Factors aff ecting lipid and fatty acid composition of Calanus sinicus in the Yellow Sea and the East China Sea in spring*
Yanqing WANG , Zhencheng TAO , Chaolun LI , Mengtan LIU
1 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
3 Engineering and Technology Department, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
4 Jiaozhou Bay National Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
5 Center of Deep Sea Research, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
6 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
7 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract The factors aff ecting lipid and fatty acid composition of copepod Calanus sinicus in the Yellow Sea (YS) and the East China Sea (ECS) were examined in this study. In spring, there were signif icant diff erences between these two regions for both environmental conditions and food availability. Such regional diff erence signif icantly inf luenced the lipid and fatty acid prof iles of C. sinicus. Our results show that C. sinicus has a higher lipids content in ECS, especially for wax ester and triglyceride lipids, indicating a more active and effi cient predation. According to BIO-ENV analysis, the variation of lipids prof iles may be inf luenced majorly by water temperature. Moreover, the fatty acids (FAs) prof iles of C. sinicus were also diff erent between YS and ECS, especially in the four major contributors, C22꞉1ω11, eicosapentaenoic acid(EPA), docosahexenoic acid (DHA), and C20꞉1ω9. The considerable amounts of self-biosynthesized FAs of herbivorous copepod (C22꞉1ω11 and C20꞉1ω9) and low DHA/EPA ratio may indicate that C. sinicus in ECS feed mainly on phytoplankton comparing to those in YS. The fatty acid prof iles of C. sinicus were aff ected by the diff erences in food availability.
Keyword: Calanus sinicus; lipid storage; wax ester; fatty acid composition; Yellow Sea and East China Sea
1 INTRODUCTION
ManyCalanusspecies, especially those from Polar Regions, can store a large amount of triacylglycerols or wax esters in oil sacs or oil droplets (Lee et al.,2006). The stored lipids play important roles in the life history of marineCalanusin reproduction,ontogeny, diapause, and coping with food scarcity, as shown by studies in various oceanic regions (Rey-Rassat et al., 2002; Mayor et al., 2009; Pond and Tarling, 2011; Clark et al., 2012). Moreover, the transport and metabolism of the carbon-rich lipids by diapausing copepods such asCalanusf inmarchicusprovide another effi cient way of sequestering carbon into the ocean f loor (Jónasdóttir et al., 2015).
The lipid accumulation process is inf luenced by both intrinsic (lipid metabolism genes) and external environmental (such as temperature, food quality and quantity) factors (Koussoroplis et al., 2014; Zhou et al., 2016; Zhou and Sun, 2017). However, existing knowledge about how these factors aff ect the lipid accumulation process inCalanusis still limited. Zhou and Sun (2017) argued that low temperature especially diurnal temperature diff erences might promote lipid accumulation inCalanussinicusby reducing the energy output at colder temperatures and extending the lipid accumulation duration. However, this was based on the result of laboratory work rather than f ield investigation. Additionally, lipid accumulation is also closely related to food availability and food composition. According to previous studies, lipid accumulation is higher in copepods cultured in high concentration diets (Hakanson, 1984; Escribano and McLaren, 1992; Hygum et al., 2000; Rey-Rassat et al., 2002). However, in the f ield,C.sinicusat copepodite stage V (CV) develops larger oil sacs in the Yellow Sea Cold Water Mass where the food is def icit (Wang et al., 2009). This indicates that other factors, apart from food availability, may also aff ect lipid accumulation inCalanus. In addition to food quantity, prey type can also inf luence lipid accumulation (Hygum et al., 2000). Pepin (2011)found that higher lipid accumulation was associated withC.f inmarchicusat CV stage from a diatom dominated region and lower lipid accumulation was associated with those from dinof lagellates and prymnesiophytes dominated area. However, in the laboratory,C.sinicusshowed a signif icantly higher level of total lipid content inProrocentrummicans(dinof lagellate) diet than inSkeletonemacostatum(diatom) diet (Liu et al., 2011).
Calanussinicusis the dominant meso-zooplankton species in the Northwest Pacif ic Ocean, contributing 73%-99% of copepod biomass in the Yellow Sea(YS) (Sun et al., 2010), and 4%-76% in the East China Sea (Xu and Chen, 2007). Due to its high abundance and wide distribution,C.sinicusplays a key role in energy transfer from phytoplankton to higher trophic levels. Our previous work has studied the role of lipids during the over-summering period ofC.sinicusin the YS (Wang et al., 2017). In this work,we collectedC.sinicusfrom the East China Sea(ECS) and the YS to explore whether the lipid accumulation traits ofC.sinicusis aff ected by diff erent environmental conditions.
2 MATERIAL AND METHOD
2.1 Sample collection and environmental conditions
The study was carried out in the YS and ECS during the cruises in spring (R/VKexue3, April 6-25,2011). Samples and environmental data were collected in 3 stations in southern YS and 4 stations in the ECS(Fig.1).
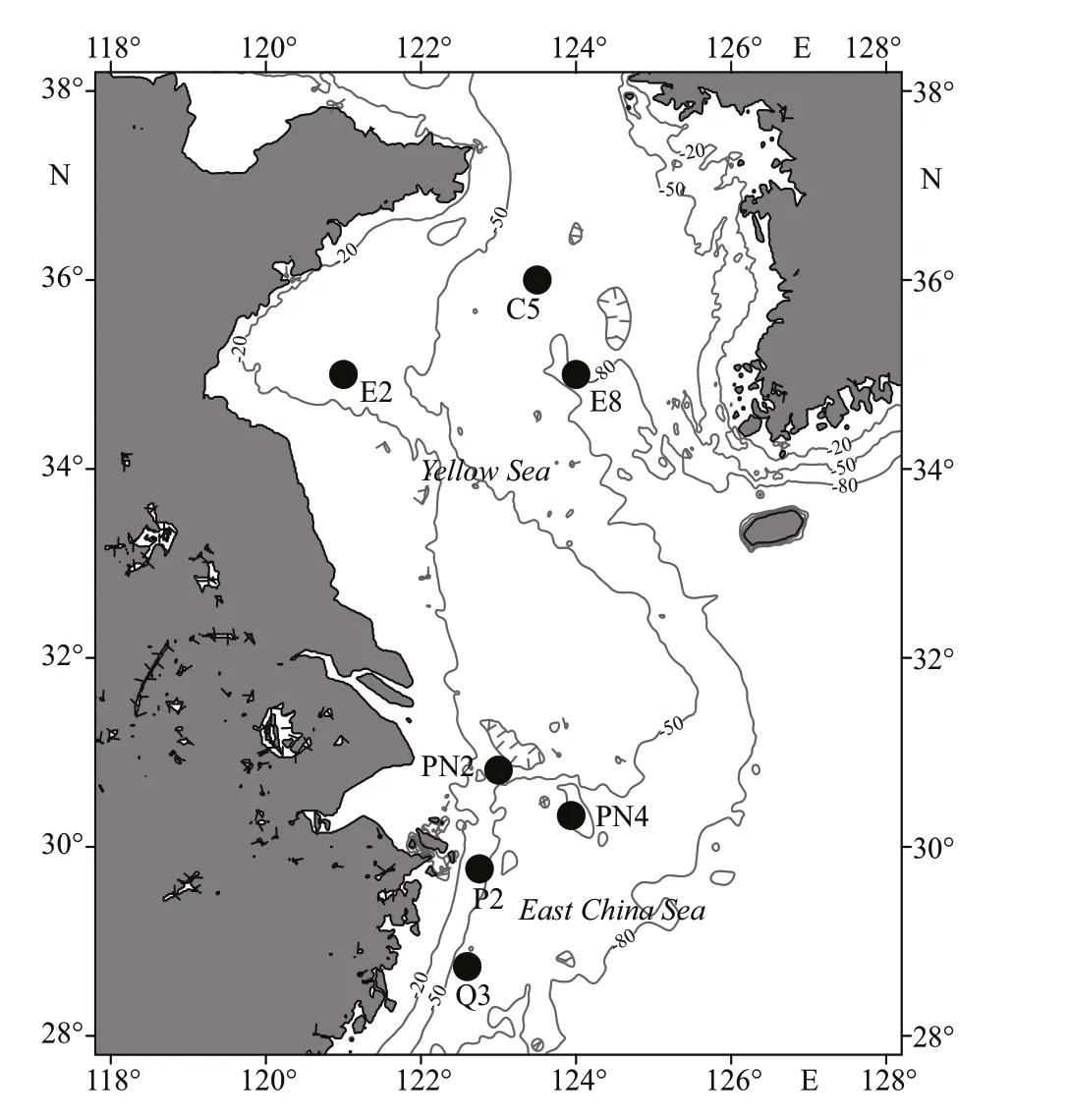
Fig.1 The sampling stations in the Yellow Sea and the East China Sea
Temperature and salinity data were collected using a Seabird Electronics 25 CTD and only the data of 5-m layer is shown in this study. Chlorophyll-a(Chla) values were determined by collecting 1 L of water from the maximum Chl-astratum using a Seabird Electronics 25 CTD. Water samples were f iltered onto 0.45-μm acetate f iber f ilters, extracted in 90% acetone for 24 h, and Chl-aconcentrations were determined using a Turner Design Fluorometer (7200).
Phytoplankton samples were collected using a plankton net (70-μm mesh size, mouth opening 0.1 m2, with a f low-meter inside), which was hauled vertically from 4 m above the seabed to the surface.The samples were preserved in 5% formalin seawater solution, and phytoplankton was analyzed microscopically. Ciliate data of this cruise from Ding and Xu (2012) was used for data analysis in the current study.
Zooplankton samples were got by another kind of plankton net (500-μm mesh size, mouth opening 0.5 m2, with a f low-meter inside), which was also hauled vertically from 4 m above the seabed to the surface. Samples were preserved in 5% formalin seawater solution.C.sinicusindividuals were counted under a dissecting microscope (Nikon SMZ645, Japan) from copepodite stage III (CIII) to adults (males and females). For lipid and fatty acid analysis, another vertical haul was conducted using the plankton net.C.sinicusindividuals were picked out according to diff erent developmental stages.However, the CIII stage and male individuals were not picked out because of their small body size and/or little abundance. Then the picked samples were rinsed with Mill-Q (Sartorius H2Opro, Germany)waters and stored in liquid nitrogen until lipid analysis.
2.2 Lipid analysis
Lipids were extracted fromC.sinicusfollowing the extraction procedures of Folch et al. (1957) and Parrish (1999). Brief ly, samples were lyophilized at-45 °C for 48 h. Total lipids were extracted using 3-mL chloroform꞉methanol (2꞉1, v꞉v) at -20 °C for 16 h. The lower chloroform phase containing the lipid extracts was transferred to a glass vial. After the addition of 0.75-mL KCl (0.88%, w꞉v), the vials were whirl-mixed and centrifuged (400×g, 2 min). The lower organic phase was transferred to a precombusted glass tube. Lipid extract was evaporated under high purity nitrogen and total lipid content was determined gravimetrically.
Wax ester, polar lipids, and triglyceride were separated by chromatography and analyzed in duplicate using an Iatroscan®MK-6 (Mitsubishi Chemical Medience, Japan) with a f lame ionization detector (Hagen, 2000). Total lipid samples were transferred to Chromarods-SIII (Mitsubishi Chemical Medience, Japan) using a microcapillary pipette(1 μL). These rods were developed twice, initially using hexane꞉benzene (1꞉1, v꞉v) and then using benzene꞉chloroform꞉acetic acid (50꞉20꞉0.7, v꞉v꞉v).The twice-developed Chromarods were then scanned on the machine at a speed of 30 s per rod and a hydrogen f low rate of 160 mL/min. Following Ohman(1997), wax ester standards for calibration were purif ied fromC.sinicusat CV stage (~500 inds.)collected from the f ield in August. Commercial standards were used for quantitative analysis:triglyceride mix for triacylglycerol and phosphotidylcholine for polar lipids.
2.3 Fatty acid analysis
Lipid classes were separated via column chromatography following Ohman (1997). Wax esters were obtained by eluting 1% diethyl ether in hexane and the eluent was collected and evaporated under high purity nitrogen. The wax ester extracts were then trans-esterif ied in 3-mL methylation reagent(methanol꞉sulfuric acid=99꞉1, v꞉v) at 50 °C for 16 h(Wang et al., 2017). After the addition of 2-mL Milli-Q water and 3-mL hexane꞉diethyl ether (1꞉1,v꞉v), samples were centrifuged (1 000×g, 10 min) for stratif ication. The upper layer was transferred to a pre-combusted vial and re-washed using 1-mL 2%(w꞉v) NaHCO3. Organic layer was separated and purif ied in thin layer chromatography(hexane꞉benzene=1꞉1, v꞉v) and fatty acid methyl esters were evaporated under high purity nitrogen.Analysis of fatty acids was carried out using an Agilent 7890A Gas Chromatography instrument equipped with a DB-FFAP capillary column (30-m length, 0.25-mm inner diameter, and 0.25-μm f ilm thickness). The temperature programming was as follows: 150 °C for 1 min, heat at 3 °C/min to 220 °C,then hold it for 33 min. The temperatures of the injector and the detector were maintained at 220 °C and 280 °C, respectively.
2.4 Data analysis
PRIMER software (Plymouth, UK) version 6(Clarke and Ainsworth, 1993) and R software version 3.2.3 were used for data analyses.
The abundance and population compositional data were subjected to q-type cluster analysis based on the Bray-Curtis dissimilarity index and group average linkage classif ication (Field et al., 1982). Based on the results of cluster analysis, nonparametric tests were used to analyze diff erences in lipid composition between diff erent cluster groups.
The fatty acid proportional data were analyzed using ANOSIM to test for diff erences between groups(cluster groups and developmental stages). Similarity Percentages ( SIMPER) and Principal Component Analysis (PCA) were used to determine the main fatty acids contribution to these diff erences (the SIMPER test was set at 70% cumulative contribution).
The BIO-ENV procedure (PRIMER software) was used to estimate which set of environmental variables(temperature, salinity, Chla, phytoplankton abundance, and ciliates) best explained the diff erences in lipid and fatty acid prof iles ofC.sinicusfrom the YS and the ECS. All the data used in this analysis were fourth root transformed. BIO-ENV analysis is based on determining the Spearman’s rank correlation coeffi cient (qw) between biological and environmental similarity matrices. A value of qw=0 would imply no match between the two matrices, while a value of qw=1 means a perfect match (Clarke and Ainsworth,1993).
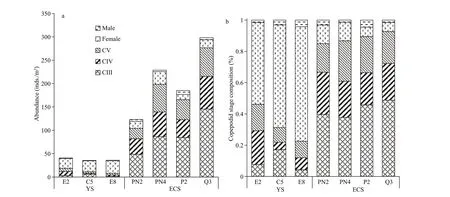
Fig.2 Abundance (a) and development stage composition (b) of C. sinicus at each sampling station in the Yellow Sea (YS)and East China Sea (ECS)
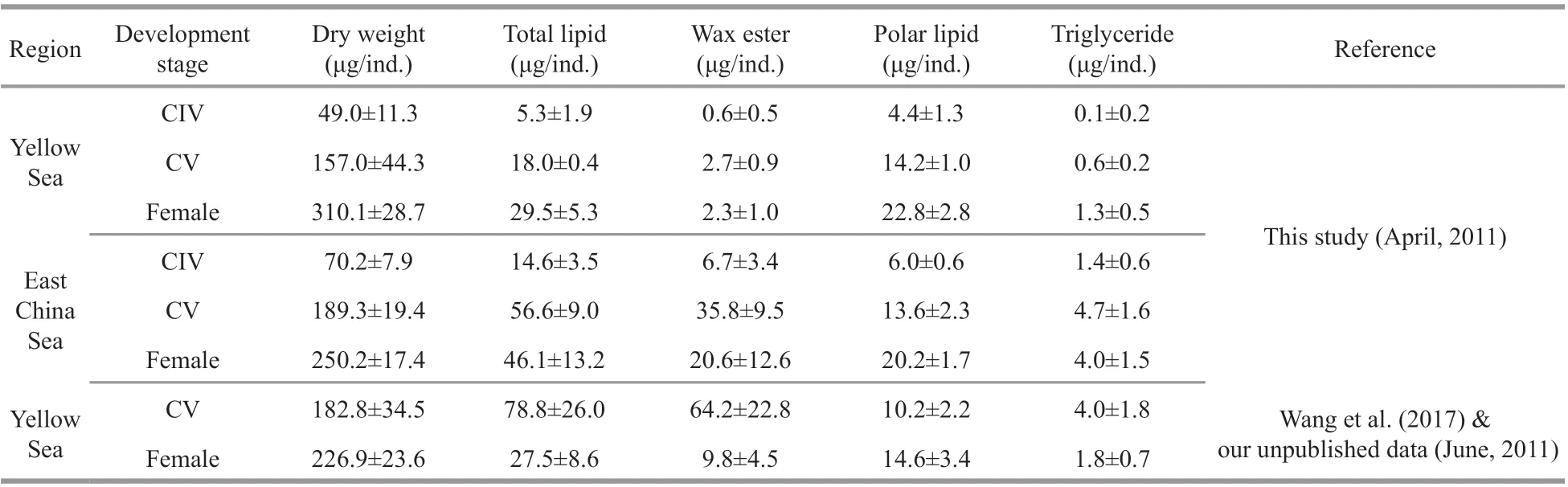
Table 1 Lipid composition of C. sinicus in the Yellow Sea and the East China Sea
3 RESULT
3.1 Abundance and development stage composition of Calanus sinicus
There were signif icant diff erences in the abundance and development stage composition ofC.sinicus(from CIII to adult) in the YS and the ECS (Fig.2).MeanC.sinicusabundance was 41 inds./m3in the YS and 224 inds./m3in the ECS.C.sinicusin the YS were dominated by female (52%-82%), while the majority (80%-94%) ofC.sinicusfrom the ECS stations was copepodites (CIII to CV).
3.2 Lipids content of Calanus sinicus
The dry weight ofC.sinicusfrom the two regions showed a similar increasing trend from early developmental stages to the adult stage (Table 1).Similarly, increasing trends were also detected in total lipid and the three lipid classes inC.sinicusfrom CIV to the adult stage, with wax ester and polar lipid being the dominant lipid class in the ECS and the YS individuals, respectively.
In addition to polar lipid, the values of total lipid,wax ester and triacylglycerol at the same stage were signif icantly higher in the ECS individuals than in the YS samples. The largest regional diff erence was observed in wax ester at the CV stage, with a mean value of 35.8 μg/ind. in the ECS samples and 2.7 μg/ind. in the YS samples. Values of wax ester content varied from 0.6 μg/ind. in the CIV stage in YS to 35.8 μg/ind. in the CV stage in ECS (Table 1).Triglyceride levels were found to be low in both sampling regions and displayed a similar variationtrend with wax esters (Table 1).
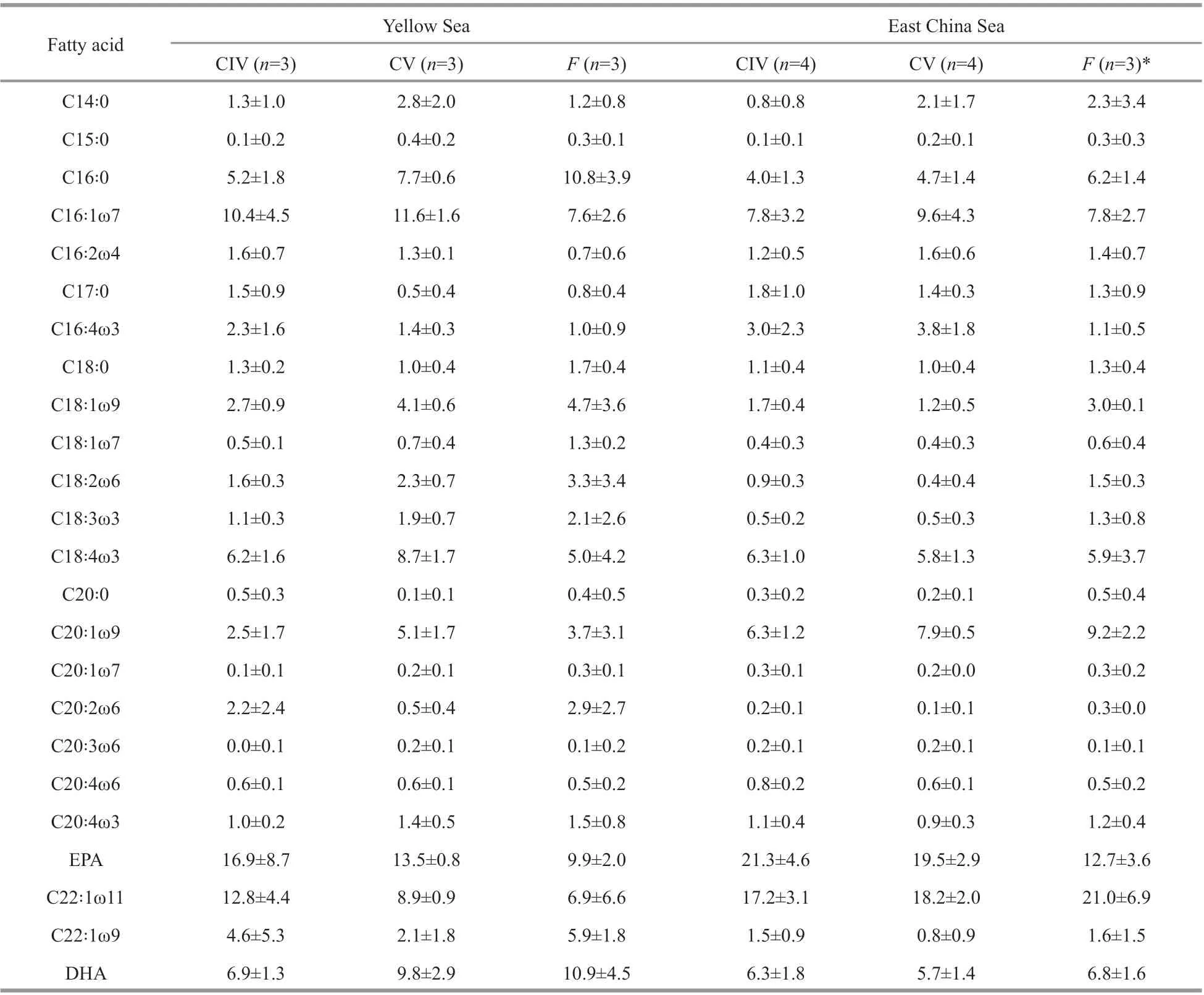
Table 2 Fatty acid composition (%) of wax esters in C. sinicus from the Yellow Sea and the East China Sea
In contrast with wax esters, polar lipids showed no regional diff erences, but did display clear diff erences between developmental stages (Table 1). Polar lipid values ranged from 4.4 μg/ind. in the CIV stage, to over 20.0 μg/ind. in adults.
Compared with data in June (Table 1), the wax ester content of CV did not reach the highest value in April in both regions. However, the wax ester content of female decreased during the next spring period(usually March to June in this study area).
3.3 Fatty acid composition of wax esters
The fatty acid compositions of wax esters inC.sinicusshowed diff erent regional characteristics between the YS and the ECS (ANOSIM,P<0.01,Tables 2 & 3). ANOSIM analysis was conducted to identify the diff erences in fatty acid compositions ofwax esters. The results showed that there were signif icant diff erences in fatty acid compositions of wax esters between the YS and the ECS. However, no diff erences were identif ied among the developmental stages in the YS or the ECS (Table 3).
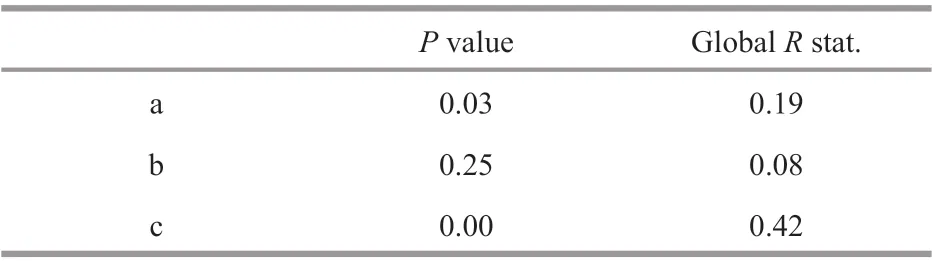
Table 3 Results of ANOSIM analysis based on the fatty acid information in Table 2
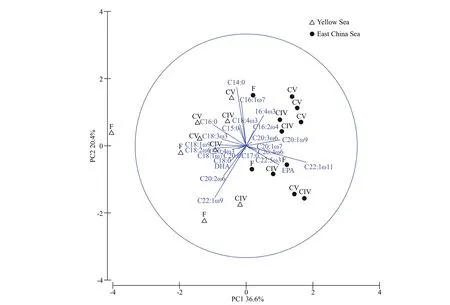
Fig.3 Principal component analysis (PCA) of C. sinicus samples based on their relative fatty acid compositions in Table 2
We identif ied the regional diff erences in fatty acid compositions using PCA analysis (Fig.3). In the PCA plot, the f irst two principal components (PC1 and PC2) accounted for 36.6% and 20.4% of the total fatty acid variations, respectively. Some kinds of fatty acid, such as EPA, C20:1ω9, and C22꞉1ω11, were more abundant in the ECS, while some fatty acids,including C22꞉1ω9, 18꞉1ω9, and DHA, were higher in the YS (Table 2, one-way ANOVA,P<0.01). The results of SIMPER analysis (Table 4) showed the main contributors (such as C22꞉1ω11, EPA, DHA,and C20꞉1ω9), which lead to the fatty acid variations between the YS and the ECS individuals. These fatty acids accounted for over 50% of total fatty acid composition.
3.4 Factors aff ecting lipid accumulation
Environmental conditions diff ered between the YS and the ECS stations. The average water temperatures were 13.3 °C in the ECS and 9.1 °C in the YS during the sampling period though there were no diff erences in salinity (Table 5). The food conditions also diff ered between the two areas. Phytoplankton cell numbers in the ECS were signif icantly lower than in the YS while ciliate abundance shown a higher level in ECS.However, the diff erences of ciliate abundance and Chl-aconcentrations were not signif icant between the ECS and the YS (Table 5).
BIO-ENV analysis was employed to identify single or combinations of environmental factors which contributed most to the variations in lipid and fatty acid compositions. The results showed that the temperature/salinity group attained the highest group score followed by temperature and temperature/salinity/ciliates group (Table 6).
4 DISCUSSION
4.1 Lipid content
Lipid prof iles diff ered between both developmental stages and regions (the YS and the ECS). Generally,Calanusbegin to synthesize wax esters at the CIII stage, reaching highest levels of lipid content at the CV stage (Kattner and Krause, 1987; Lee et al., 2006).As copepodid develops into an adult (or enters diapause in deep water), stored lipids are usually depleted gradually for reproductive or energy needs,resulting in the decline of stored lipids (Irigoien,2004; Lee et al., 2006; Mayor et al., 2009). This leads to diff erences in lipids prof iles among developmentstages ofC.sinicus(Table 1). However, regional variation in lipid content ofC.sinicusbetween the YS and the ECS may be inf luenced by internal and external factors, such as population state, food,temperature, and salinity conditions.
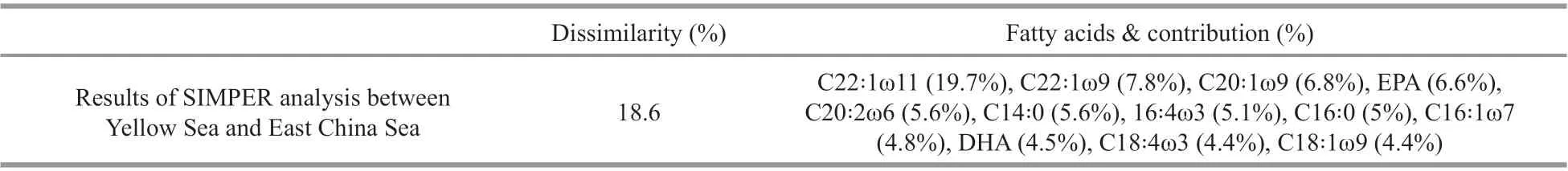
Table 4 Results of SIMPER analysis based on the fatty acid information in Table 2
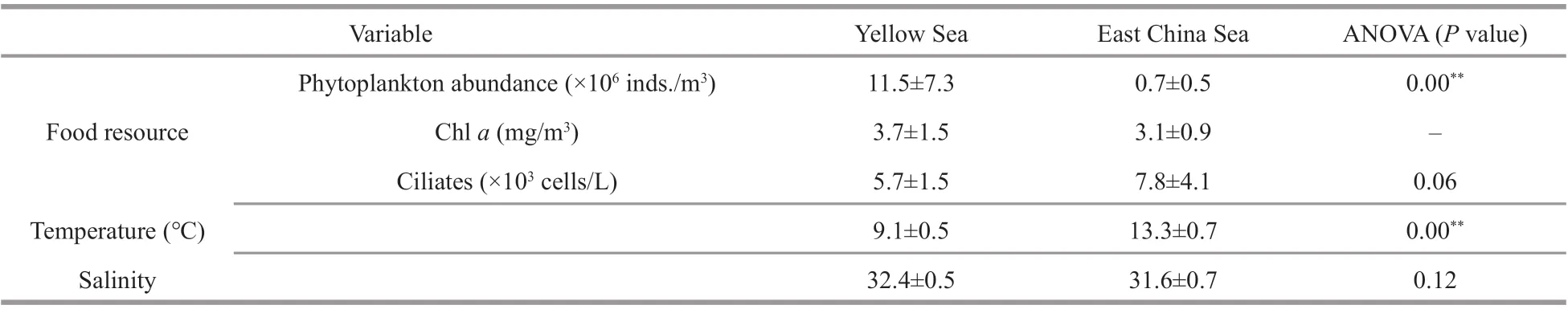
Table 5 Environmental conditions and food availability in the Yellow Sea and the East China Sea
Population state is an internal factor which may inf luence lipids prof iles ofCalanus. Seasonal diapauseCalanus, such asC.f inmarchicus, usually accumulate a large amount of lipids in spring when the population recovers from the food limited winter (Lee et al.,2006). In this study, the high proportion of copepodite stage in the ECS indicates thatC.sincusmay be recovered earlier from winter population than in the YS. Earlier studies showed that hatching speed and development of nauplii and copepodites increased as temperature elevating (Uye, 1988). The higher temperature in the ECS might be the main reason to explain the earlier population recovery ofC.sincusin the ECS. TheC.sincuspopulation in the YS and the ECS were clearly in diff erent states. Though the population state is diff erent, we compared the same development stages, from CIV stage to adult, to analyze the major external factors which may aff ect the lipid and fatty acid prof iles.
For external factors, the BIO-ENV analysis showed that temperature played an important role in determining lipid content. Temperature can aff ect metabolic rates (Arts et al., 1993; Gillooly et al.,2001) and therefore inf luence the lipid usage (e.g.during a period of resource limitation or fasting) or restore (during refeeding). In this study, reginal diff erences were mainly identif ied in wax esters and triglycerides. When food is abundant,Calanusis capable of storing large amounts of wax esters. Forexample,C.f inmarchicushas been reported to be able to store more than 20% of its body weight in wax esters (Diel and Tande, 1992). Stored wax esters are required during curial life-stage periods, such as dormancy or reproduction (Lee et al., 2006;Baumgartner et al., 2017). In this study, the food concentrations were high in both the YS and the ECS.However, the wax ester contents ofC.sinicuswere signif icantly low in the YS. This indicated that there are other factors, apart from food availability,inf luenced the wax ester contents. Previous studies have found thatC.sinicushas an optimum temperature range of 10.0-20.0 °C (Huang and Zheng, 1986), andC.sinicusshow a higher accumulation effi ciency of lipids in 13.0 °C than 10.0 °C in laboratory incubation experiment (Zhou and Sun, 2017). In this study, water temperature in the ECS was more suitable forC.sinicus. And it might helpC.sinicusto accumulate a higher level of storage lipid.
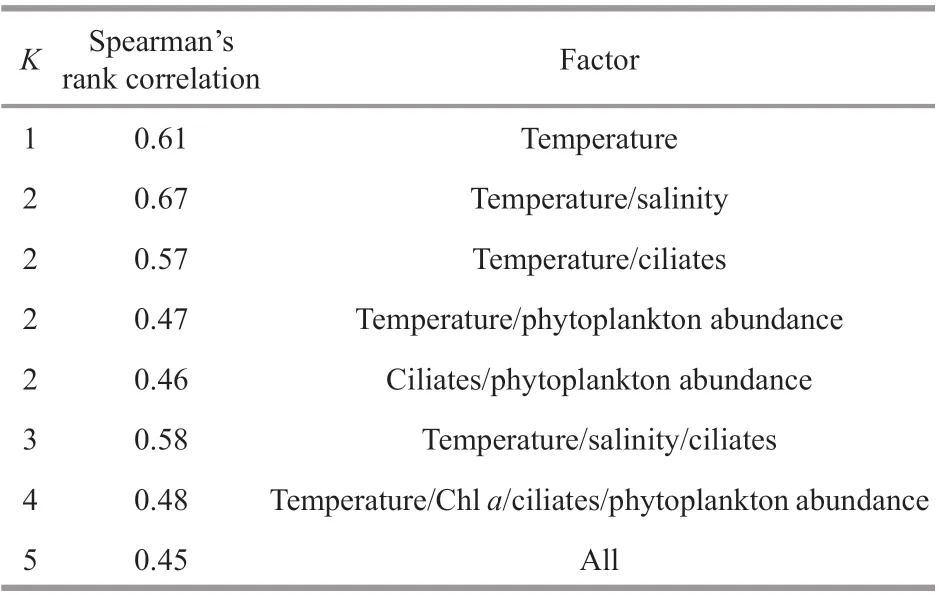
Table 6 Results of BIO-ENV analysis comparing biological data shown in Tables 1 & 2 and external condition data shown in Table 5
Unlike wax esters, triglyceride is indicative of short-term feeding (Lee et al., 2006). Previous studies have shown that triacylglycerols are utilized more rapidly than wax esters in zooplankton and the fatty acids of triacylglycerol may be more ref lective of recent food, whereas wax ester fatty acids and alcohols ref lect both dietary inf luences and de novo synthesis (Lee et al., 2006). Because of the Assimilation of dietary fatty acids and de novo biosynthesis are rapid processes (Graeve et al., 2005),higher triglyceride content could indicate a more favorable feeding state. In this study, the triglyceride content shows a higher level in the ECS than in the YS. This indicates that the feeding environments are more favorable forC.sinicusin the ECS in comparison to the YS.
4.2 Feeding diff erence
In this study, wax ester fatty acid prof iles(percentage composition) diff ered signif icantly between the YS and the ECS individuals. The main fatty acid classes ofC.sinicuswere similar to those in polar/subpolarCalanussp. (such asC.f inmarchicus).The main fatty acids recorded inC.sinicuswere C14꞉0, C16꞉0, C16꞉1ω7, C18꞉1ω9, C18꞉4ω3,C20꞉1ω9, EPA, C22꞉1ω11, and DHA (Lee et al.,2006). It has been suggested thatCalanuscan incorporate unmodif ied dietary fatty acids into storage lipids (Lee et al., 1971), with fatty acid compositions varied with food availability. Therefore, variations in food conditions between the YS and the ECS stations might be partly responsible for the regional diff erences in fatty acid prof iles.
SIMPER analysis results revealed that C22꞉1ω11,EPA, DHA, and C20꞉1ω9 were the top four contributors to the regional diff erences in fatty acid prof iles. Previous studies (Napolitano, 1999;Dalsgaard et al., 2003) have reported that C22꞉1ω11 and C20꞉1ω9 are self-biosynthesized fatty acids of herbivorousCalanusspecies, while the DHA/EPA ratio can be used for trophic position comparison. In this study, the high levels of C22꞉1ω11, C20꞉1ω9 and low DHA/EPA ratio ofC.sinicusin the ECS indicate thatC.sinicusare feeding mainly on phytoplankton in this location. Though f ield studies have reported thatC.sinicuswas an omnivorous feeding species(Sun et al., 2006), it still feeds mainly on phytoplankton.Although grazing data was not analyzed in this study,it seems thatC.sinicusin the YS feeds more nonphytoplankton food sources than in the ECS, such as ciliates, and exhibits higher C18꞉1ω9/∑herb (∑herb:the sum of 16:1ω7, 18:4ω3, and 18:1ω7) and DHA/EPA ratios. BIO-ENV analysis also showed that ciliates were important biological factors inf luencing the fatty acids prof iles ofC.sinicusthough it does not show signif icant diff erences of Chl-aconcentration and ciliate abundance between the ECS and the YS.Sun et al. (2006) found thatC.sinicusfeeds diff erently in diff erent seasons and omnivorous feeding played a more important role forC.sinicusfrom summer to autumn. In this study, the environment was diff erent between the ECS and the YS. ThenC.sinicusmay show diff erent feeding selectivity in these two regions.In addition, this f inally leads to the diff erences in fatty acids prof iles ofC.sinicus.
5 CONCLUSION
The spring phytoplankton bloom is an important period for coastal marine ecosystems. Copepods can store high levels of lipids during bloom periods. The results from this study indicate that temperature and nutritional variation directly inf luence lipid and fatty acid prof iles inC.sinicus. This is the case for many polarCalanusspecies. Temperature may be the limiting external factor aff ecting lipid storage inC.sinicus, while regional food diff erences may aff ect fatty acid composition prof iles ofC.sinicus.
6 DATA AVAILABILITY STATEMENT
The data used in the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We appreciate the assistance and enthusiasm of the crew of R/VKexue3and the team of scientists onboard.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
