Recovery time of macroinvertebrate community from Cd pollution in Longjiang River, Guangxi, China*
Yongde CUI , Baoqiang WANG ,2, Yongjing ZHAO , Nick R. BOND , Hongzhu WANG ,**
1 State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences,Wuhan 430072, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Murray-Darling Freshwater Research Centre, La Trobe University, Wodonga 3690, Australia
Abstract Estimating recovery times from pollution incident is an important issue of targeted biomonitoring programs. In the present study, the impact and recovery of macroinvertebrate communities from a cadmium wastewater discharge in the Longjiang River, Guangxi, China, in early January 2012 were studied based on 83 samples collected in f ive surveys within 20 months after the incident. The pollution aff ected seriously the local aquatic biota, and consequently, the invertebrate abundance and species richness were reduced considerably. Twelve months later, the taxonomic number of macroinvertebrates began to increase. However, sensitive taxa remained rare. Twenty months later, the taxon richness and abundance of macroinvertebrates increased signif icantly compared to those in the previous four time points. To explore the possible time-scale over which pre-disturbance conditions might occur, we chose four diff erent typical metrics of taxa richness (total taxa number, cumulative taxa number, taxa number per samples, and the Shannon-Wiener diversity index) and extrapolated modeled recovery trajectories. Target values for the four metrics were set at average values for sites from the nearby Lijiang River, which were used as a reference.Assuming a continued linear trajectory, the recovery times were estimated to be 52, 39, 39, and 31 months,respectively, which was roughly 3-5 years. This is consistent with results from recovery times from other studies of acute pollution cases, but contrasted strongly to the much longer recovery times associated with chronic pollution from groundwater contamination and mine-tailing runoff .
Keyword: macroinvertebrate community; recovery time; heavy metal pollution; cadmium; Longjiang River
1 INTRODUCTION
Heavy metal pollution of streams and lakes is recognized as one of the most important environmental problems worldwide. Active and abandoned mines are the primary source of heavy metals (including Hg, Cr,Cu, Pb, Zn, Cd, Ni, As, etc.) pollution in aquatic habitats (Smith, 1987). Many studies have shown that heavy metal contamination of streams can reduce the water quality and harm numerous organisms (Feris et al., 2004; Jia et al., 2017; Khan et al., 2019), which ultimately aff ects the aquatic ecosystem and human health (Smith, 1987; Miao et al., 2020). In many regions, heavy metal pollution continues to occur in spite of environmental protection measures. For example, in China, although the Ministry of Environmental Protection has imposed strict environmental regulations on the mining sector, heavy metal pollution events continue to occur because of emergency wastewater discharge and accidental spills from local mines (Zhang et al., 2013). Consequently,despite regulatory eff orts, there is still a great need to understand the impact on, and recovery potential of,the aquatic ecosystems from mine-related pollution.
As the major group of organisms representing the diversity and biomass in stream ecosystems,macroinvertebrates are relatively sedentary, have long life cycles, perform a variety of ecological functions, and exhibit a range of sensitivities to contaminants; and thus can serve as sentinels of change in local conditions (Rosenberg and Resh,1993). Having these attributes, the organisms are used frequently to monitor and assess the impact of metal pollution in lotic ecosystems (Winner et al., 1980;Clements et al., 2000; Gray and Delaney, 2008), and to evaluate the eff ectiveness of stream restoration and recovery rates after decrease or removal of stressors(Armitage et al., 2007; Clements et al., 2010; Gunn et al., 2010; Schmidt et al., 2010; Verdonschot et al.,2013). Overall, they have been shown widely as an indicator to both the initial impact and subsequent recovery trajectories.
The likelihood that degraded ecosystems can recover following the removal of a stressor and the length of time required for systems to return to predisturbance conditions remain critical research questions in applied ecology (Millennium Ecosystem Assessment, 2005). Many studies of macroinvertebrate recovery times from single events, such as toxic chemicals spills, f loods, drought, and organic pollution, indicate recovery times of a few months to several years (Niemi et al., 1990; Wallace, 1990).Other disturbances, such as runoff from mine tailings,can lead to chronic pollution, after which the changes in community characteristics may persist for decades(Niemi et al., 1990; Wallace, 1990; Clements et al.,2010; Gunn et al., 2010). The recovery of communities from anthropogenic disturbance is inf luenced by numerous factors, including the specif ic nature of the perturbation (e.g., physical vs. chemical stressor;Rapport et al., 1985), the amount of natural variation that a community experiences (Kiff ney and Clements,1996), the presence of other stressors (Paine et al.,1998), the availability of colonists (Niemi et al.,1990), the condition of the community at the time of the impact (Feris et al., 2004), life-history characteristics of dominant species (Gunderson,2000), and functional redundancy (Bellwood et al.,2004). As such, the recovery trajectories can be very complex.
In the present study, we evaluated the impact of,and recovery of, macroinvertebrate communities from a serious cadmium wastewater pollution in the Longjiang River, Guangxi, China. The wastewater was illegally discharged into the river from a local mine-processing plant in early-mid January 2012 in Hechi City, Guangxi. The concentration of cadmium was estimated 80 times higher over the offi cial benchmark in some reaches of the river, reaching 0.408 mg/L, and the total cadmium amounted equivalently to 21 t (Zhang et al., 2013). The impacts of the pollution incident were severe and potentially long-term. Our aim was to extrapolate the expected timeframe for macroinvertebrates to recover to a putative reference condition in contrast to neighboring rivers.
2 MATERIAL AND METHOD
2.1 Study area and cadmium pollution event
The Longjiang River Basin (24°16′N-25°45′N,107°43′E-109°15′E) has an area of 16 878 km2, a total length of 358 km (Fig.1), and mean annual discharge of 418.7 m3/s. Longjiang River originates in Sandu, Guizhou, and f lows through Lizhi, Nandan,Huangjiang, Hechi, and Yizhou before pouring to Liujiang River. The climate is subtropical monsoon,with an average annual precipitation of 1 200-1 600 mm and average annual temperature of 16.9-21.5 °C. The Longjiang Basin landscape is mostly karst topography with many subterranean streams in carbonate rock regions (Editorial Committee of Encyclopedia of Rivers and Lakes in China, 2013).
As the cadmium pollution occurred in mid-January 2012 in the Longjiang River that is a large river, it is not feasible to apply treatment technologies.Therefore, reduction in cadmium concentration of the wastewater released into the river depended primarily on deposition in the sediment and dilution by upstream water. After the relief of metal stressor in the end of 2012, the average cadmium concentration in the water column and sediments gradually decreased from 6.75 to 0.27 μg/L, and from 34.98 to 17.5 μg/g, respectively,in the f irst year after the pollution incident (Table 1).
2.2 Macroinvertebrate sampling
The cadmium pollution in the Longjiang River was an isolated incident, and thus assessing the impacts of cadmium pollution on macroinvertebrate communities, and predicting the recovery time are a challenge because of the lack of pre-disturbance data.Although there were no data before the incident,sampling of macroinvertebrates began in one month after the incident. However, the site in the upstream of the pollution scene was considered unsuitable as a reference system because the discharge and the geography diff erentiate them from the mainstream.Therefore, we choose the neighboring Lijiang River(25°59′N-23°23′N, 110°18′E-111°18′E), the tributary of Xijiang River, as the reference river, and macroinvertebrate data from 2008 (Table 2) (Cao,2010) were used as the reference to assess the recovery. We thus assumed that a well-def ined and temporally stable reference condition existed for these rivers, which is well supported by previous studies in this region in terms of species composition.
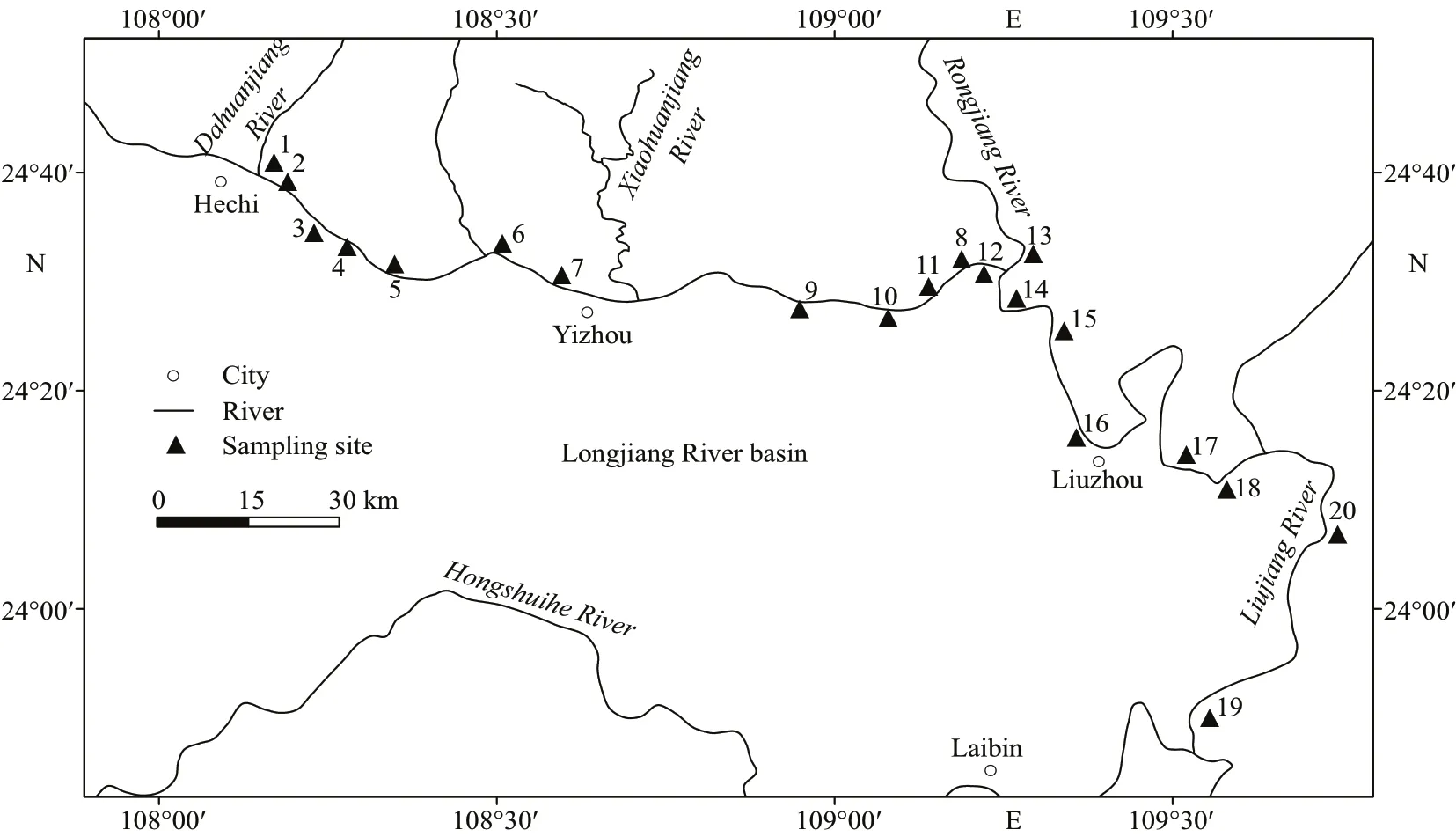
Fig.1 Position of the Longjiang River basin and deployment of sampling sites
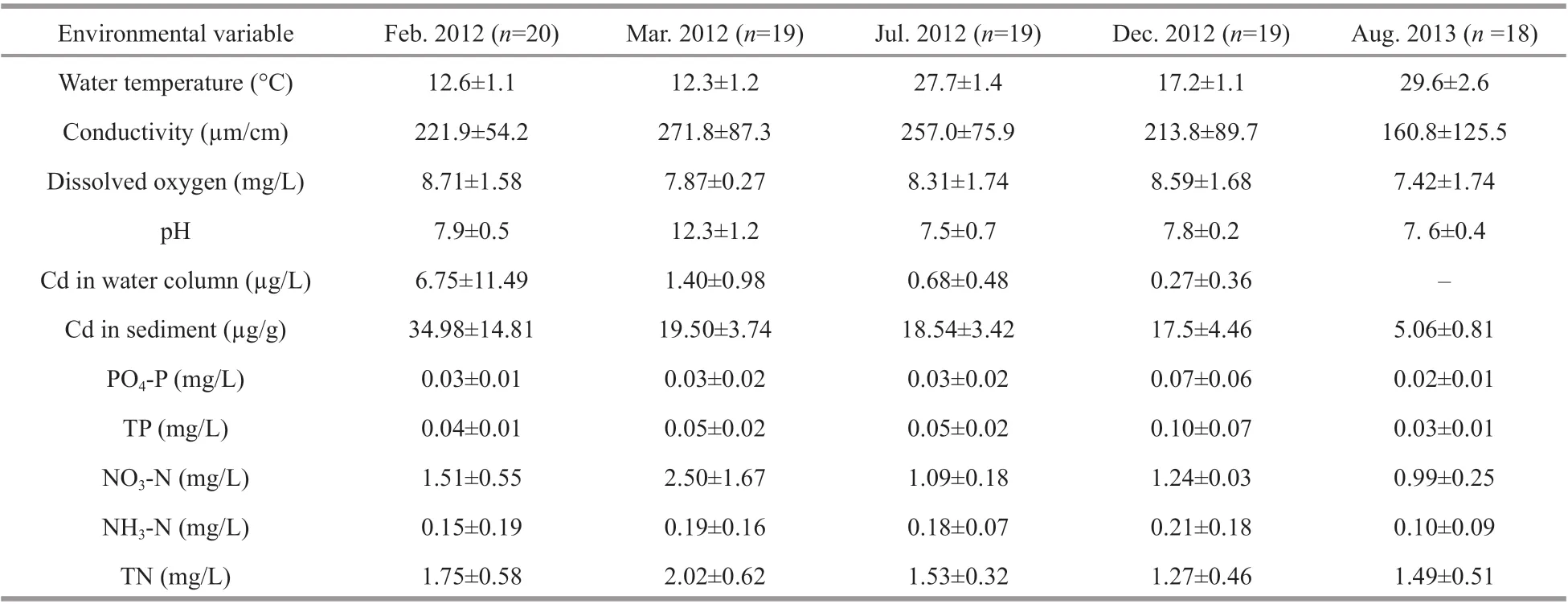
Table 1 Environmental variables in f ive surveys of Longjiang River in 2012 (mean value±SE)
Samplings were conducted in f ive surveys during February 5-8, 2012 (15 sites, except S1, S12, S17,S18, S20), March 1-3, 2012 (18 sites, except S17,S19), July 10-12, 2012 (17 sites, except S15, S17,S20), December 12-15, 2012 (18 sites, except S19,S20), and August 9-11, 2013 (15 sites, except S10,S15, S17, S18, S20), which were in approximately 1,2, 6, 12, and 20 months after the pollution occurred. A total of 83 samples were collected in 20 sampling sites with three samples per site downstream from the pollution scene (Fig.1). Quantitative samples were collected using a weighted Peterson grab and D-frame kick net in the profundal and littoral zones,respectively, and gastropods were sampled by hand from the rocks or boats as qualitative samples. The sampling eff ort (time) was apportioned as equally as possible in the sampling section. Samples were f ield washed through 350-μm sieve; large organic and inorganic particles were removed, the samples were placed into sealed plastic containers, and preserved in 10% formalin. We picked all animals from the entire samples in naked eyes or with the aid of microscopes.
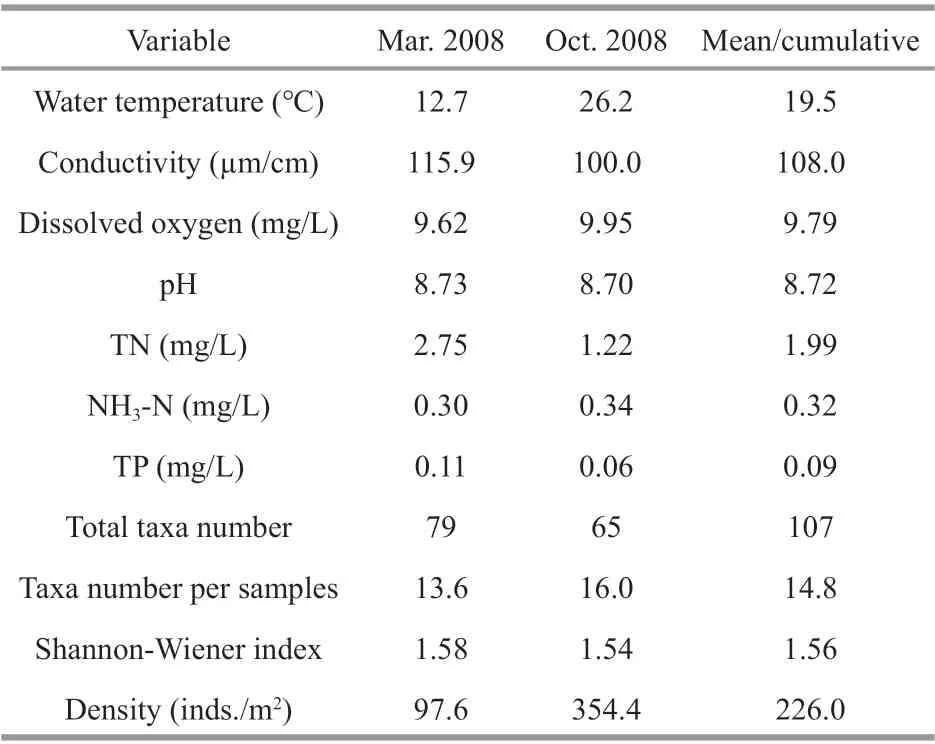
Table 2 Environmental variables and macroinvertebrate assemblage in the reference river (Cao, 2010)
Macroinvertebrate individuals were counted and identif ied to the lowest taxon possible, usually genus,using taxonomic keys (Sperber, 1948; Brinkhurst and Jamieson, 1971; Liu et al., 1979; Morse et al., 1994;Wang, 2002). All the taxa were assigned to functional feeding groups (FFGs): shredders, collector-gatherers,collector-f ilterers, scrapers, predators, and omnivores,according to related materials (Morse et al., 1994;Liang and Wang, 1999). When a taxon had several possible feeding activities, its functional designations were equally proportioned, e.g. if a taxon can be both collector-gatherer and scraper, and its abundance was divided 50꞉50 into these groups. The occurrence (%of samples) of taxa in f ive surveys from Longjiang River are shown as Supplementary Table S1 in the Supporting Information.
2.3 Environmental variable
On each sampling occasion, some physicochemical variables of water temperature (WT, ° C),pH, dissolved oxygen (DO, mg/L), and conductivity(COND, μS/cm) were measured using a YSI-6600V2(Xylem Analytics, White Plains, NY, USA) prior to sampling the macroinvertebrates.
Water samples collected for determination of dissolved Cd were f iltered (0.45 μm) and acidif ied with nitric acid to a pH of <2. The concentration of Cd was determined using a standard atomic absorption spectrophotometric method (APHA, 1992) in a spectrophotometer (PE800A, Perkin-Elmer, Waltham,MA, USA). Total phosphorus (TP, mg/L), total nitrogen (TN, mg/L), phosphate (PO4-P, mg/L),nitrate (NO3-N, mg/L), and ammonia nitrogen(NH4-N, mg/L) were determined according to the Standard Methods for Water and Wastewater Monitoring and Analysis (Chinese Environmental Protection Chief Bureau, 2002).
Sediments were randomly collected with a grab from each site, placed in a zip-lock freezer bag, and stored at 4 °C. Each sample was homogenized, and 1-g samples were dried at 80 °C for 24 h and weighed again to determine the mean percentage water content(%). Sediments were digested in 50% (v/v) nitric acid,20% (v/v) hydrochloric acid, and the concentration of Cd was determined using standard atomic absorption spectrophotometric method (APHA, 1992) in a spectrophotometer (PE800A; Perkin-Elmer).
2.4 Data analysis
Assemblage composition data was used to calculate 30 commonly used indices describing the benthic invertebrate assemblage structure (Supplementary Table S2). However, because of the high level of correlation and redundancy among these metrics, we focused on a subset only for our results. In particular,we describe the results for average species richness,total density and biomass, and Shannon-Wiener diversity, as well as diversity within major taxonomic groups and partitioning of individuals among FFGs.At the site scale, we additionally calculated the total taxon richness across all samples. One-way analysis of variance (ANOVA) was used to detect the diff erences in environmental and biological variables among the f ield survey dates. Bonferroni correction on the alpha value of the test was carried out before performing these comparisons. All of these analyses were performed using SPSS statistical software(Version 13.0; IBM Corp., Armonk, NY, USA).
3 RESULT
3.1 Taxonomic richness and composition of macroinvertebrates
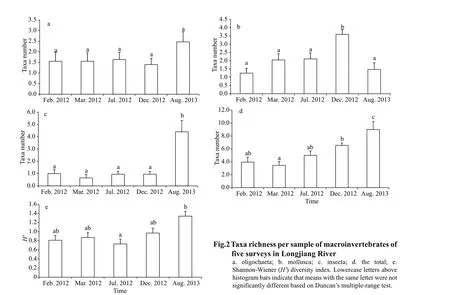
A total of 64 macroinvertebrate taxa belonging to 4 phyla, 8 classes, 30 families, and 58 genera(Supplementary Table S1) were collected from Longjiang River during the f ive surveys. Insecta contributed 45.3% of the total richness, with Chironomidae (26.6%, 17 taxa overall) as the taxonomically richest group. Oligochaeta and Gastropoda contributed 25.0% and 15.6% of the total richness, respectively (Table 3). The most common taxa (occurrence frequency exceeding 15% in all sites) were all known to be tolerant to pollution, and they included Tubif icidae (Branchiurasowerbyi,Limnodrilushoff meisteri), Gastropoda (Bellamyasp.,Stenothyraglabra, andSemisulcospiralibertina),Bivalvia (Limnopernalacustris,Corbiculaf luminea),and Chiromonidae (Polypedilumsp.,Tanypussp.). In contrast, sensitive taxa, such as EPT species(Ephemeroptera, Plecoptera, and Trichoptera) were rare (Table 3). Diff erences among the f ive surveys were statistically signif icant, largely because of changes in the f ifth survey, which was signif icantly diff erent to all other surveys, according to post-hoc tests. Comparing the total taxa number across the f ive surveys, diff erences among the f irst four surveys were small, with the total taxa number ranging from 22 to 28; however, the average total richness increased to 45 by the f inal survey, mainly caused by additional oligochaetes and insects (Table 3). Similar patterns were observed for other common taxa, with the exception of oligochaetes (Fig.2a-d). Shannon-Wiener diversity also increased signif icantly over time (ranging from 0 to 2.14), again caused by changes occurring before the f inal survey (Fig.2e).
3.2 Density and biomass
The total density and biomass of macroinvertebrates in Longjiang River were 860±229 inds./m2(mean±SE)and 55.8±26.3 g/m2, respectively. Oligochaetes and molluscs were the most abundant organisms,accounting for 53.3% of the density and 97.3% of the biomass. Figure 3 shows the density and biomass of each taxonomic group of macroinvertebrates across the f ive surveys. The density of insect taxa was higher in the f ifth than in the four previous surveys, (F=5.464;P=0.001) (Fig.3c) as was the total macroinvertebrates density (F=9.162;P<0.001) (Fig.3d). In contrast,oligochaetes and molluscs densities were not signif icantly diff erent over time, although this might ref lect a lack of statistical power, as there were still upward trends apparent (Fig.3a-b). Dominance was also high, with just six taxa accounting for 64.8% of total density and 77.0% of total biomass (Table 4).L.hoff meisteri,B.sowerbyi,C.f luminea, andS.libertinawere dominant in all f ive surveys, whilePolydorasp. andPolypedilumsp. were dominant in the fourth and f ifth surveys, respectively.
3.3 Functional feeding group
The functional feeding groups were dominated by collector-gatherers, collector f ilterers, and scrapers,which comprised respectively 69.8%, 12.8%, and 9.6% in terms of density, and 1.8%, 70.4%, and 27.0%of the biomass. The relative density of shredders(F=3.424;P=0.013) showed a signif icant diff erence among the f ive surveys, while the relative biomass of collector-gatherers (F=2.934;P=0.026) diff ered among the f ive surveys (Fig.4). Other parameters showed no diff erence and their comparisons between the f ive surveys are shown in Fig.4.
3.4 Trends in macroinvertebrate indices after metal pollution events
Our goal was to predict the recovery time of macroinvertebrate community from the cadmium pollution event in Longjiang River, therefore, we analyzed the relationships of 30 common macroinvertebrate metrics (Supplementary Table S2),including taxa richness, abundance of taxonomic groups, and FFGs, with time elapsed (in month) after the cadmium pollution event. We found that many metrics increased with the time after cadmium pollution, but only insect taxa number, total taxa number, taxa richness per samples, cumulative taxa number, Shannon-Wiener diversity index, anddensities of molluscs, scrapers, and collectorgatherers showed a signif icant and positive correlation with time after the cadmium pollution event (Table 5).The relationships of the metrics of taxa richness with time (months) after cadmium pollution events are shown in Fig.5.
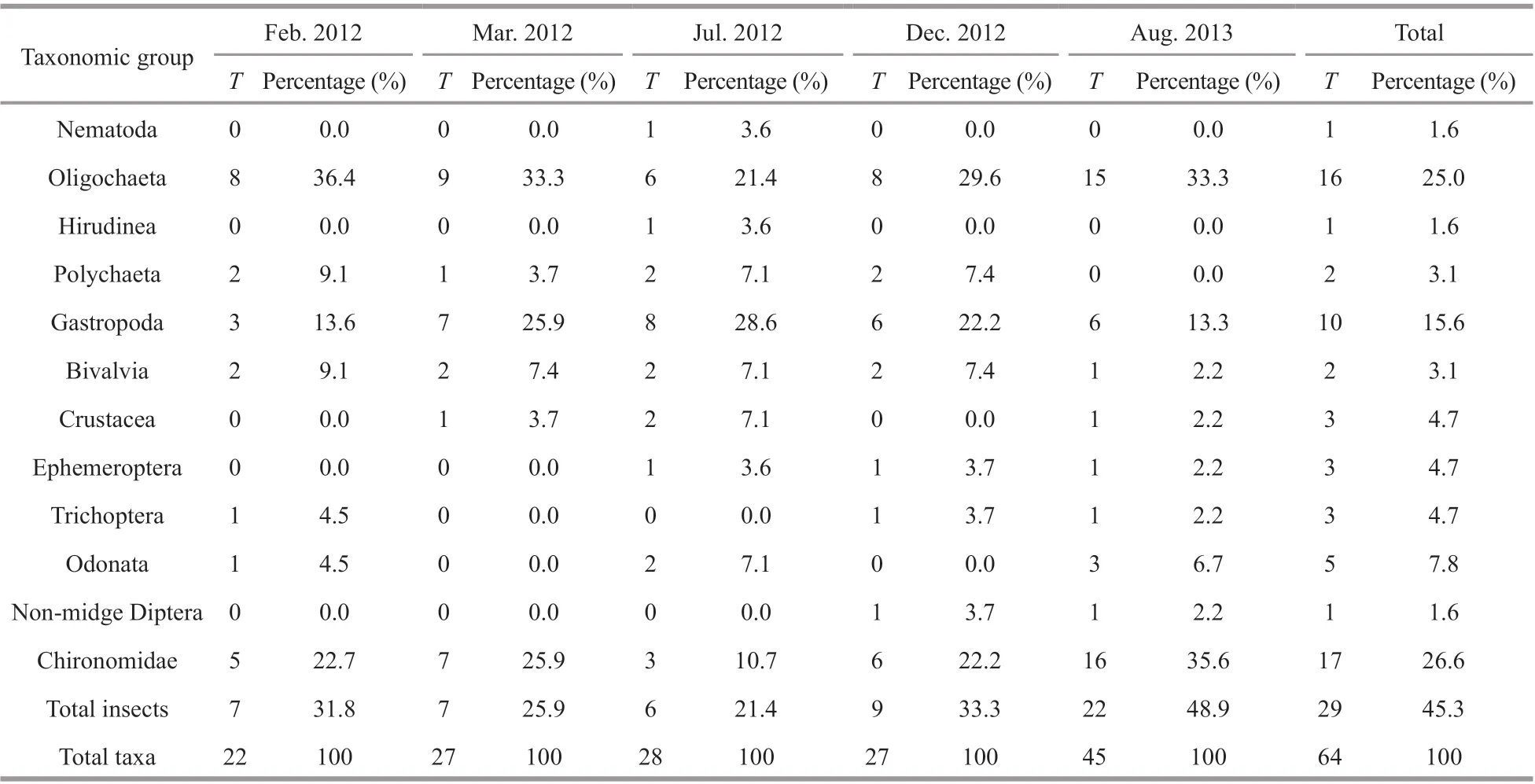
Table 3 The number of taxa ( T) and percentage summarized by major taxonomic groupings for f ive surveys in Longjiang River
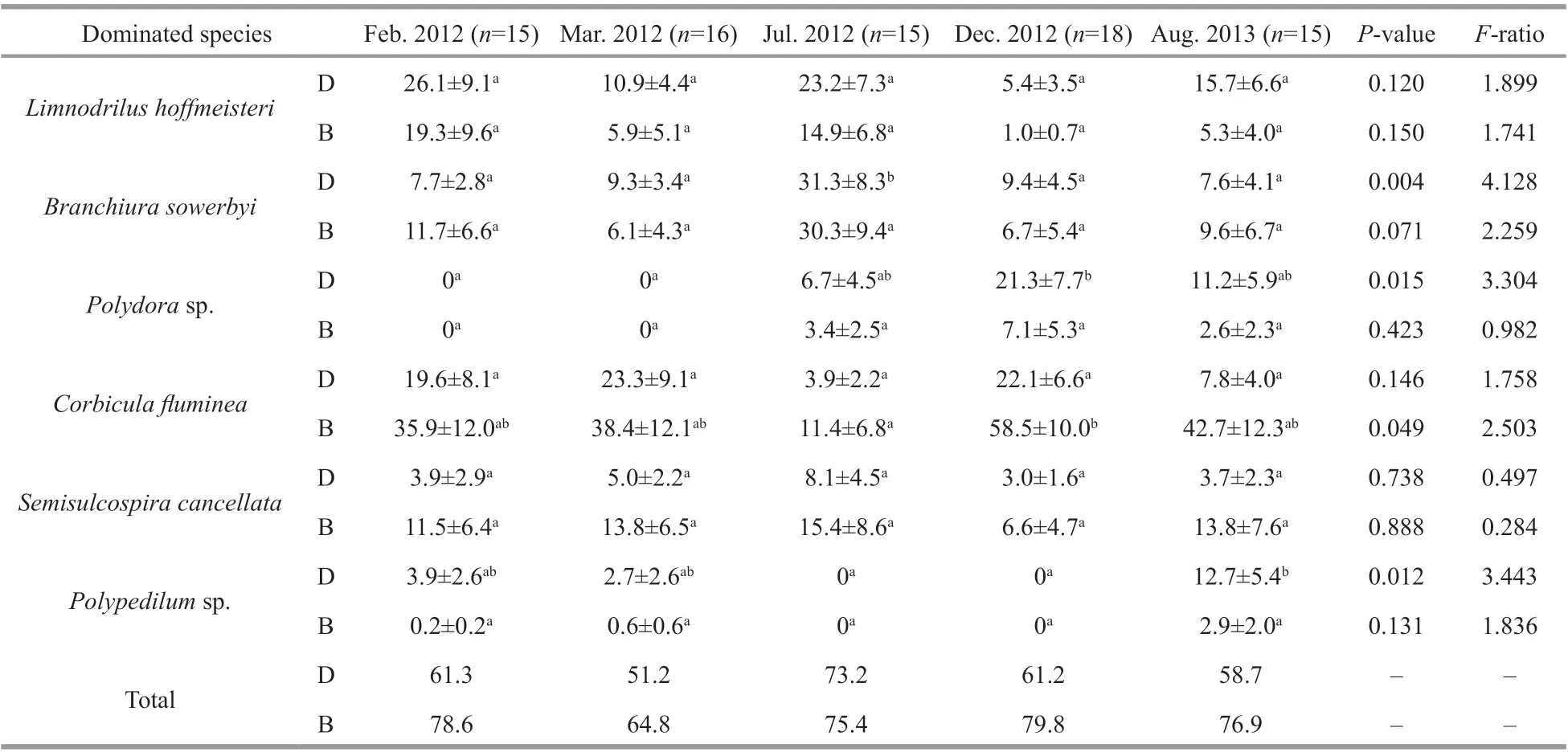
Table 4 Relative abundance of dominated species of f ive surveys in Longjiang River (>10%)
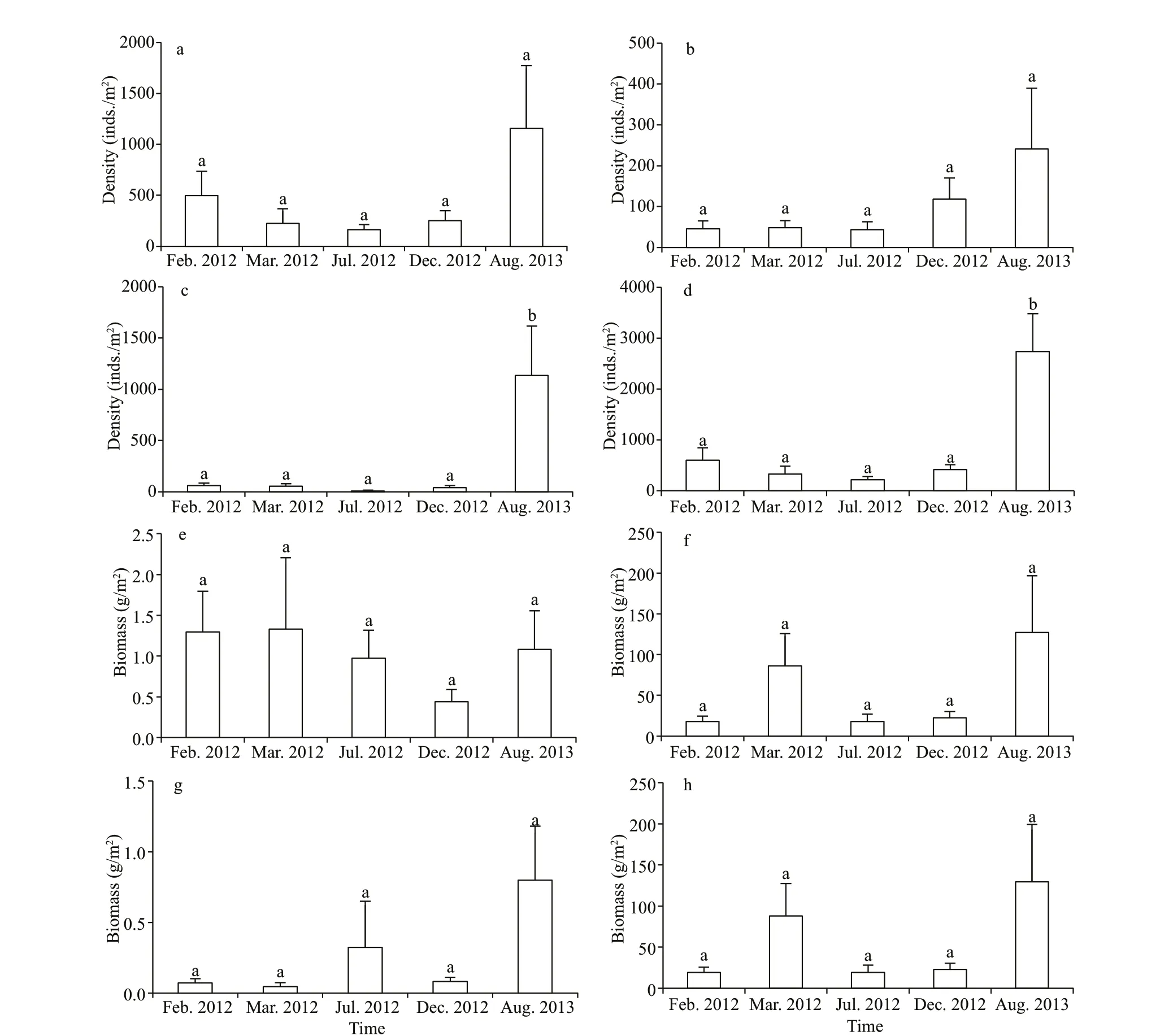
Fig.3 Density (a-d) and biomass (e-h) of taxonomical groups of macroinvertebrates in f ive surveys in Longjiang River
4 DISCUSSION
4.1 Macroinvertebrates assemblage variations after cadmium pollution
Heavy metal pollution can have dramatic eff ects on community structure (e.g., reduced density, reduced species richness, and shifting of the community to more-tolerant species) because of the eff ects on individuals, i.e., mortality, reduced growth, and reduced reproductive f itness (Winner et al., 1980;Cairns and Pratt, 1993). Community level responses are often used to evaluate the eff ects of metals on macroinvertebrates (Clements, 1991). Where metal concentrations are suffi ciently high, the local macroinvertebrates might be entirely absent or their abundance could be greatly reduced (Clements, 1991;Armitage et al., 2007). Where metals concentrations do not entirely eliminate the community, however,measures of taxa richness or abundance of metalsensitive taxa provide the most sensitive and reliable measures of community level eff ects (Barbour et al.,1992; Clements and Kiff ney, 1995; Carlisle and Clements, 1999; Gray and Delaney, 2008; Qu et al.,2010). At one month after the cadmium pollution event, the species diversity of macroinvertebrate was much lower in the Longjiang River than the values previously observed in the nearby river. One year after the cadmium pollution, taxa richness had only just begun to increase (Fig.2); however, sensitive taxa(such as those from the EPT families) were still rare(Supplementary Table S1), and remained lower than those of Lijiang River (Cao, 2010; Chen et al., 2014).These results are comparable to those from other rivers that subjected to single pollution events(Heckman, 1983; Hynes, 1960; Mciwem et al., 2010).These results contrast with the eff ects of chronic exposure, even at low levels, which seem to have a reduced impact on invertebrates, especially on some sensitivity species (Clements et al., 1988; Clements,1994; Gray and Delaney, 2008; Qu et al., 2010).
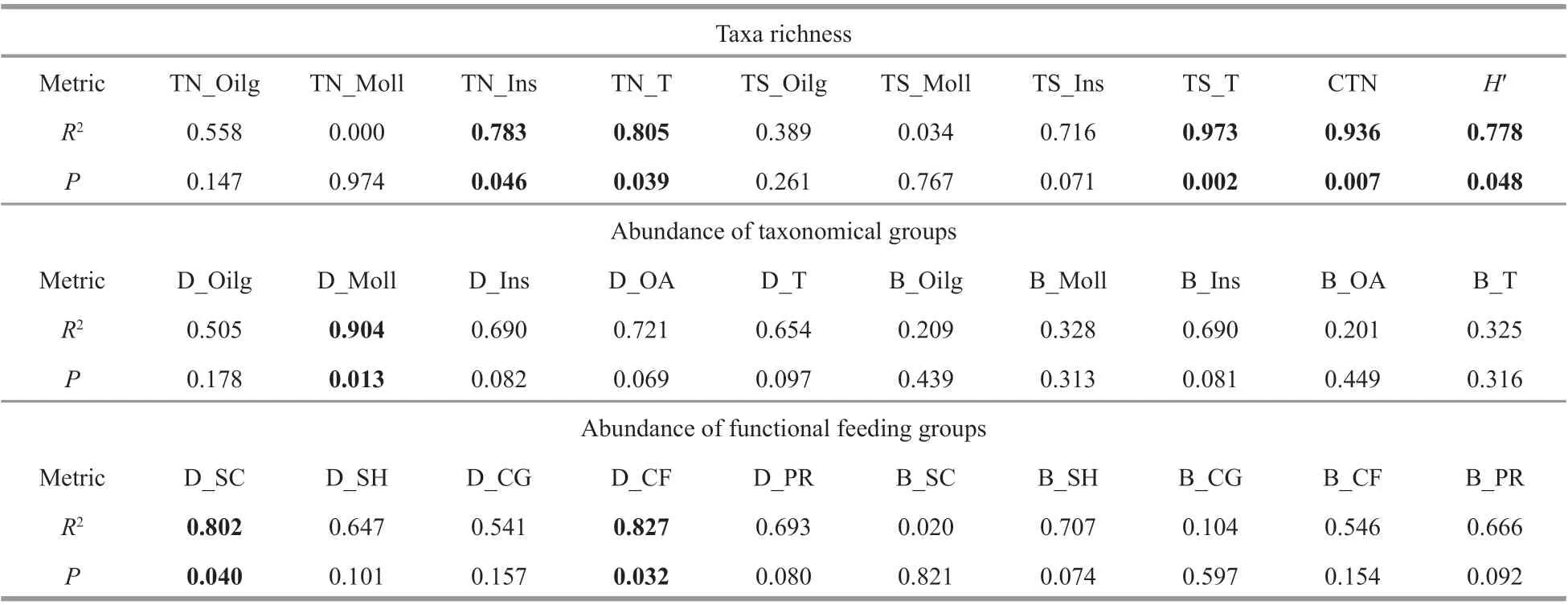
Table 5 Relationships of macroinvertebrate metrics with time elapsed (in months) after cadmium pollution ( n=5)
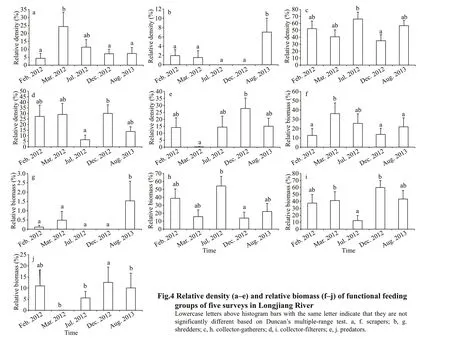
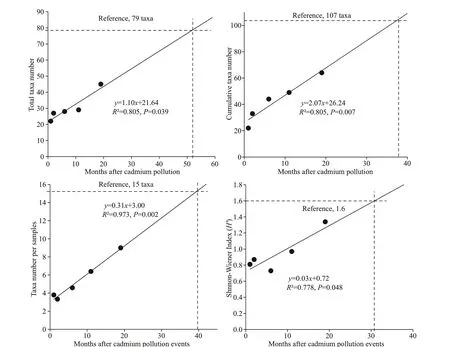
Fig.5 Relationship of taxa richness with the time (months) after cadmium pollution events
Among the dominant species of macroinvertebrates in Longjiang River (Table 4), except for Polychaeta,their tolerance to cadmium is unknown. Although heavy metal pollution can lead to the disappearance or decline of the species sensitive to heavy metals,the species tolerant to heavy metal pollution become dominant (Barbour et al, 1992; Clements and Kiff ney,1995; Carlisle and Clements, 1999; Anderson, 2007),which is the only reason for the dominant species pattern of macroinvertebrates in Longjiang River,but not the main factor. Longjiang River f lows through many cities and towns, receives a large amount of urban sewage, and has serious organic pollution. In addition, there are many projects of water conservation in the main stream; some rivers become reservoirs, and sediments deposit, which are likely the main reasons for the high density of Oligochaeta and chironomid larvae.
Heavy metals might also have an eff ect on ecosystem function. Clements et al. (2000) recorded a change in the FFGs of macroinvertebrates, with a lower abundance of scrapers, shredders, collectors,and predators at stations with medium- and highconcentrations of heavy metals compared with the reference stations. Our results show a lower abundance of scrapers, shredders, and predators during periods with high concentrations of cadmium, which is consistent with previous studies (Fig.4).
4.2 Estimation of the recovery time of macroinvertebrates from cadmium pollution
We found that the metrics of the macroinvertebrate assemblage structure were largely unchanged from approximately 1-12 months after a major pollution spill, and evidence of recovery was only observed after twenty months, although most metrics had still not converged on the putative reference conditions def ined from samples in the undisturbed Xijiang River (Fig.5).
To explore the possible time-scale over which the reference conditions might occur, we chose four diff erent typical metrics of taxa richness: total taxa number, cumulative taxa number, taxa number per samples, and Shannon-Weiner diversity index, and extrapolated modeled recovery trajectories (Fig.5;Table 4). Target values for the four metrics were set at the average values for the Lijiang sites, which were used as a reference (79, 107, 15, and 1.6, respectively).Assuming a continued linear trajectory, the recovery times were estimated as 52, 39, 39, and 31 months,respectively, for these indices. These estimates assume a continued linear trajectory, but are also well below the timeframes often observed for recovery from severe heavy metal contamination. Although stream ecosystems are generally thought to be quick response systems that rapidly recover from a stressor(Niemi et al., 1990), many studies of macroinvertebrate recovery times from single events, such as toxic chemicals spills, f loods, pesticides, drought, and organic pollution, indicate recovery times of a few months to several years (Wallace, 1990). In contrast,invertebrate assemblages often take several decades to recover from heavy metal pollution, largely because of the persistence of toxic chemicals within the environment, especially bed sediments (Wallace,1990; Armitage et al., 2007; Clements et al., 2010).Although the cadmium pollution in the Longjiang River was a single episodic event, the aqueous cadmium concentrations had decreased to below the detection limit by August 2013 (six months after the pollution initiation). Not surprisingly, sediment concentrations remained higher throughout our surveys.
While speculative, the observed trends in assemblage metrics would suggest a minimum of 3-5 years before invertebrate communities in the Longjiang River could recover to their likely predisturbance status.
5 CONCLUSION
Macroinvertebrate assemblages have been used extensively to monitor and assess the eff ects of contamination on aquatic systems, and taxa richness and biodiversity are regarded as a reliable measure of heavy metal impacts. In the present study, we used four measures of taxa richness to extrapolate the expected timeframe for macroinvertebrates to recover to a putative reference condition (based on a neighboring river). These predictions are speculative because community recovery trajectories may be nonlinear, even once recovery has commenced, and might be subjected to subsequent disturbances and other unforeseen inf luences. The above estimates of 3-5 years are thus likely to represents a minimum timeframe for complete recovery of the Longjiang River. However, these estimates are helpful to guide stakeholder awareness and responsibilities, and for planning of ongoing monitoring programs.
6 DATA AVAILABILITY STATEMENT
All data used to support the f indings of this study are available from the corresponding author upon request.
7 ACKNOWLEDGMENT
We are indebted Dr. Ke CUI of South China Agriculture University, and Mr. Siyang LI and Ms.Ling’ai YAO of South China Institute of Environmental Science, Ministry of Environmental Protection for assistance in the f ield sampling.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
