays an essential role in the formation of emulsions, and is also a key condition for emulsif iers(Rouimi et al., 2005). The lower surface tension of WSC is on a par with its higher emulsifying ability.For all these reasons, WSC and ASC have the potential to be used as emulsif iers in the production of surimi,sausages, etc. and WSC is the better of the two.
The limited content of protein in WSC exhibited inadequate cohesion and elasticity of the protein monolayer f ilm formed at the interface, which leads to poor foamability. However, polysaccharides can be used to enhance the stability of protein foams, because of their ability to increase bulk viscosity (Patel, 2018).This is higher thanKappaphycusalvareziiprotein(53.33%±2%),Chlorellapyrenoidosaprotein(36.88%±6%) andArthospiraplatensisprotein(54.02%±6%) (Suresh Kumar et al., 2014; Chen et al., 2019). Nonetheless, ASC foam, like other vegan protein foams, is incapable of being highly stable(Söderberg, 2013). The foaming stability is aff ected by pH and time. In this study, after 90 min the foam was reduced to around 10% at all tested pH values.The weak foaming stability could be due to the protein concentration, is not high enough to cause a failure of the formation of a multilayer, cohesive protein f ilm at the interface (Damodaran, 1997).
Oxidative stress caused by accumulation of reactive oxygen species could induce cell apoptosis and ultimately lead to neurodegenerative diseases (Emerit et al., 2004). Antioxidant activity is a widely validated bioactivity of natural extracts, which was reported in many studies, including proteins and polysaccharides(Isaka et al., 2015; Alizadeh and Aliakbarlu, 2020;Khan et al., 2020). However, compared to the positive control (ascorbic acid), like other plant extracts, WSC and ASC did not show better scavenging ability at the same sample concentration (Isaka et al., 2015;Alizadeh and Aliakbarlu, 2020; Khan et al., 2020). On the whole, WSC and ASC exhibit decent antioxidant activity, which raises the possibility of further applications in the f ield of functional food.
5 CONCLUSION
We have provided an uncomplicated protocol for the extraction of biomacro-molecules inP.yezoensis,which is accessible at a low cost for future food processing applications. The results of this study are vital in f illing the lack of knowledge concerning the functional properties of biopolymers inP.yezoensis.The characterization the functional properties of WSC and ASC indicates that they both have the possibility of being used as emulsif iers. As a polysaccharide concentrate, WSC is likely to have further bioactivities that may be elucidated in further studies. Nevertheless,ASC not only could be used as a protein supplement;it also performs well in improving water-holding and fat-absorbing capacities of foods. Furthermore, the antioxidant activity of WSC and ASC observed in this study could imply the application of WSC and ASC in the area of functional food. Therefore,Pyropiasp.should be given more attention as an inexpensive and readily available plant with a high biopolymer content.
6 DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
