PSI-driven cyclic electron f low partially alleviates the peroxidation of red alga Gelidium amansii (Gelidiaceae)caused by temporary high temperature*
Yongfu LI , Jianguo LIU ,**, Litao ZHANG
1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Marine Biology and Biotechnology Laboratory, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
Abstract PSI-driven cyclic electron f low (CEF-I) helps higher plants avoid severe heat damage.Gelidium amansii, a red seaweed used in the production of agar, inhabits subtidal rocks but can be found in the intertidal zone. The biological role of CEF-I is still unclear in this organism. Wild G. amansii was exposed to 30 °C heat stress for 12 h with continuous lighting. The results showed that treatment at 30 °C gradually decreased maximal PSII photochemical effi ciency ( F v/ F m), linear electron transfer rate, and activity of photosynthetic reaction center. Both the maximal photochemical effi ciency under light ( F v′/ F m′)and maximum quantum yield of light-adapted PSII ( Φ PSII) were maintained at a relatively stable level during the initial 6 h and then signif icantly decreased at 12 h. The up-regulated CEF-I helps to enhance proton gradient transfer across thylakoid membrane to protect oxygen-evolving complex against heat damage.Following the addition of a CEF-I inhibitor to plants, the F v/ F m greatly decreased, suggesting that the CEF-I alleviates degree of photoinhibition caused by strong light. The results of measurement of antioxidant enzymes, including superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT), and the contents of H 2 O 2 and malonaldehyde (MDA) provided additional evidence that CEF-I plays a protective role to a certain extent for G. amansii to manage stress at 30 °C. Therefore, it can be concluded that CEF-I enables G. amansii to survive in intertidal zones by protecting it from the heat damage caused by high temperature stress.
Keyword: cyclic electron f low; Gelidium amansii; heat stress; chlorophyll f luorescence; quantum yield;natural distribution
1 INTRODUCTION
Gelidiumamansii, a highly used commercial red seaweed that produces the f inest quality agar, is widely distributed along the east coast of China(Huang, 2010). To date, the large-scale cultivation of this alga has not been successful, despite the many eff orts to cultivate it in a variety of places (Li et al.,2019). The supply ofGelidiumresources can only be collected from wild habitats, resulting in a signif icant decline of the wild stocks.
The zonation pattern of seaweed has been proven to be related to their physiological responses to environmental factors, including nutrient level,temperature, light intensity, and desiccation(Zaneveld, 1969; Davison and Pearson, 1996).Typically,G.amansiiis considered to be a subtidal,perennial red seaweed that primarily grows on calcareous substrate in subtidal waters (Lee and Shiu,2009; Xie and Sun, 2018). Our surveys along the Jiaozhou Bay coast during 2016-2018 showed thatG.amansiicould also survive in the tidal pools that were 0.3-m deep where the water temperature can reach as high as 30 °C during low tides in summer (Li et al., 2019). During the survey, samples of macroalgae were collected for identif ication in the supratidal zone, intertidal zone, and subtidal zone with water depth <1 m at the sampling section at the lowest tide of each season. The tidal pools are about 2-5 m² in size, and are not aff ected by tide (i.e. the whole pond is exposed to the air) during the 2 h of the low tide.We sought to understand the mechanism that ensures survival ofG.amansiiin these sites. The performance traits and tolerance of algae under diff erent temperatures are two principal aspects determining the natural distribution and cultivation prospects of seaweed, especially the economic ones (Eggert,2012). Understanding the responses and possible mechanisms for acclimation to low or high temperature is crucial for a sustainable seaweed cultivation industry, e.g.,Kappaphycus(Li et al., 2016a, b;Kumar et al., 2020) andGracilaria(Yang et al.,2015). The inf luence of temperature on the algae is time dependent, i.e. the rules of short-term physiological regulation and long-term acclimation or adaptation of algae is often diff erent (Eggert, 2012). It involves whether the damage caused by temperature is recoverable (Sampath-Wiley et al., 2008; Blouin et al., 2011). For high temperature, thermal stress may not be enough to cause harm to organisms in a very short time scale, but long-term eff ects irreversibly reduce growth. Generally, temperature optima of photosynthesis are situated well above the temperature optima of growth (Eggert and Wiencke, 2000). This shows that temperature eff ects on a specif ic physiological process (i.e., photosynthesis in this case) do not necessarily correspond to the temperaturegrowth pattern as growth integrates the eff ect of temperature on the total metabolism. However, there is also useful information to be gained by investigating the photosynthetic response to temperature.Photosynthesis is the most temperature-sensitive process in plants and growth temperature is a key factor as it determines the CO2f ixation capacity as well as the activity of photosynthetic apparatus(Sharkey and Schrader, 2006; Mathur et al., 2014;Brestic et al., 2018). More conveniently, the photoreaction activity can be detected non-invasively by chlorophyll f luorescence probe, as many researches adopted nowadays (Alemu, 2020).
In our previous study, we also reported that the optimal temperature for photosynthetic oxygen evolution ofG.amansiiranged from 22 °C to 26 °C,which is consistent with the previous results obtained based on growth performance (Xie and Sun, 2018).Exposure to 30 °C induced signif icant losses of chlorophyllaand carotenoids and an increased phycobiliproteins content. The carbon assimilation process, light harvesting pigments, PSII reaction centers, and PSII acceptor side ofG.amansiiwere damaged under 30 °C at 12 h; however, the PSII donor side (oxygen-evolving complex, OEC) was relatively stable during the initial 12-h treatment.Previous studies on other algae and higher plants,e.g.,Arthrospirasp. (Zhang and Liu, 2016), wheatTriticumaestivum(Mathur et al., 2011a, b), and potatoSolanumtuberosum(Dou et al., 2014) have suggested that high-temperature stress may lead to the dissociation of OEC, resulting in an imbalance between the electron f low from OEC to the PSII acceptor side in the direction of the PSI reaction center (De Ronde et al., 2004). This interesting phenomenon further encouraged us to investigate the protective mechanism ofG.amansiiagainst photoinhibition under high temperature stress.
In some seaweeds, CEF-I has been found to be a key photo-protective mechanism under various environmental stresses (Larkum et al., 2017). The assimilation of one CO2molecule consumes three ATP and two NADPH molecules in one cycle of the Calvin-Benson cycle. Meanwhile, only 2.6 ATP are generated when 2 molecules of NADPH are produced in linear electron f low. This means a shortfall of 0.4 ATP for sustained operation of the Calvin-Benson cycle (Yamori and Shikanai, 2016). As an important alternative electron f low, PSI-driven cyclic electron f low (CEF-I) is essential for photosynthesis in many higher plants and algae. In CEF-I, electrons are recycled from the stromal reducing pool to the plastoquinone (PQ) pool, generating ΔpH and consequently ATP without accumulation of NADPH(Shikanai, 2007). The CEF-dependent generation of proton gradient across thylakoid membranes is considered to be the main way to increase the ATP/NADPH ratio as well as to protect the photosystem from photoinhibition through activating nonphotochemical quenching and stabilizing OEC(Shikanai, 2007, 2016; Takahashi et al., 2009; Huang et al., 2012; Wang et al., 2015). CEF-I includes NAD(P)H dehydrogenase complex (NDH) and PGR5(Proton Gradient Regulation 5) / PGRL1 (Proton Gradient Regulation Like 1) pathways. Among them,PGR5/PGRL1-dependent CEF is considered to be the key pathway in C3 plants (Huang et al., 2018), which can be specif ically inhibited by Antimycin A (AA)(Shikanai, 2007). Under high temperature stress, the enhanced photorespiration resulted in an increased of ATP demand. CEF-I is activated to increase ATP supply. Meanwhile, HCO3ˉ inf lux across cell membranes in the CO2concentrating mechanism(CCM) of algae is also ATP-required (Price, 2011).CCM further intensif ies the CO2demand for cell photosynthesis. Aihara et al. (2016) have reported that temperatures above the normal tolerance range induced CEF-I inSymbiodinium. Moreover, the induction of reactive oxygen species (ROS) and oxidative stress by high temperature has also been observed in macroalgae (Lee and Shiu, 2009; Eggert,2012). The antioxidant system is the main way for plants to remove ROS. Antioxidant enzymes induced by heat stress could help to ameliorate the dramatic increase in oxidative stress (Guo et al., 2006).Information derived from the measurement of ROS and antioxidant enzymes may aid in understanding inf luence of temporary high-water temperatures on this seaweed.
In this study, the variations of CEF-I and antioxidant enzymes ofG.amansiibefore and after treatment at high temperature were recorded to investigate the possible protective eff ect of CEF-I on photosynthesis of this alga.
2 MATERIAL AND METHOD
2.1 Plant material and pretreatment
Gelidiumamansiithat inhabits the Second Beach,Qingdao, China was collected for the experiment in which algae were exposed to high temperature.Samples were collected at a depth of about 1 m at low tide. Sixty healthy individuals (without showing signs of disease and discoloration on the thalli) were used for the experiment. Collected algae were kept in seawater using an ice cooler at about 15 °C and transported to the laboratory within a half hour.Before the treatment, algae that had been carefully rinsed with fresh f iltered seawater (salinity 32) were placed in a growth chamber with 16-L modif ied f/2 medium (without pH adjustment, composition of the elements were shown in Li et al. (2016c) for 5 days under natural lighting. The water temperature in the tank was controlled at 20 °C. Based on the identif ication method provided by Huang (2010),tetrasporophytes of this alga were used for the next heat treatment.
2.2 Analytical procedure
Glass beakers containing 400-mL fresh seawater were placed into an incubator at 30 °C for 12 h with continuous low light (LL) 40 μmol photons/(m2·s)and continuous high light (HL) with 1 000 μmol photons/(m2·s), respectively. Samples treated at 20 °C for the same period were utilized as controls. Healthy fronds (each 2.0 g, 3-4 cm in height) were chosen as test samples. Four individuals were placed in the incubator as a parallel sample. Samples treated for 6 h and 12 h at 20 and 30 °C, respectively, were used to determine the chlorophyll f luorescence and maximum light utilization effi ciency of PSII (Fv/Fm) and other photosynthetic parameters, i.e., qP,Fv′/Fm′, andΦPSII,representing the photochemical quenching, light adapted maximum quantum yield of PSII, and PSII eff ective quantum yield, respectively, were used to ref lect the activity of photoreaction as described by Zhang et al. (2011). The measurement for each treatment was repeated for four times with darkadapted (for 15 min) leaf disks at room temperature.Brief ly, the initial f luorescenceF0of fronds was recorded in low modulated measuring light. Next, a 0.7-s pulse of saturating light (8 000 μmol photons/(m2·s)) was applied to detectFm(the maximum f luorescence). The diff erence betweenFmandF0is the variable f luorescence, abbreviated asFv. The steadystate f luorescence level (Fs) and the maximum chlorophyll f luorescence level (Fm′) during exposure to illumination with actinic light at 40 μmol photons/(m2·s) were also measured, respectively.Fm/F0, an indicator of linear electron transfer rate, was calculated as previously described (Ibrahim and Jaafar, 2012).Fv/F0, the ratio of variable f luorescence to unquenchable portion of f luorescence was also determined to indicate activity of photosynthetic reaction centers (González-Mendoza et al., 2013;Kula et al., 2017). In an attempt to display the response of photosynthesis to high (30 °C) and standard temperatures (20 °C), the parametersFv/Fm,Fv/F0,Fm/F0, qP,Fv′/Fm′, andΦPSIIin the thalli that had been treated for 6 and 12 h under LL condition were also recorded.
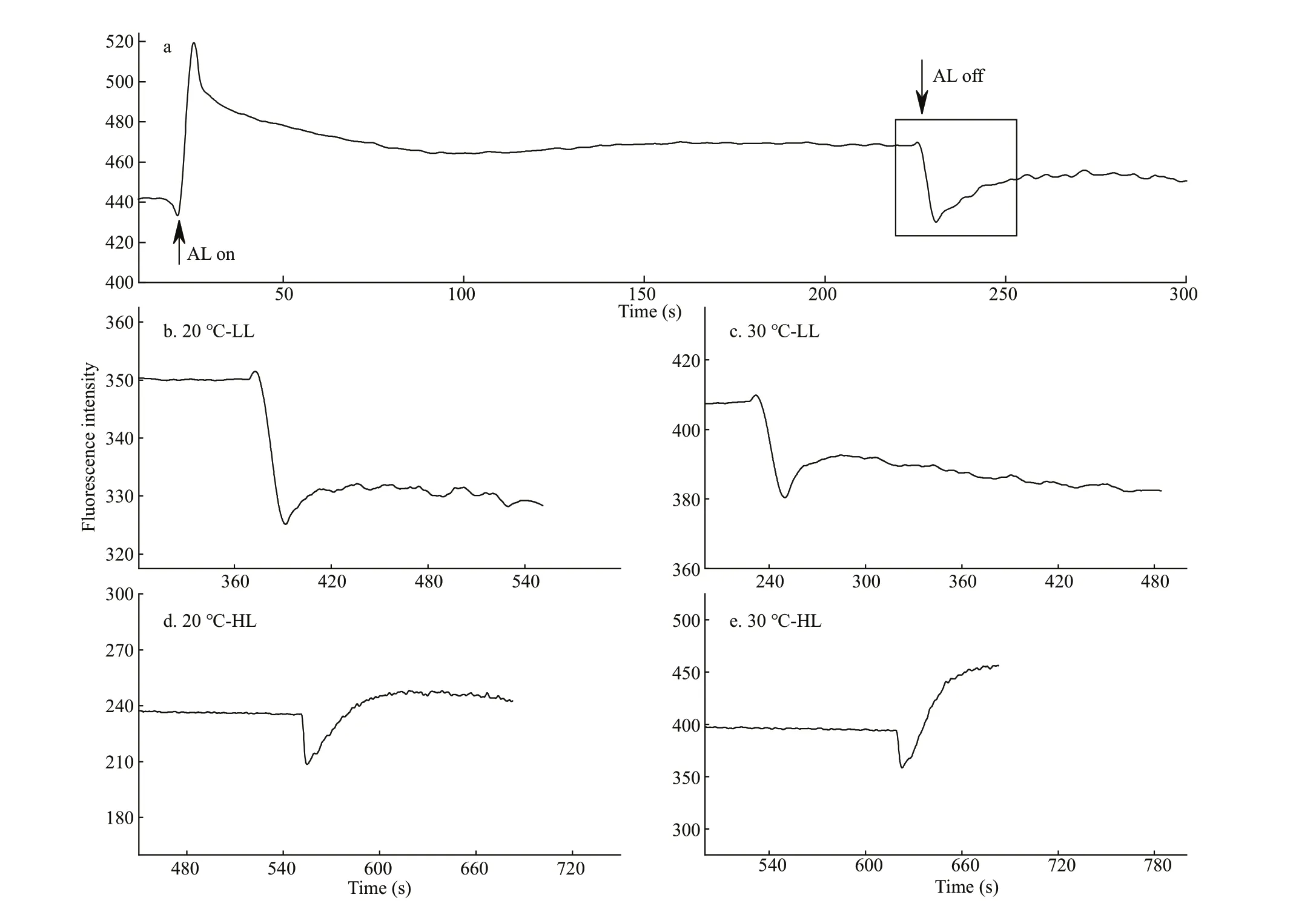
Fig.1 The post illumination increase in chlorophyll f lorescence of Gelidium amansii exposed to high temperatures under low(LL, 40 μmol photons/(m 2·s)) and high (HL, 1 000 μmol photons/(m 2·s)) light for 6 h
The post illumination increase in the intensity of chlorophyll f luorescence was measured as described by Wang et al. (2006) and Zhang et al. (2017). Before the measurements, the four 6 h heat-treated fronds of specif ic conditions (20 °C-LL, 20 °C-HL, 30 °C-LL,and 30 °C-HL, respectively) were combined as a single sample and adapted to dark for 15 min at same temperature as that of exposure. The transient post illumination was recorded after termination of the illumination by white actinic light with wavelength 400-750 nm for 2 min (AL, 40 μmol photons/(m2·s)and 1 000 μmol photons/(m2·s), respectively) (Fig.1a).The post illumination increase was quantitatively described using the enhancement of chlorophyll f lorescence (ΔF) and initial rate (S) after the AL termination.
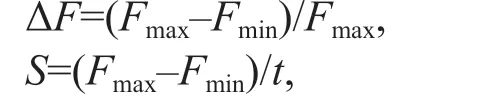
whereFmaxandFminare the maximum and minimum f luorescence intensity after the AL treatment,respectively;trepresents the time betweenFmaxandFmin.Swas obtained by f itting the data using a linear least square method based on Origin 8.0 (OriginLab,Northampton, MA, USA).
Antimycin A (AA) is a specif ic inhibitor of CEF-I(Shikanai, 2007). To monitor the CEF-I, 10 μmol/L of AA was used to incubate with algal fronds pre-treated at 30 °C for 6 h.
Fronds treated at high temperature for 6 h and 12 h,respectively, were used to determine the antioxidant enzyme system. The activities of ascorbate peroxidase(APX), superoxide dismutase (SOD), and catalase(CAT), and content of malonaldehyde (MDA) were determined using an ascorbate peroxidase assay kit, a total superoxide dismutase assay kit, a hydroperoxide dehydratase assay kit, and a malondialdehyde assay kit, respectively, provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) as described by Li et al. (2016a, b).
Diff erences between the measurements were analyzed using a one-way ANOVA complemented by Ducan post hoc test using the program SPSS 17.0(IBM, Inc., Armonk, NY, USA). Statistical signif icance was evaluated at a probability level ofP<0.05.
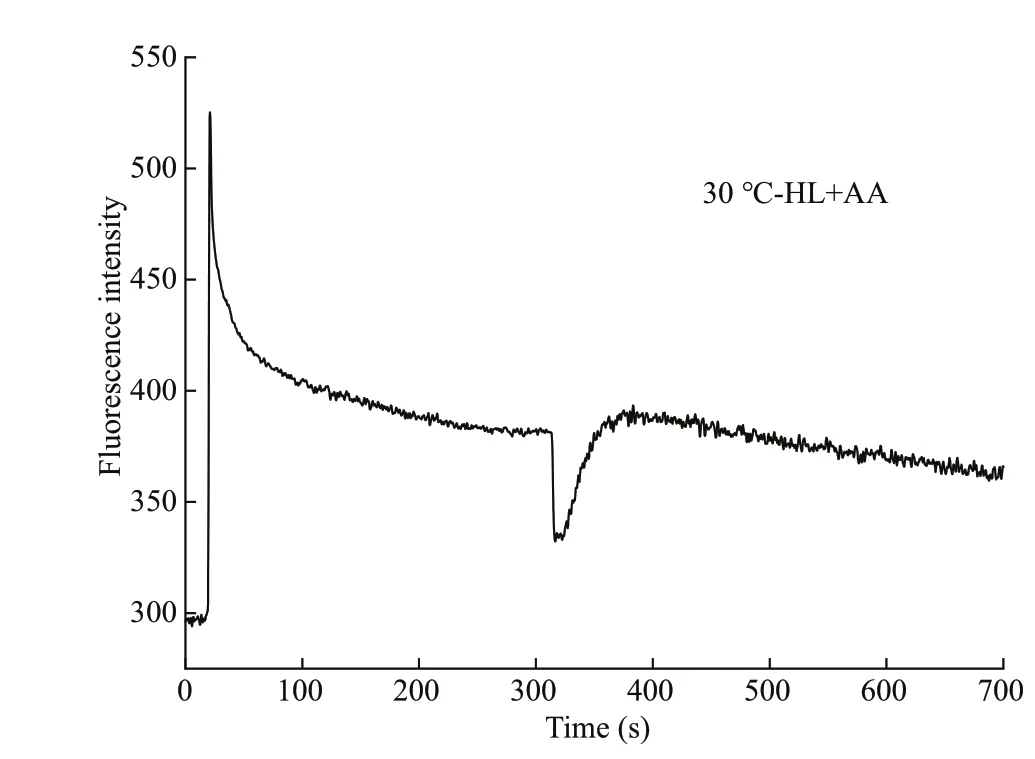
Fig.2 The post illumination increase in chlorophyll f lorescence of Gelidium amansii exposed to high temperature under high light that can be further treated using 10 μmol/L of inhibitor antimycin A for 5 min
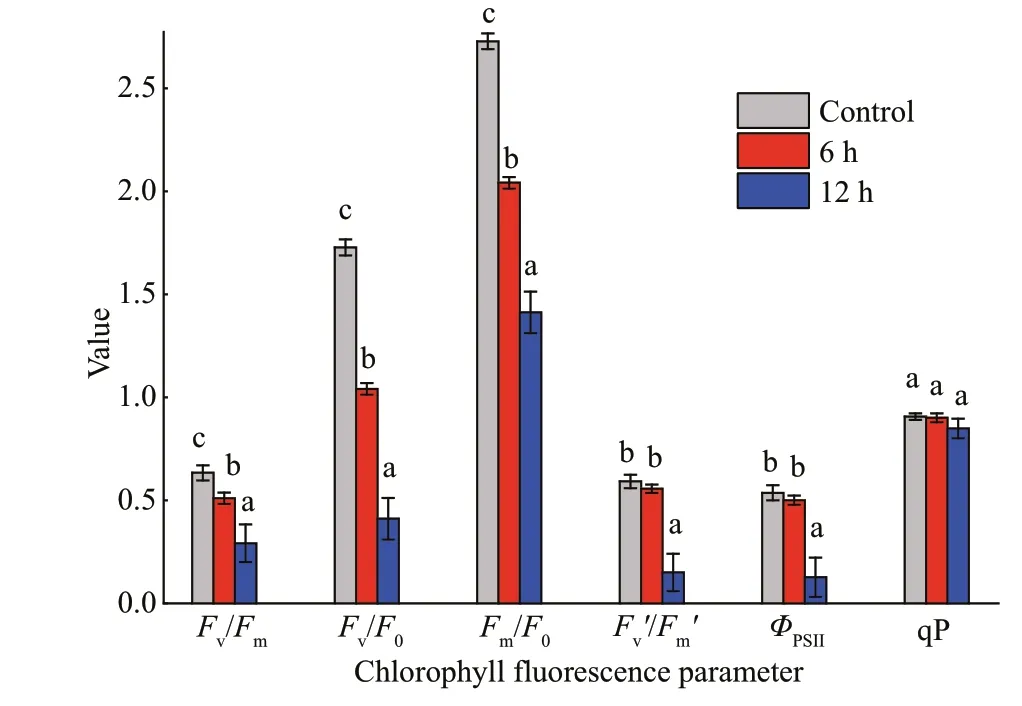
Fig.3 The variations of chlorophyll f luorescence parameters in fronds of Gelidium amansii at 30 °C with light intensity of 40 μmol photons/(m 2·s) for diff erent times (i.e., 6 h and 12 h)
3 RESULT
3.1 Heat-induced changes on cyclic electron f low at diff erent light intensities
When AL was removed, an instantaneous enhancement of chlorophyll f luorescence intensity occurred (Fig.1a). The quantitative result of this visible increase showed that when the algae were treated at 30 °C, both the ΔFandSwere enhanced,under either LL or HL conditions (Fig.1b-e; Table 1).Otherwise, the CEF-I can be decreased by AA (Fig.2).After treatment with10 μmol/L of AA for 5 min, the enhanced CEF-I at 30 °C with high light decreased to the level of that at 20 °C with the same light intensity(Table 1).
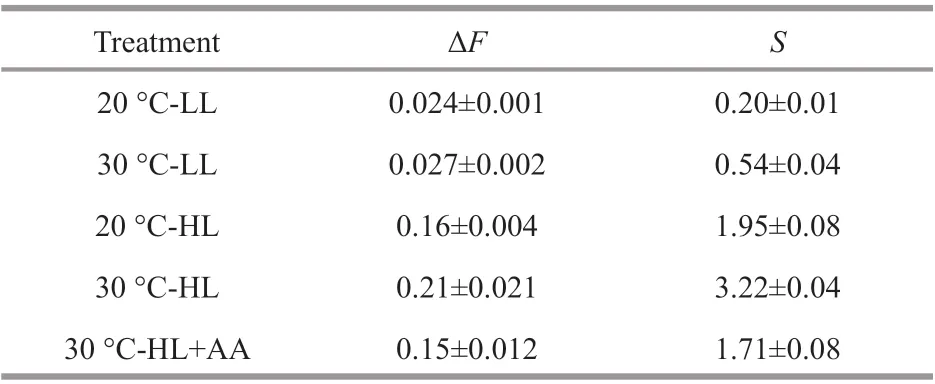
Table 1 The amplitude of the post illumination increase in chlorophyll f lorescence (Δ F) and the initial rate of the increasing phase ( S) in G. amansii exposed temperatures under low (LL, 40 μmol photons/(m 2·s)) and high (HL, 1 000 μmol photons/(m 2·s))light for 6 h
3.2 Changes in PSII photochemical activity in response to temperature stress
It has been widely proven that chlorophyll f luorescence is a convenient and particularly noninvasive probe to evaluate the photosynthetic activity under various abiotic stresses (Baker, 2008).With the goal of exploring eff ect of high temperature on the photosynthesis ofG.amansii, chlorophyll f luorescence parameters of algae treated at 30 °C for diff erent periods are shown in Fig.3.Fv/Fm,Fv/F0, andFm/F0all decreased with the time of exposure.However, the lowest decrease was all recorded at 6 h,with the degrees of 19%, 40%, and 25%, respectively,compared with those of the control. At 12 h, these three parameters decreased by more than 54%, 76%,and 48%, respectively, in comparison with the control.With regard toFv′/Fm′ andΦPSII, the values were both relatively stable during the initial 6 h period and then decreased sharply at 12 h. qP ref lects the share of light energy absorbed by antenna pigments used for photochemical reactions, i.e., the fraction of open PSII centers (Várkonyi et al., 2005).
3.3 Photoinhibition at diff erent temperatures and its response to the inhibitor

Fig.4 The changes of maximal photochemical effi ciency of PSII ( F v/ F m) of Gelidium amansii exposed to diff erent temperatures with low and high light
Fv/Fmis an indicator of photoinhibition in plants(Takahashi et al., 2009; Zhang et al., 2015). It is indicated that HL induces a signif icant inhibition ofG.amansiiat 20 °C (Fig.4a). Exposure at 30 °C also reduced theFv/Fmof this alga, either under low light or high light conditions (Fig.4b & c). These reductions were further intensif ied when the inhibitor of CEF-I was added. This phenomenon suggests that CEF-I may alleviate the photoinhibition caused by high temperature or strong light.
3.4 Changes in H 2 O 2 and the antioxidant system in response to temperature stress
Heat treatment resulted in a similar response of the activities SOD, APX, and CAT, and the contents of H2O2and MDA. Each parameter increased slightly during the f irst 6 h of exposure at 30 °C, with the exception that MDA remained stable as shown in Fig.5. At 12 h, all the indices increased signif icantly.
4 DISCUSSION

Fig.5 The variation in H 2 O 2 concentrations, activities of superoxide dismutase (SOD), catalase (CAT),and ascorbate peroxidase (APX), and contents of malondialdehyde (MDA) in Gelidium amansii exposed to 30 °C with 40 μmol photons/(m 2·s) for diff erent times
CEF-I, as an electron f low pathway in the thylakoid membranes of algae, has proven to help intertidal algae resist abiotic stress. For example,Ulva(Gao et al., 2011) andPorphyra(nowPyropia)yezoensis(Gao and Wang, 2012) are resistant to desiccation stress,Sargassumfusiformeto saline stress (Huan et al., 2014; Gao et al., 2016), andPyropiayezoensisto high light (Niu et al., 2016). In higher plants, studies conducted using tobacco (Huang and Hu, 2015) and spinach (Agrawal et al., 2016) leaves have conf irmed that the CEF-I can protect PSI and PSII from high temperature damage. There is little information regarding the CEF-I of subtidal macroalgae,particularly with respect to the response of this physiological process to high temperature. As shown in Fig.1a, a transient increase in post illumination when the AL was removed can also be observed inG.amansii. Such occurrence can be attributed to the reduction of plastoquinone (PQ) mediated by reducing substances that have accumulated in the presence of incident light (Muraoka et al., 2006; Wang et al.,2006). ΔFandSare used to quantitatively describe the intensity of CEF-I. After AA treatment, these two parameters were both signif icantly lowered,suggesting that AA is a potent inhibitor of CEF-I inG.amansiiand can be used to measure the involvement of CEF-I in photosynthetic reaction. The PGR5/PGRL1-dependent CEF-I can be specif ically inhibited by AA (Shikanai, 2007). We further believe that PGR5/PGRL1-dependent CEF-I at least is present inG.amansii. To the best of our knowledge, this is the f irst study that conf irms the existence of CEF-I inG.amansii(Fig.1a).
In our previous study, we reported that the oxygenevolving complex (OEC) was stable at 30 °C stress for 6 h (Li et al., 2019). The results of this study further indicate that the photochemical effi ciency of PSII, the size and the number of active photosynthetic reaction centers (as indicated byFv/F0), and the linear electron transfer rate (as indicated byFm/F0) are all gradually inhibited by high temperature. Interestingly,both the maximal photochemical effi ciency under light (Fv′/Fm′) and maximum quantum yield of PSII after light adaptation (ΦPSII) changed only slightly during initial 6 h.ΦPSIIis multiplied by qP andFv′/Fm′.qP decreased slightly throughout the high temperature treatment for 12 h, suggesting that the loweredΦPSII(from 0.54 to 0.13) was primarily a result of decrease inFv′/Fm′ at 12 h. However, all the parameters, i.e.ΦPSII,Fv′/Fm′, and qP, changed little when the fronds treated at 30 °C for 6 h. Presumably, there is a physiological process that dissipated the light energy absorbed excessively during the f irst 6 h and helpedG.amansiimaintain a relatively high maximum photochemical effi ciency under light. Our results showed that CEF-I plays an important role in mitigating the heat damage ofG.amansiicaused by high temperature. As shown in Fig.1b-e and Table 1,both the initial rate of the increasing phase and the amplitude were up-regulated in theG.amansiithat had been exposed to high temperature, either at low or high light intensity. This leads to the determination of possible cause of inconsistent response between theoretical maximum value after dark adaptation and that of the actual value measured under light. It is highly likely that the light-activated CEF-I relieves the pressure of photosynthetic system caused by high temperature and protects OEC from temporary heat damage (Huang et al., 2015; Yang et al., 2017).
A transient post illumination increase in the f luorescence intensity of chlorophyll is considered to arise from reduction of PQ (Burrows et al., 1998;Kofer et al., 1998). Two alternative pathways have been demonstrated for the PSI-driven CEF pathway.The main pathway is mediated by PGR5, a proton gradient regulation protein (Munekage et al., 2002).The second pathway is mediated by the NADH dehydrogenase (NDH) complex, a homolog of mitochondrial complex I (Johnson, 2011). AA is typically a potent inhibitor of CEF in those organisms that have a strong component of PGR- or NDHdependent CEF, and where it is active, it can be used to measure the involvement of CEF in photosynthetic reactions (Endo et al., 1997). Both the ΔFandSof AA fed-thalli ofG.amansiidecreased to the levels of control (20 °C-HL) at the end of 6 h of heat stress,suggesting that this inhibitor can be used to clarify the CEF in this alga. The addition of CEF-I inhibitors usually led to a signif icant increase in nonphotochemical quenching, which implied that CEF-I was an essential ATP supply mechanism that used the energy absorbed by photosynthetic apparatus (Zhang et al., 2017). The qP of heat-exposedG.amansiimaintained a stable level during the treatment period.However, theΦPSIIdecreased at 12 h. It can be deduced that the reduction of photochemical effi ciency is partly attributed to the up-regulated nonphotochemical quenching, i.e., heat dissipation (data not shown) (Yin et al., 2010; Naeem et al., 2020). Our previous study showed that the OEC indicates a high tolerance to 30 °C stress within 12 h. This study provides an explanation for this phenomenon. The stabilization of OEC requires a necessary transmembrane proton gradient (ΔpH) (Wang et al.,2006; Takahashi et al., 2009). The formation of ΔpH caused by increased CEF-I could suppress the damage of OEC and then alleviate the reduction of PSII activity. During heat stress, the drop ofFv/Fmin AAfed fronds intensif ied signif icantly in comparison with that without the inhibitor, suggesting that the photoinhibition caused by heat stress was intensif ied when the CEF-I was down-regulated.
An oversupply of light energy accelerates the production of ROS and leads to occurrence of photoinhibition. Plants have evolved antioxidant enzyme systems (e.g., SOD, APX, and CAT), which can be used to remove ROS and thus avoid the damage of excessive light energy (Mittler, 2002). No signif icant change in MDA, a marker of lipoperoxidation, was found at 6 h, although the H2O2content and antioxidant enzyme activity had increased(Fig.5). At 12 h, all the antioxidant enzymes of heattreated plants measured in this study were fully activated, resulting in a high level of H2O2and a rapid increase in lipoperoxidation. It can be further deduced that the protective eff ect of CEF-I is not suffi cient to resist the heat-induced damage of linear electron transfer in photosynthetic apparatus ofG.amansii.Therefore, the high temperature of 30 °C is ultimately lethal to this alga. It should be noted that the results of this study are based on short-term laboratory measurements (on hours timescales) of CEF-I and antioxidant enzyme responses ofG.amansii.We expect that the results are helpful in ref lecting the acclimation ofG.amansiito high temperature.Extrapolation of our results must be done with caution, and we acknowledge that studies on a longer timescale are needed to verify our inferences,especially in term of growth adaptation in diff erent temperatures. Moreover, heat shock proteins (HSP)are present in organisms and its activation is triggered by high temperatures to prevent protein denaturation(Wang et al., 2004). Enhanced production and accumulation of free and conjugated polyamines as well as increased activities of their biosynthetic enzymes in plants also have been associated with heat stress (Kuznetsov et al., 2006). Nowadays, study on temperature eff ects on HSPs and polyamines ofG.amansiistill remains scarce and it is an interesting subject needs further study.
A semi-diurnal tide is the tidal type along the east coast of China (Teng et al., 2017), which is the area in whichG.amansiiis primarily distributed. This type of tide results in two high tides and two low tides every day. The diff erence between the f irst high tide and the next low tide is approximately 6 h.G.amansiithat inhabits the intertidal rocky zones will not be exposed to high temperatures for less than 6 h, even at very low tides. These results showed that activated CEF-I partially protected the photosynthetic apparatus from high temperature that causes ROS damage after 6 h of exposure to high temperature. The up-regulated CEF-I is an important physiological process that enablesG.amansiito adjust to f luctuating natural high temperature. It is still unclear whether there are other factors that dominate the geographical distribution of this species, such as the content of osmotic solutes and soluble proteins, e.g., proline,glycine betaine, and phycobiliprotein. This is an interesting subject that merits further study.
5 CONCLUSION
CEF-I was up-regulated inG.amansiiexposed to 30 °C, regardless of whether it was coupled with high or low light, compared with that grown at 20 °C. The enhanced CEF-I alleviated the photoinhibition of PSII in this alga. The enhancement of CEF-I activity at high temperature aids in the generation of ΔpH and protects the donor side of PSII, i.e., oxygen-evolving complex, against heat damage. However, the protection of CEF-I is not suffi cient forG.amansiito adapt to high temperature stress. Therefore, CEF-I is regarded as an important strategy byG.amansiito acclimate to high temperatures in a relatively short time.
6 DATA AVAILABILITY STATEMENT
Full information developed from this study is available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENT
The authors would like to thank Dr. Hu LI, Institute of Oceanology, Chinese Academy of Sciences, for his kind help in collecting the seaweed.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
