Roles of interleukins in antibacterial immune defense of the brood pouch in the lined seahorse H ippocampus erectus*
Han JIANG , Chunyan LI , Bo ZHANG , Yongli WU , Qiang LIN ,**
1 CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
2 Guangdong Provincial Key Laboratory of Applied Marine Biology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract Seahorse embryos are brooded in the enclosed nutrient-rich environment of the male brood pouch, which may be prone to bacterial infection. The immune responses of interleukin (IL) genes in the brood pouch have not been well studied. We identif ied 13 interleukins in the lined seahorse Hippocampus erectus. Tissue-specif ic expression analysis revealed increased mRNA expression levels of il- 1β, il- 18, and il- 8 in the brood pouch. When challenged with lipopolysaccharide or Vibrio parahaemolyticus, il- 1β and il-18 were active as part of the acute and chronic inf lammatory responses, respectively. Importantly, i l- 8 may be involved in powerful antibacterial immune responses and may be induced by il- 1β and il- 18 via a process involving the nuclear factor-κB signaling pathway. These results suggest that il- 1β, il- 18, and il- 8 may play key roles in the antibacterial immune defense of the brood pouch in male seahorses.
Keyword: lined seahorse; brood pouch; interleukin; antibacterial immunity
1 INTRODUCTION
Seahorses utilize the charismatic reproduction strategy of male pregnancy (Wilson et al., 2001; Lin et al., 2016). The male seahorse possesses a brood pouch, in which the female seahorse deposits her eggs before they are fertilized and undergo embryonic development (Laksanawimol et al., 2006; Rosenqvist and Berglund, 2011). The brood pouch of seahorse is located along the ventral midline of the tail posterior to the anus. The outer epithelium of the pouch is composed of stratif ied cuboidal epithelial cells,whereas the lumen of the pouch is surrounded by three layers containing abundant blood vessels: two dermis layers and the pseudoplacenta. The outer dermis layer consists of a loose network of connective tissue and the inner dermis layer consists of tightly connected collagenous f ibers. The pseudoplacenta is beneath the inner dermis layer and composed of connective tissue with a mesh-patterned structure and abundant blood vessels (Kawaguchi et al., 2017).
Seawater can directly f low into the brood pouch of seahorse during the non-pregnancy state (Whittington and Friesen, 2020). Previous studies had indicated that crucial immune molecules were expressed at high transcriptional levels in the seahorse brood pouch, such as secretion of C-type lectin (Melamed et al., 2005), expression of major histocompatibility proteins MHC IIa and IIb (Luo et al., 2016; Roth et al., 2020), and expression of interleukin IL-20RB(Whittington et al., 2015; Roth et al., 2020). Recent studies revealed that interleukins play crucial roles in the innate immune responses of mammals and teleosts(Kaiser et al., 2004; Secombes et al., 2011; Zou and Secombes, 2016). However, the immune responses of interleukin genes in the seahorse brood pouch are unclear.
Produced by activated monocytes and macrophages,interleukins are a highly diverse subgroup of cytokines present as more than 40 diff erent types in mammals(Zou and Secombes, 2016). Diff erent subfamilies of interleukins have diff erent conserved domains and were def ined in previous studies (Secombes et al.,2011; Zou and Secombes, 2016). These proteins are divided into pro-inf lammatory and anti-inf lammatory cytokines (Kaiser et al., 2004; Secombes et al., 2011).In teleosts, challenge with bacteria or lipopolysaccharide (LPS) can increase the transcription levels of pro-inf lammatory cytokines.For example, the mRNA expression of interleukin(IL)-8 is rapidly upregulated afterV ibrioparahaemolyticuschallenge in the spleen, head kidney, and liver inLarimichthyscrocea(Wang et al.,2019). Interleukin (IL)-1β can induce proinf lammatory gene expression to further cause pathological inf lammation involving activation of the nuclear factor (NF)-κB pathway inMiichthysmiiuy(Hayden and Ghosh, 2011; Yang et al., 2017).
In this study, we identif ied and characterized members of the interleukin gene family at the genome and transcriptome levels in the lined seahorse(Hippocampuserectus) and built a phylogenetic tree with representative genes. Further, we evaluated the potential roles ofil-1β,il-18, andil-8in antibacterial immunity in the brood pouch of seahorses following challenge with LPS andV.parahaemolyticus.
2 MATERIAL AND METHOD
2.1 Ethics statement
All experiments were conducted in accordance with the guidelines and with approval from the animal research and Ethics Committees of the Chinese Academy of Sciences (approval number: SCSIOIACUC-2019-000140).
2.2 Interleukin gene family
To identify interleukin genes in lined seahorse,genome annotations f iles ofHippocampuscomes,H.erectus, andMicrophismanadensiswere initially searched. The reference genomes ofH.comes(LVHJ00000000) andM.manadensis(QODF00000000) and transcriptomic data ofH.erectus(SRA392578, SRA392580) were searched with TBLASTN of parameter “tblastn.exe -query -db-out -evalue 0.000 01” using sequences of interleukin genes from humans and zebraf ish obtained from the National Center for Biotechnology Information(NCBI) (https://www.ncbi.nlm.nih.gov/) as query sequences (Supplementary Table S1). We then conf irmed the acquired interleukin gene ofH.erectusfrom the conserved domain structures of Pfam(https://pfam.xfam.org/) (Supplementary Fig.S1).
2.3 Phylogenetic analysis
At least one representative interleukin from each subfamily was selected (il-1β,il-18,il-17d,il-8,il-10,il-15, andil-6), and phylogenetic analysis was carried out using the amino acid sequences of interleukin genes fromH.erectusand several representative f ish(Supplementary Table S1). These sequences were downloaded from the NCBI for zebraf ish (Daniorerio), tiger tail seahorse (H.comes), manado pipef ish(M.manadensis), medaka (Oryziaslatipes), fugu(Takifugurubripes), and stickleback (Gasterosteusaculeatus). Multiple amino acid sequences were aligned using Muscle software (https://www.ebi.ac.uk/Tools/msa/muscle/) with default parameters(Edgar, 2004). We constructed phylogenetic trees using two methods. The phylogenetic tree determined by the maximum-likelihood method was constructed using MEGA7 and that based on the Bayesian method was constructed using Mrbayes (Heled and Drummond, 2010). Bootstrapping was conducted with 1 000 and 10 000 000 replications to evaluate the phylogenetic trees, respectively.
2.4 Animal husbandry
Fifty-six reproductively mature seahorses (average body weight, 13.9±0.5 g) were purchased from Zhangzhou Seahorse Breeding Center (Fujian, China)and maintained in a large tank with a 31-32 salinity and pH 8.2-8.3 at 25-27 ℃. The tank was connected to a central circulation system for 1 week prior to the experiments for acclimatization. The system provided mechanical and biological f iltration, ultraviolet sterilization, and protein skimmer for the seahorse.
2.5 Tissue-specif ic expression analysis
To analyze the levels of interleukin gene transcription in various tissues under normal conditions, six male seahorses that were not injected were selected. Their tissues, including the liver, gill,kidney, intestine, muscle, gonad, and brood pouch,were collected in liquid nitrogen and stored at -80 ℃until RNA extraction.
2.6 Immune challenge
Fifty seahorses were randomly divided into three groups/tanks for further injection: control (16 seahorses) and two experimental groups (17 seahorses for each group). Control seahorses were injected with 100-μL phosphate buff er saline (PBS) into the sidewall tissue of brood pouch. Experimental seahorses were equally subdivided into LPS or bacterial challenge groups and injected with 100-μL LPS (2 μg/μL) (Sigma, St. Louis, MO, USA, 0111:B4) orV.parahaemolyticus(108CFU/mL) (Oh et al.,2017; Tharuka et al., 2019), respectively.
The f ish were euthanized prior to dissection by anesthetization with tricaine methanesulfonate (MS-222, Sigma, 20 mg/L) (Qin et al., 2016). The intact brood pouch, with both the outer epithelium and three inner layers (two dermis layers and the pseudoplacenta), was sampled at 6, 12, 24, and 48 h post-injection (hpi) from four individuals per time point per group.
2.7 RNA isolation, cDNA synthesis, and qRT-PCR assay
Total RNA was extracted from the tissues using TRIzol Reagent (Life Technologies, Carlsbad, CA,USA), and RNA quality was assessed by both agargel electrophoresis (three clear strips without degradation) and with a nucleic acid analyzer (A260/A280 ranged from 1.8 to 2.0). First-strand cDNA was synthesized using a Genome Erase cDNA Synthesis Kit with gDNA Eraser (TaKaRa, Shiga, Japan)following the manufacturer’s protocol, and 1-μg RNA was used for each reaction. A real-time quantitative reverse transcription polymerase chain reaction (qRTPCR) was performed with a Roche Light-Cycler 480 real-time PCR system (Basel, Switzerland) according to the manufacturer’s protocol.
The standard curve was constructed using a series of 10-fold dilutions of quantified pMD18-T vector(TaKaRa) with the target gene of interest. Two qRTPCR experiments (tissue-specif ic expression and injection) were conducted, and β-actin was used as the reference gene for each experiment because of its stable expression in diff erent seahorse tissues (Cq value ranges from 15-18). The sequences and locations of all primers are shown in Supplementary Table S2 and the amplif ication sequence of each pair of primers spans at least an intron-exon boundary to avoid the amplif ication of genomic DNA. No primers had non-specif ic amplif ication for the following three reasons: 1) There is only one specif ic peak in the melt curve; 2) one clear strip in the agar-gel electrophoresis;3) both no template control and no reverse transcriptase control were conducted per run with three replicates,and they both showed no specif ic amplif ication(Cq>35) (Bustin et al., 2009). The f inal sample for qRT-PCR (10 μL) contained 1 μL of cDNA template,5 μL of SYBR®Green Realtime PCR Master Mix(Roche), 0.4 μL of each primer (10 pmol/μL), and 3.6 μL of nuclease-free H2O. The qRT-PCR thermal prof ile comprised an initial cycle at 95 °C for 3 min,followed by 40 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 20 s.
The relative expression of interleukin mRNAs was calculated using the 2-ΔΔCtmethod (Livak and Schmittgen, 2001). For tissue-specif ic expression analysis, the liver tissue was used for normalization and for the immune challenge experiment, and the sample from the 6-h PBS-injected control was used to normalize the expression level. Three technical replicates were evaluated.
2.8 Statistical analysis
All data are presented as the mean relative mRNA expression ±SD and analyzed with SPSS software(version 22.0, SPSS, Inc., Chicago, IL, USA).Analysis of variance (one-way ANOVA) followed by Duncan's multiple range test was used to determine the statistical signif icance of mRNA expression in healthy and challenged tissues.
3 RESULT
3.1 Phylogenetic analysis of seven interleukin sequences in H. erectus
We identif ied 13 interleukin genes in theH.erectusgenome (Supplementary Table S1), seven of which were further conf irmed by phylogenetic analysis of the lined seahorse, tiger tail seahorse, manado pipef ish, and other close species. As shown in Fig.1,seven interleukin proteins of the lined seahorse were clustered with their respective homologs in other bony f ishes. A conserved phylogenetic relationship was identif ied within syngnathids: for each selected interleukin member,H.erectusshowed the closest relationship withH.comes, and the two seahorses were then clustered with the pipef ishM.manadensisand other tested teleosts (Fig.1). Similar results were obtained from the phylogenetic tree constructed using Bayesian method (Supplementary Fig.S2).
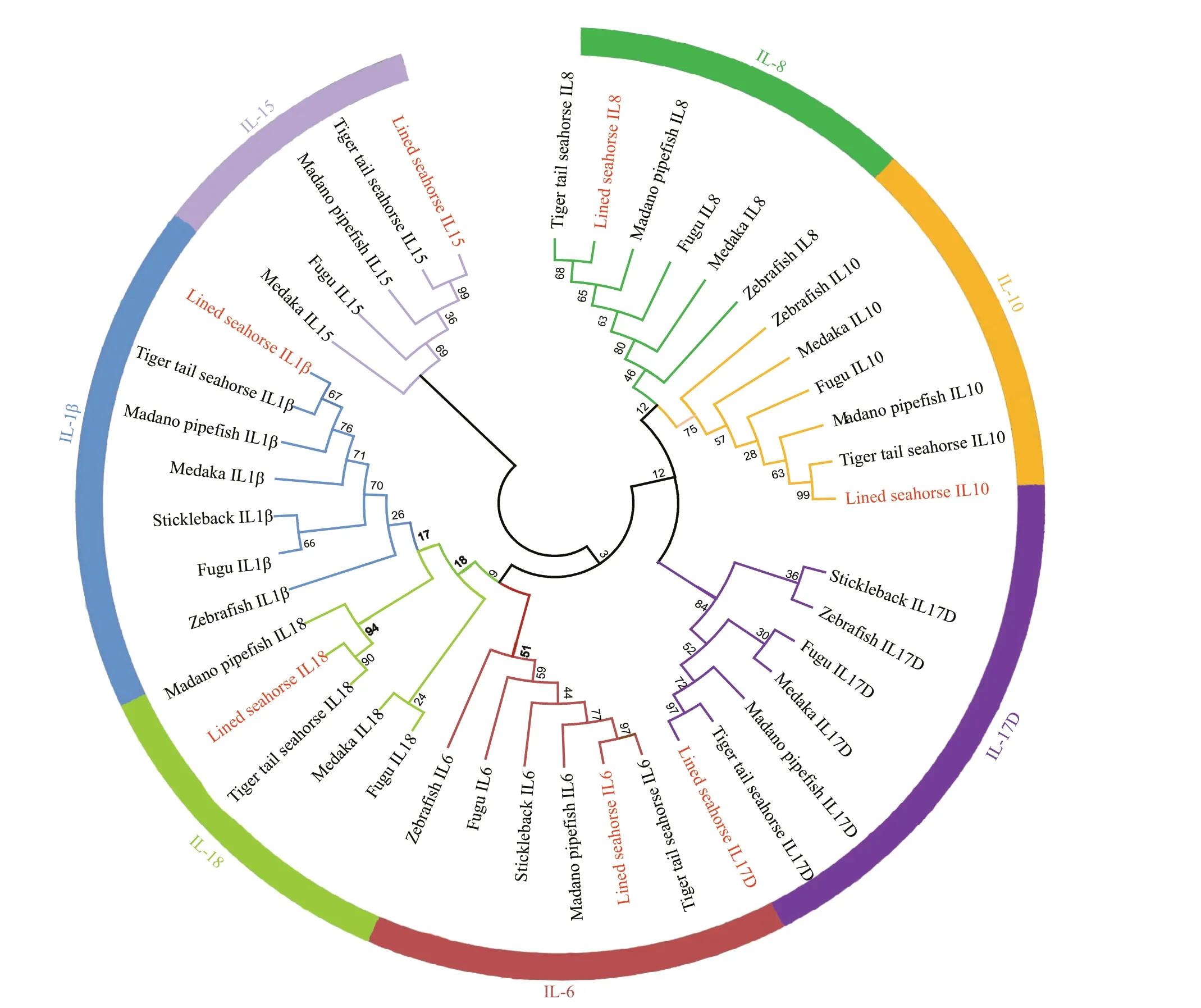
Fig.1 Phylogenetic relationships of IL-15, IL-1β, IL-18, IL-6, IL-17D, IL-10, and IL-8 in the lined seahorse and other selected teleosts
3.2 Expression analysis of interleukin genes in the brood pouch of H. erectus
The expression level of 13 interleukin genes was quantif ied in the seahorse brood pouches (Fig.2a).The maximum expression was observed foril-8and the minimum was observed foril-6, showing a diff erence of approximately 300-fold (Fig.2a). To further detect the expression of key interleukin genes in the brood pouch of seahorse, we conducted tissuespecif ic expression analysis of four genes (Fig.2b-e).First, we selected the top three genes showing the highest expression levels in seahorse brood pouch(il-8,il-15, andil-18) (Fig.2a). Second,il-1βwas chosen because it not only showed signif icant responses after LPS and bacterial challenge inCtenopharyngodonidella(Bo et al., 2015) andMicropterussalmoides(Ho et al., 2016), but also showed relatively high expression in the brood pouch of seahorse (Fig.2a). Our results showed thatil-8,il-15,il-18, andil-1βwere expressed at relatively high levels in the brood pouch;il-18in the brood pouch showed the highest expression level among all selected tissues (Fig.2b-e).
3.3 Immune responses of il- 1β, il- 8, and il- 18 in seahorse brood pouch
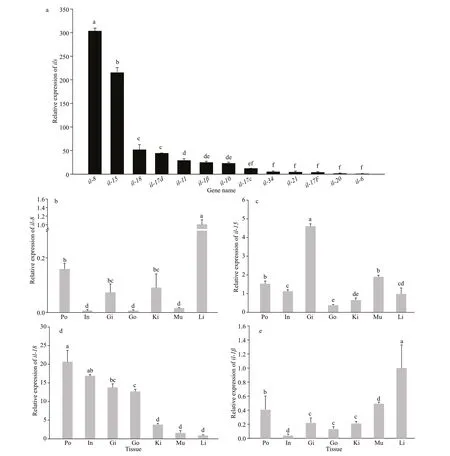
Fig.2 Interleukin gene expression patterns in diff erent tissues
We evaluated the potential immune responses of interleukins in the seahorse brood pouch after challenge with LPS andV.parahaemolyticus. In addition toil-15, signif icantly changes inil-1β,il-8,andil-18all were detected after injection withV.parahaemolyticusor LPS (Fig.3a-c and Supplementary Fig.S3).il-1βmRNA was signif icantly upregulated at 6 hpi and then gradually downregulated followingV.parahaemolyticuschallenge but there was no signif icant response to LPS stimulation(Fig.3a). Bothil-1βandil-18belong to the sameil-1subfamily but responded diff erently to exposure to LPS andV.parahaemolyticus. The expression ofil-18was unchanged within the f irst 24 hpi and then signif icantly upregulated at 48 hpi in response to LPS(P<0.05); however, no signif icant response toV.parahaemolyticuswas observed (Fig.3b).
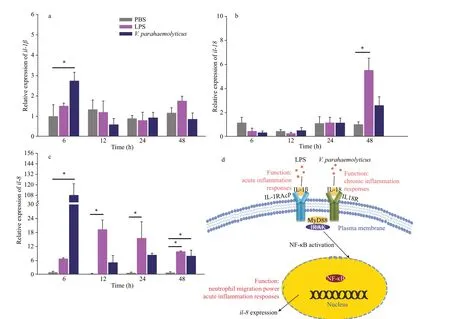
Fig.3 Expression analysis of il- 1β, il- 18, and il- 8 post-LPS or post- V. parahaemolyticus challenge in the lined seahorse brood pouch
Compared withil-1βandil-18,il-8showed remarkable responses following stimulation by LPS andV.parahaemolyticus.il-8transcripts were upregulated starting at 6-h post-LPS injection, reaching a maximum at 12 hpi (18-fold above the control;P<0.05). After 12 hpi,il-8expression decreased but remained signif ciantly higher than that in the control(P<0.05) until 48 hpi. In contrast,il-8transcripts were signif ciantly higher at 6-h postV.parahaemolyticusinjection than in the control (P<0.05). After 6 hpi,il-8expression decreased but remained higher than that in the control until 48 hpi (Fig.3c).
4 DISCUSSION
Interleukin genes play important roles in the innate immune response (Secombes et al., 2011; Zou and Secombes, 2016). In mammals and teleosts, research into the functional roles of interleukins has focused on immune organs, such as the blood and gills (Herath et al., 2016), but little is known about the seahorse brood pouch. In this study, we identif ied 13 interleukins in the lined seahorse and investigated the immune responses ofil-1β,il-18, andil-8following challenges with LPS andV.parahaemolyticusdirectly to the brood pouch.
The independent cluster pattern of each of the seven selected interleukin genes indicated by phylogenetic analysis conf irmed the accuracy of the identif ied interleukin sequences in the lined seahorse(Fig.1). Additionally, compared to the inconsistent tree topology of diff erent interleukin genes among other teleosts, species within syngnathids (H.comes,H.erectus, andM.manadensis) showed conserved phylogenetic relationships in all 7 selected interleukins. Discrepancies between trees of a single gene and species-level tree may result from incomplete lineage sorting (Nichols, 2001; Wang et al., 2018) and the conserved tree topology of interleukins within syngnathids and between syngnathids and other teleosts indicate the unique role of interleukins in syngnathids with a brood pouch.
il-8,il-18, andil-1βencode pro-inf lammatory cytokines that mediate immune responses and induce inf lammatory reactions to infections in mammals and teleosts (Biet et al., 2002; Bird et al., 2002; Li and Yao, 2013; Wang et al., 2016). Previous studies reported that these genes are highly expressed in typical immune tissues, such as the blood and gills, in zebraf ish (Liao et al., 2018) and large yellow croaker(Li et al., 2013). Our results showed thatil-8,il-15,il-18, andil-1βwere highly expressed in seahorse brood pouch (Fig.2a & b). As the brood pouch has important immune functions (Melamed et al., 2005;Whittington et al., 2015), high expression of theseilsin the brood pouch indicates their importance in the immune response of brood pouches against pathogens.Additionally,il-1βandil-8showed high expression levels in the liver (Fig.2b & e), indicating a role for these genes in regulating immune responses in this organ (Xiao et al., 2019).
Previous studies investigating the response to bacteria in other immune tissues (Zou et al., 2004; Xu et al., 2016; Wang et al., 2019) showed a trend toward a stronger, localized, pro-inf lammatory response at the site of infection or injury (Herath et al., 2016).il-1βandil-18belong to the il-1 subfamily which, in teleosts, mediates the host response to microbial invasion, inf lammation, and immune reactions (Akira,2000; Herath et al., 2016). Interestingly,il-1βandil-18responded to diff erent challenges(V.parahaemolyticusand LPS, respectively) in the brood pouch of seahorse. LPS is a main component of the cell walls in gram-negative bacteria, comprising lipid A, core polysaccharides, andO-specif ic chains(Wang and Quinn, 2010). Previous studies showed that lipid A is responsible for the major bioactivity of endotoxins (Wang and Quinn, 2010), In addition to LPS, the outer membrane protein ofHelicobacterpylori(a gram-negative bacterium) can also induce characteristic inf lammatory responses (Sugimoto et al., 2009). The diff erent sensitivities ofil-18andil-1βpost-LPS and post-V.parahaemolyticuschallenge may have resulted from their protective functions against diff erent bacterial components. For example,il-18may only act on lipid A of LPS, whereasil-1βmay have to be induced by the whole cell wall ofV.parahaemolyticus, including LPS and outer membrane protein. A similar tendency was found in mammals, whereas the outer membrane protein ofH.pylori(a gram-negative bacterium) induced characteristic inf lammatory responses (Sugimoto et al., 2009). In humans, lipid A compounds can strongly induceIL-18overIL-1B(Shimoyama et al., 2011). In addition, the bacterial outer membrane protein (OipA)plays an important role in the expression ofIl-1b,Il-17, andTnf-a in mice (Sugimoto et al., 2009).
Il-1βandIl-18were previously shown to be involved in acute and chronic inf lammatory responses in mammals, respectively (Shimoyama et al., 2011).For example,Il-1βmRNA was signif icantly upregulated at 3 h in blood cells and at 6 h in the spleen afterStreptococcusiniaechallenge (Herath et al., 2016). Our data showed thatil-1βmRNA was increased at 6 h followingV.parahaemolyticusinfection in the brood pouch, whereas theil-18mRNA response was slower and was increased at 48 h in response to LPS stimulation, supporting the results of previous studies. Unlikeil-1βandil-18, the expression ofil-8mRNA, a chemokine gene, rapidly increased at 6 h and was sustained from 6 to 48 h following LPS andV.parahaemolyticusstimulation (Fig.3c). This suggests its involvement in a powerful, acute,inf lammatory reaction, which is consistent with previous reports of chemokines. InHippocampusabdominalis, mRNA expression of the CXC chemokine gene (ShCXCL) was upregulated in the blood and kidney tissues after immune stimulation by live bacteria such asS.iniaeandEdwardsiellatarda(Oh et al., 2017). Additionally, rapid upregulation ofil-8expression was observed in the immune tissues inL.croceaafterV.parahaemolyticusinjection (Li and Yao, 2013; Wang et al., 2019) and LPS stimulation(Li and Yao, 2013). In addition, a previous report in mammals illustrated that IL-18 and IL-1β can stimulate IL-8 production via the NF-κB signaling pathway (Biet et al., 2002; Geisert et al., 2012; Herath et al., 2016). NF-κB family transcription factors have been shown to regulate tissue immune function and inf lammatory responses in humans (Ghosh and Hayden, 2008; Hayden and Ghosh, 2011). In addition to direct bacterial stimulation, our result may indicate that upregulatedil-8mRNA followedil-1βandil-18induction by activating the NF-κB signaling pathway(Fig.3d, Biet et al., 2002; Geisert et al., 2012; Herath et al., 2016), further indicating thatil-8plays an important role in the antibacterial immunity of the seahorse brood pouch.
5 CONCLUSION
In conclusion, we demonstrated thatil-1β,il-18,andil-8play key roles in the antibacterial immune defense of the brood pouch in the lined seahorse(H.erectus).il-1βandil-18responded toV.parahaemolyticusand LPS, and they could induce acute and chronic inf lammatory responses,respectively.il-8may be involved in a more powerful antibacterial immune responses and may itself be induced by IL-1β and IL-18, involving the NF-κB signaling pathways. These f indings provide valuable information that will be helpful for further analyzing antibacterial immune and initial inf lammatory responses.
6 DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the f indings of this study are available within the article and its supplementary f iles.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
