Low genetic diversity in the endangered marine alga Silvetia siliquosa (Ochrophyta: Fucaceae) and the implication to conservation*
Yanshuo LIANG , Jie ZHANG , Xiaohan SONG , Han-Gil CHOI , Xu GAO ,Delin DUAN ,**, Zi-min HU
1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 University of Chinese Academy Sciences, Beijing 100049, China
4 Faculty of Biological Science and Institute for Environmental Science, Wonkwang University, Iksan 54538, Korea
5 Ocean School, Yantai University, Yantai 264005, China
Abstract Although signif icant research eff orts have been targeted toward conservation and management of endangered terrestrial f lora and fauna, attempts have been limited to conserve threatened seaweeds.Silvetia siliquosa is an ecologically and commercially vital brown alga that is uniquely distributed in the Yellow-Bohai Sea and along the southwest coast of Korea. A massive decline in its distribution range and biomass from the mid-1990s onward indicates that this species has become endangered. In the present study, we used nuclear internal transcribed spacer and concatenated mitochondrial cytochrome oxidase I subunit+intergenic spacer to estimate the genetic diversity, population connectivity, and degree of genetic diff erentiation of S. siliquosa in China and Korea. The molecular results exhibited strikingly low levels of haplotype/ribotype and nucleotide diversity in S. siliquosa populations, with only three mitochondrial haplotypes and nuclear ribotypes detected in 136 and 143 specimens, respectively. The analysis of molecular variance revealed 85%-95% of genetic variance among populations. Population diff erentiation coeffi cient( F ST) and gene f low ( N m) suggested that two populations (JIN and GWA) along the southern coast of Korea are highly divergent from the others, with weak genetic exchange. No signif icant genetic diff erentiation was observed among populations either in China or along the geographically proximate west coast of Korea.Thus, four independent management units were designated for sustainable management: the LII and RUS populations in China, the YEO and CHA populations along the west coast of Korea, and each of the GWA and JIN populations along the south coast of Korea. We suggest that artif icial cultivation and transplantation of S. siliquosa are the eff ective approaches for restoration and conservation.
Keyword: biodiversity conservation; endangered seaweed; genetic diversity; habitat loss; Silvetia siliquosa
1 INTRODUCTION
Delineation of genetic diversity of endangered species has become a primary task for conservation biologists due to increasing global environmental change and anthropogenic impacts (Crandall et al.,2000; Spielman et al., 2004). Although numerous studies have been conducted on endangered terrestrial f lora and fauna, research on the conservation and management of endangered seaweeds is still limited(Brodie et al., 2009; Couceiro et al., 2011). A major problem in addressing this paucity of literature is the def iciency of data on taxonomy, genetics, and phylogeography (Brodie et al., 2009). Conservation genetics provides vital theoretical and practical guidelines to maintain population sustainability and avoid endangered species from becoming extinct(Fagan and Holmes, 2006), as exemplif ied in the endangered red seaweedAhnfeltiopsispusilla(Couceiro et al., 2011). Maintaining a high level of genetic diversity is crucial for the sustainability of viable populations (Reed and Frankham, 2003),including the identif ication of evolutionarily signif icant units (ESUs) (Li and Ge, 2002). However, ESUs are sometimes diffi cult to manage because of their large geographical distribution (Li et al., 2020). The concept of management units (MUs) is to determine the appropriate population unit for the suffi cient targeting of management and monitoring programs toward independent populations (Moritz, 1994).
The brown algaSilvetiasiliquosa(Tseng et Chang Serrão, Cho, Boo and Brawly) is a perennial and functionally constructive species in the intertidal ecosystem (Mineur et al., 2015). This species was previously known asPelvetiasiliquosaand was transferred into a new genus,Silvetia, after phylogenetic reassessment (Serrão et al., 1999). The historical distribution range ofS.siliquosais restricted to the Yellow-Bohai Sea (Tseng and Chang, 1953,1958) and the south and west coasts of Korea (Yoo and Kim, 2003; Oh et al., 2005; Park et al., 2007; Song et al., 2011; Hwang et al., 2015; Han et al., 2016).Interestingly, theS.siliquosamorphology is greatly aff ected by its dwelling environment; it exhibits more branches and larger fronds in a wave-sheltered habitat than in a wave-exposed habitat (Tseng and Chang,1953). The genusSilvetiais monoecious. The species of this genus exhibit only sporophytic generation in their life history without an independent gametophytic stage, and the gametophytic generation is replaced by the conceptacles, antheridium, and oogoniums (Cho et al., 2001). From early October to late November when seawater temperature ranges from 14 °C to 18 °C,S.siliquosanaturally undergoes spermatozoids and releases eggs, which develop into juvenile sporophytes after in vitro fertilization (Huang et al., 2008).Although preliminary research has investigated the biological characteristics ofS.siliquosa, its population genetic connectivity and genetic structure remain poorly understood.
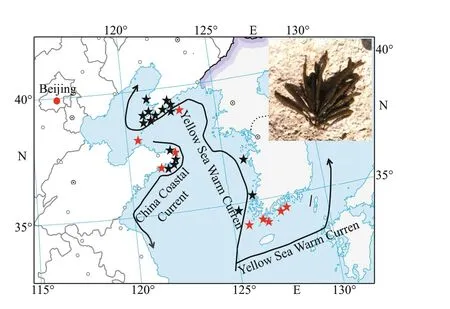
Fig.1 The main current systems in the Yellow-Bohai Sea and adjacent areas
Population biomass and distribution range ofS.siliquosain East Asia have declined dramatically since the 1990s due to habitat fragmentation and anthropogenic impacts (Fig.1; Tseng and Chang, 1958;Baek et al., 2007). Thus, this species has been listed as endangered in the ecological region of Yellow-Bohai Sea, with a high extinction risk (Kim et al., 2012). The market price ofS.siliquosaas a commercial seaweed is approximately 10 USD/kg wet weight, which is much higher than those of other edible seaweeds such asSaccharinajaponicaandUndariapinnatif ida(Gao et al., 2017). Therefore, overf ishing is a crucial reason for the rapid decline of population resources (Hwang et al.,2015). Additionally, temperature is a key factor that restricts the growth and development ofS.siliquosa.The temperature in its growth habitat generally does not exceed 25 °C in summer (Tseng and Chang, 1958).When temperature is between 21 °C and 23 °C from early August to September,S.siliquosareleases spermatozoids and eggs from the conceptacles;however, they do not survive (Huang et al., 2008).After October, when the water temperature drops to 18.4 °C, some fertilized eggs attach, germinate, and grow well (Huang et al., 2008). Global warming is estimated to be approximately 1.0 °C (±0.2 °C) above pre-industrial levels, and the warming level is likely to reach 1.5 °C between the year 2030 and 2052 if it continues to increase at the current rate(Intergovernmental Panel on Climate Change, 2018).Therefore, the rising seawater temperature may exert a disastrous eff ect on the growth, development, and geographical distribution ofS.siliquosa. The restoration and protection ofS.siliquosaare an imperative task due to the severe diversity loss and range contraction.
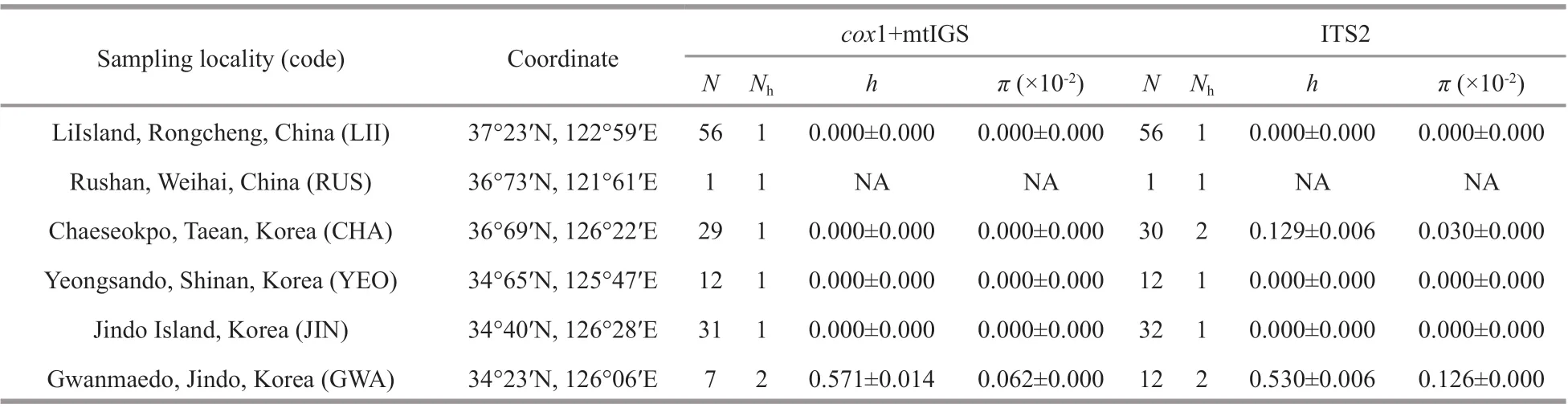
Table 1 Genetic diversity indices of S. siliquosa populations in China and Korea inferred from mitochondrial cox 1+IGS and nuclear ITS2
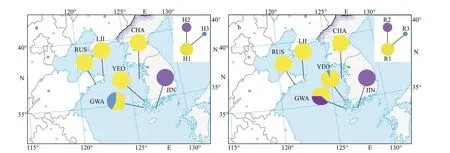
Fig.2 Geographical distribution of cox 1+mtIGS haplotypes (a) and ITS2 ribotypes (b) of S. siliquosa in China and Korea
In the present study, we obtained partial DNA sequences of three gene markers [mitochondrial cytochrome oxidase I subunit (cox1) + intergenic spacer (IGS) and ribosomal internal transcribed spacer II (ITS2)] ofS.siliquosafrom China and Korea. The present study had the following main goals: (i) to describe population genetic diversity and diff erentiation and identify MUs, (ii) to reveal population genetic connectivity in the Yellow-Bohai Sea by estimating gene f low. Our f indings may provide the basic guidelines for the government and policy-makers to formulate conservation and management plans forS.siliquosain East Asia.
2 MATERIAL AND METHOD
2.1 Sampling, DNA extraction, polymerase chain reaction and sequencing
Historically,S.siliquosawas widely distributed along the Shandong and Liaodong peninsulas of China and the western and southern coasts of Korea.However, its distribution range continuously declined from the mid-1990s onward (Song et al., 1996; Lee et al., 1997; Baek et al., 2007). From 2014 to 2018, we collectedS.siliquosaspecimens (n=145) from the Shandong peninsula and the southwest coast of Korea(Table 1 & Fig.2). We randomly collected ≥12 individuals form localities where the specimens were commonly distributed and easily accessible, with an interval transect >10 m. We only found a small patch of biomass (ca 40 cm×30 cm) in Rushan, China. Thus,only one individual was collected. A 3-5-cm tip of apical tissue was excised from each individual collected from all the localities and stored in silica gel for molecular analysis.
Total genomic DNA was extracted using a Fast Pure Plant DNA Isolation Mini Kit (Vazyme Biotech Co., Ltd., Nanjing, China). Mitochondrialcox1,23S/trnK IGS (mtIGS), and nuclear ITS2 were chosen based on previous phylogenetic and phylogeographical studies of the order Fucales (Hu et al., 2007; Li et al.,2017a, b). Three primer sets (Supplementary Table S1) were developed based on the available sequences ofS.siliquosa(GenBank accession number:AF102960.1), the congenericSilvetiacompressa(HQ990495.1), andFucusvesiculosus(AY494079.1)by using the Primer-BLAST Program in the National Center for Biotechnology Information database. PCR amplif ication was performed in 50 μL of reaction volume, containing 25 μL of 2×Taqplus Master Mix II (Vazyme Biotech Co., Ltd., Nanjing, China), 2 μL of forward primer (10 μmol/L), 2 μL of reverse primer(10 μmol/L), 1 μL of template DNA, and 20 μL of ddH2O. The thermo-cycling parameters were set according to the instructions of 2×TaqPlus Master Mix II, and the annealing temperatures were set at 58 °C forcox1, 55 °C for mtIGS, and 60 °C for ITS2.Electrophoresis, purif ication, and sequencing were conducted following the available protocols (Hu et al., 2007, 2011). Sequences were aligned and manually edited using Bioedit v7.2.5 (Hall, 1999) and MEGA v7.0 (Kumar et al., 2016).
2.2 Population genetic diversity and diff erentiation
Nuclear ribosomal ITS2 and concatenated mitochondrialcox1+IGS were used separately for the following analysis. Population genetic diversity was estimated using DnaSP v6 (Rozas et al., 2017) by assessing the number of haplotypes (Nh), haplotype diversity (h), nucleotide diversity (π), and the number of polymorphic sites (S). Pairwise population genetic diff erentiations were estimated by computingFSTby using ARLEQUIN v3.5 (Excoffi er and Lischer, 2010).The signif icance of covariance components for all results were tested using 104permutations. Analysis of molecular variance (AMOVA) was computed in ARLEQUIN to partition genetic diversity among and within populations, and the signif icance was assessed through 104random permutations. To assess the evolutionary relationships among mitochondrial haplotypes or ribosomal ribotypes, parsimony median-joining networks were generated using Network v10 (Bandelt et al., 1999).
2.3 Population connectivity
Gene f low is a vital quantitative indicator of interpopulation connectivity that facilitates the conservation and management of endangered species.We used DnaSP v6 (Rozas et al., 2017) to evaluate gene f low between populations according to the methods described by Hudson et al. (1992) and assessed whether population connectivity is aff ected by surface currents in the Yellow-Bohai Sea. In the method, each polymorphic site was considered a separate locus and theFSTfrom the frequencies of alleles at each locus in diff erent geographic locations was f igured out. According toFST, the gene f lowNmwas measured by using the formulaNm=(1-FST)/NFST,whereNmdenotes the number of migrants per generation andFSTis the population f ixation index,N=2 or 4 for mtDNA or nuclear DNA, respectively(Hudson et al., 1992). IfNm>1, gene f low can resist the eff ect of genetic drift and prevent population diff erentiation, whereas ifNm<1, genetic drift will become the dominant factor for divided population genetic structure (Slatkin, 1985).
3 RESULT
3.1 Molecular diversity
Concatenated mitochondrialcox1+IGS fragments were obtained for 136 individuals with an aligned length of 921 bp, including 525 bp ofcox1 and 396 bp of mtIGS. The concatenated dataset contained two parsimony informative sites and yielded three haplotypes. Of these haplotypes, H1 was the most abundant and shared by 102 specimens, which accounted for 75% of all individuals (Table 1 &Fig.2), whereas H2 and H3 were site-specif ic. The three haplotypes were unevenly distributed among the sampling sites. H1 dominated along the Shandong peninsula and the west coast of Korea, whereas H2 dominated in the populations of Jindo Island, Korea(JIN). The population of Gwanmaedo, Korea (GWA),was characterized by the richest haplotype (h=0.571±0.014) and nucleotide diversity (π=0.062±0.000;Table 1).
We obtained 143 sequences, with an aligned length of 427 bp containing two parsimony informative sites, which yielded 3 ribotypes for ITS2. Of these ribotypes, R1 was shared among the f ive locations(except for Jindo Island), whereas R2 and R3 were site-specif ic and mainly distributed along the south coast of Korea (Table 1 & Fig.2). Similarly, the populations from Gwanmaedo, Korea, harbored the highest ribotype (h=0.530±0.006) and nucleotide diversity (π=0.126±0.000), whereas other populations exhibited low or no genetic diversity.
3.2 Population genetic structure and diff erentiation
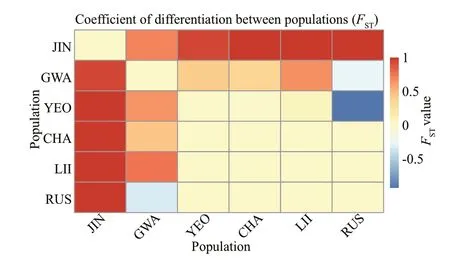
Fig.3 TheaverageFSTmatrixestimatedfromconcatenated cox 1+mtIGS(lowerleft) and ITS2 (upperright)
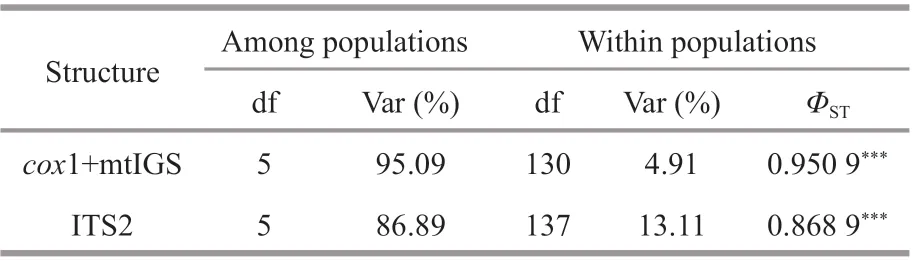
Table 2 Hierarchical analysis of molecular variance to partition genetic variance in S. siliquosa populations based on cox 1+mtIGS and ITS2
Haplotype network indicated no genetic structure in sixS.siliquosapopulations (Fig.2). Concatenatedcox1+mtIGS indicated that the populations JIN and GWA signif icantly diverged from others, withFSTvalues ranging from 0.444 to 1.000 (Fig.3;Supplementary Table S2). Despite relatively short geographical distance between JIN and GWA, theFSTvalue between them was as high (0.925). No genetic diff erentiation (FST=0.000) was observed between the populations from China (LII and RUS) and the west coast of Korea (YEO and CHA). ITS2 revealed a similar pairwiseFSTpattern among populations, with the populations from the southern coast of Korea (JIN and GWA) highly diverged from others (FST=0.364-1.000). Moderate divergence (FST=0.069) was detected between the LII and YEO populations;however, this diff erence was statistically nonsignif icant (P=0.121).
We def ined the six populations as one group to partition genetic variance between and within populations. AMOVA results based oncox1+mtIGS indicated that most of the genetic variance (95.09%)occurred among populations, whereas only 4.91%occurred within populations (ΦST=0.950 9,P<0.000 1;Table 2). Similarly, ITS2 exhibited that genetic variation among populations accounted for 86.89% of the total variance (ΦST=0.868 9,P<0.000 1; Table 2).
3.3 Gene f low
Gene f low based on mitochondrial and nuclear markers yielded similar results. We observed a weak gene f low (Nm) between the GWA population in Korea and others (cox1+mtIGS: 0.041-0.626; ITS2: 0.105-0.437; Table 3). Similarly, either none or weak gene f low was observed between the JIN and other populations (cox1+mtIGS: 0.000-0.041; ITS2:0.000-0.105; Table 3). Although ITS2 revealed a high gene f low (Nm=3.373) between the LII and YEOpopulations, the result was statistically nonsignif icant. Moreover, no gene f low was observed among the three populations (YEO, CHA, and LII)based on both mitochondrial and nuclear datasets.Finally, we observed that the gene f low between the YEO and the CHA population was negative (Nm=-22.98). If the sum of diff erences among sequences from diff erent populations is less than the sum of diff erences among sequences within a single population, it will result inNm<0 (Hudson et al.,1992). When the inter-population level ofNmis less than 0, it can be assumed that no gene exchange occurs between populations (Hudson et al., 1992).
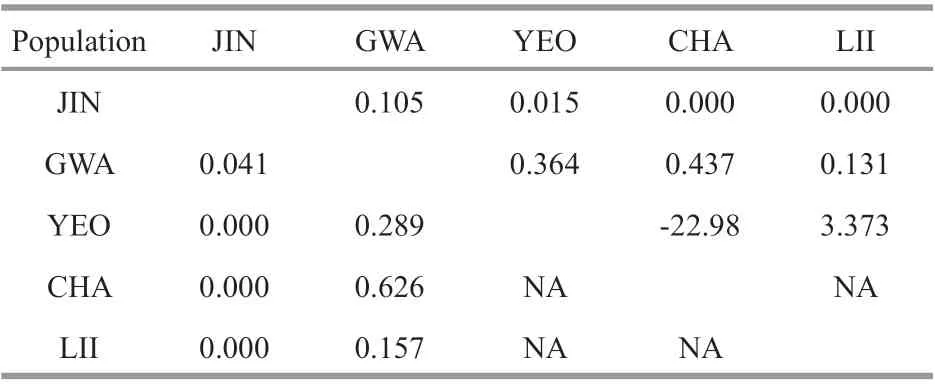
Table 3 Gene f low ( N m) among S. siliquosa populations based on concatenated cox 1+mtIGS (lower left)and ITS2 (upper right) data set
4 DISCUSSION
In the present study, mitochondrialcox1+IGS and nuclear ITS2 consistently revealed a strikingly low population genetic diversity of the endangeredS.siliquosain East Asia, indicating a high risk of extinction. Based on population-level connectivity and genetic diff erentiation, we designated four MUs in East Asia for better conservation and restoration of naturalS.siliquosaresources.
4.1 Low genetic diversity and range contraction
A high level of genetic diversity is essential for maintaining the evolutionary potential and viability of species. However, mitochondrialcox1+IGS and nuclear ribosomal ITS2 detected few genotypes,indicating that genetic variation in theS.siliquosapopulations from China and Korea is strikingly low.Most threatened populations have lower average heterozygosity, evolutionary potential, and reproductive f itness than those of their non-threatened counterparts (Spielman et al., 2004). Thus, the low genetic diversity inS.siliquosamay aggravate the decline of species f itness and adaptability, eventually leading to the decrease in reproduction and increase in mortality. This pattern of no or low local genetic diversity has also been reported in other endangered algae such asAegagropilalinnaei(Soejima et al.,2009) andAhnfeltiopsispusilla(Couceiro et al.,2011). The endangeredS.siliquosapopulations are likely to become extinct because the assessment of some known extinct cases suggests that species extinctions can be preceded by a complete loss of genetic diversity (Johnson and Dunn, 2006).
Several reasons may account for the low genetic diversity and distributional contraction ofS.siliquosa.Ocean currents and inappropriate environmental conditions are crucial in reducing genetic diversity and distribution range of species. According to the hypothesis of Tseng and Chang (1958), the historical distribution ofS.siliquosawas shaped by mainly oceanic currents in the Yellow-Bohai Sea. Ocean currents can produce highly homogeneous algal genetic lineage in a wide space as a medium for gene migration and exchange (Muñiz-Salazar et al, 2005;Mitarai et al, 2009; Li et al, 2017b). In this case, we detected no clear genetic divergence among populations in both China (LII and RUS) and along the west coast of Korea (CHA and YEO, Fig.3;Supplementary Table S2), indicating that the genetic structure of theS.siliquosapopulation is inf luenced by ocean currents and leads to a high degree of genetic homogeneity. On the other hand, the two geographically proximate populations (JIN and GWA) from Jindo, Korea highly diverged from others with a weak genetic exchange (Fig.2; Table 3),indicating that the two isolated populations may be subject to the decrease in genetic diversity (Woff ord et al., 2005; Riley et al., 2006). Unsuitable environmental conditions, such as storms, aff ect the zygote attachment and the growth and development of juvenile thalli.S.siliquosagenerally germinates in a wave-sheltered habitat, where it grows abundantly and the individual is large. On the other hand,S.siliquosathat grows on exposed rocks susceptible to wind and waves has fewer branches and smaller fronds (Tseng and Chang, 1958).S.siliquosacultures in laboratory indicate that the zygotes should adhere to the substrate after fertilization because the attachments of the zygotes are not f irm and it must be cultured in still water (Li et al., 2007). If it fails to adhere to the substrate, the fertilized eggs will wither up and discolor, and eventually fail to develop into young thalli (Yoon et al., 2003). Therefore, storm might interfere with the growth and development ofS.siliquosa. Alternatively, seawater temperature can not only aff ect the release and survival ofS.siliquosasperm and egg (Huang et al., 2008), but also aff ect its growth, development, and recruitment (Hwang et al.,2015; Gao et al., 2017). TheS.siliquosareceptacles develop and mature from March to August when seawater temperatures range from 8 °C to 26 °C(Hwang et al., 2015), and the most suitable water temperature for the release of sperm and egg is below 18.4 °C every year (Huang et al., 2008). However, the sea surface temperature (SST) in the Yellow-Bohai Sea has increased by 0.67-0.89 °C from 1982 to 2006(Belkin, 2009). This increase in the SST may be partly responsible for the reduction in the geographical range ofS.siliquosain East Asia and hence the loss of genetic diversity. High-temperature stress resulted in the loss of genetic diversity and adaptability ofFucusserratus(Jueterbock et al., 2014), a brown alga closely related toS.siliquosa. Thus, the low level of genetic diversity further reduces individual f itness and population adaptability (Kaljund and Jaaska,2010), which eventually results in small populations.Thus, future work should concentrate on the eff ect of increased temperature and irradiation on the fertilization, settlement, growth, and reproduction ofS.siliquosa. Furthermore,S.siliquosais commonly used as food and possesses medicinal value in China(Lee et al., 2003; Kang et al., 2005; Spavieri et al.,2010), and the current market price far exceeds other brown algae. Consequently, commercial interest results in overf ishing of naturalS.siliquosaresources,which accelerates the decline in its biomass and hence causes its extinction, which renders the species endangered with low genetic variation.Gadusmorhus(Reynolds et al., 2005) andAegagropilalinnaei(Soejima et al., 2009) are two comparable cases in which overf ishing made them endangered.
4.2 Population connectivity and the conservation of S. siliquosa
Understanding connectivity is the key to manage and conserve marine ecosystems (Bradbury et al.,2008). MUs provide a solution for independent populations aff ected by human activities (Palsbøll et al., 2007), particularly for the short-term management and monitoring of natural populations (Schwartz et al., 2007). If the degree of population connectivity is suffi ciently low, each population should be monitored and managed separately (Taylor and Dizon, 1999).Alternatively, MUs can be assigned when the observed genetic divergence is signif icantly greater than a predef ined threshold value (Palsbøll et al., 2007). In the present study, JIN and GWA from Jindo, Korea,exhibited higher levels of genetic diff erentiation than other populations, indicating a poor gene f low and low connectivity (Nm=0.041-0.105,FST=0.705-0.925,Table 3; Supplementary Table S1). Thus, these two populations (JIN and GWA) can be designated as two independent MUs. Additionally, a considerable geographical distance is present from the west coast of Korea to the Shandong peninsula. Absence of a signif icant genetic diff erentiation and weak gene f low imply that both the LII and RUS populations in China and the YEO and CHA populations along the west coast of Korea should be designated individually as a single MU.
The delineation of the underlying genetic data of the MUs provides a theoretical basis for the monitoring and management of specif ic target species and relevant conservation objectives. However, the strikingly low genetic variation and weak population connectivity in East Asia may lead to a low evolutionary potential ofS.siliquosa. The selfsustainment of future populations may not be suffi cient to support long-term survival, which necessitates human intervention to increase its abundance and distribution range. Transplantation provides technical support for increasing natural populations and restoring the degradation of natural habitats. The extensive transplantation experiments with brown seaweeds such asFucusgardneri,Macrocystispyrifera, andCystoseiracompressa(Stekoll and Deysher 1996; Hernández-Carmona et al., 2000; Susini et al., 2007) have been undertaken successfully in the f ield to restore natural resources.Because theS.siliquosapopulation in Gwanmaedo,Korea, is characterized by the highest genetic diversity (h=0.571±0.014;π=0.062±0.000; Table 1),seedlings can be harvested in the region and transplanted to other regions after artif icial incubation. The optimum time for seedling collection is between early October and late November each year when the SST drops to less than 18.4 °C, and it can be cultured in still water and natural light after zygote formation for better adhesion to the glass or other substrates (Li et al., 2007; Huang et al., 2008).Additionally, the optimum salinity for culturing sporophytes is 18 g/L. The rhizoid hairs that are conducive to attachment develop abundantly, and the young sporophytes grow well under these conditions(Huang et al., 2008). A study indicates that the transplants developed using polyethylene ropes grow and mature well in new environments, which suggests that population restoration ofS.siliquosamay be achieved through polyethylene rope transplantation(Gao et al., 2017). However, the growth ofS.siliquosapopulations may be hindered due to competition and grazing (Gao et al., 2017). In addition to preserving the regions with high genetic diversity, other unique populations or regions should receive special attention. For example, we only found one small patch ofS.siliquosain Rushan, Weihai. Thus, the ex situ conservation may sustain survival ofS.siliquosa.Future studies are required to better understand the eff ect of environmental factors on survival and recruitment ofS.siliquosaafter the native reciprocal transplantation.
5 CONCLUSION
The present study reveals a strikingly low population genetic diversity of the endangeredS.siliquosain East Asia, with a high risk of extinction.In view of the scenario of diversity loss and habitat fragmentation, our work provides preliminary insights into diversity conservation and population restoration.Based on the degree of genetic diff erentiation,population connectivity, and geographical distance between China and Korea, we propose that four independent MUs can be designated for the management and protection ofS.siliquosain East Asia. Artif icial transplantation may be a feasible step for sustaining manageable resources and functions of seaweeds. To maximize the effi cacy of conservation,future research should link the genetic information to the transplantation and restoration of outdoorS.siliquosaresources.
6 DATA AVAILABILITY STATEMENT
The datasets of sequences analyzed in this study can be found in the GenBank (cox1: MW093753-MW093890; mtIGS: MW093891-MW094035; ITS2:MW082642-MW082784).
7 ACKNOWLEDGMENT
We thank Drs. Zhongmin SUN and Jing WANG for providing assistance during f ield collection and specimens’ preservation in Korea.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
