Inf luences of aeration induced turbulence on growth and competition of Microcystis and Scenedesmus in the presence of sediments with varying particle sizes*
Qiang HE , Yiyao WANG , Ming LI ,**
1 College of Natural Resources and Environment, Northwest A&F University, Yangling 712100, China
2 Chengdu Environmental Protection Research Institute, Chengdu 610072, China
Abstract Aeration is an important measure to prevent cyanobacterial growth in eutrophic lakes and reservoirs. The purpose of this study is to clarify the inf luence of aeration induced turbulence on growth and competition of Microcystis and Scenedesmus in the presence of sediments with varying particle sizes.Microcystis aeruginosa and Scenedesmus obliquus were selected as the model organisms. Sediments with varying particle sizes were added into mono and mixed cultures of the organisms. In the absence of sediment,both low- and high-intensity aerations (the turbulent dissipation rates were 1.60×10 -6 and 1.16×10 -5 m 2/s 3,respectively) promoted the growth of Scenedesmus, but the growth of Microcystis was inhibited particularly obvious under the high-intensity aeration conditions. In the presence of sediment, Scenedesmus was promoted under all aeration conditions, while Microcystis was inhibited. The inhibition rate of Microcystis decreased with the increase of sediment size when treated with low-intensity aeration in the nighttime.The highest inhibition rate of M. aeruginosa (89.2%) was identif ied under the condition of low-intensity aeration in the nighttime with small sediment addition. Furthermore, our results indicated that the impacts of sediment-induced light intensity reduction on algal growth were insignif icant. In the mixed culture, the growth of Microcystis was inhibited by Scenedesmus in all treatments with aeration. Our results provided a theoretical basis for the practice in controlling cyanobacteria by aeration.
Keyword: aeration induced turbulence; suspended sediment; phytoplankton community; cyanobacteria;Scenedesmus
1 INTRODUCTION
Cyanobacterial blooms, especiallyMicrocystisblooms, is a major water environmental issue around the world (Jacoby et al., 2000; Paerl and Otten, 2013).Artif icial mixing such as aeration has been used as a measure to prevent cyanobacterial blooms in eutrophic lakes and reservoirs for many years (Visser et al., 2016). Lehman (2014) demonstrated that a shift from cyanobacteria to green algae was often found in lakes after long-term aeration, thus the cyanobacterial blooms was controlled. However, the utilization of aeration for cyanobacterial blooms management has not always been successful. It was identif ied that there were no signif icant eff ects of aeration on the cyanobacterial blooms in Lake Yogo (Japan) and Lake Sheldon (the United States) (Oberholster et al.,2006; Tsukada et al., 2006). Therefore, it is still required to further understand the mechanisms of cyanobacteria control using aeration, which will eff ectively guide the implementation of aeration for water environments management.
Aeration and agitation can aff ect the growth and physiology of algae by means of changing the hydrodynamic conditions (Sitanggang and Lynett,2010; Fouxon and Leshansky, 2015; Sengupta et al.,2017). As a microcosmic index to directly evaluate the turbulence intensity, the turbulence dissipation rate can eff ectively ref lect the magnitude of hydrodynamic conditions. Li et al. (2017)demonstrated that turbulence promoted the growth ofAphanizomenonf los-aquaewhen the turbulent dissipation rate increased from 0 to 0.022 59 m2/s3but was inhibited when the value increased to 0.050 58 m2/s3.Xiao et al. (2016) found that the growth rate ofAnabaenaf los-aquaeincreased f irstly and then decreased when the turbulent dissipation rate increased from 0 to 8.01×10-2m2/s3. They also found that the growth rate ofAnabaenaf los-aquaereached its maximum value when the turbulent dissipation rate was 2.26×10-2m2/s3. O’Brien et al. (2004)reported a similar phenomenon and the critical turbulent dissipation rate toMicrocystisaeruginosawas 7×10-6m2/s3. Song et al. (2018) found that the superoxide dismutase (SOD) and catalase (CAT)activities ofMicrocystiswere lower at the lower energy dissipation rate compared to those at the higher energy dissipation rate. All the above results indicated that the increase of turbulence intensity f irstly promoted the algal growth and then inhibited the growth of algae.
Turbulence may also change phytoplankton community composition (Jungo et al., 2001; Zhu et al., 2013; Barton et al., 2014). Heo and Kim (2004)found that cyanobacteria were replaced by diatoms after aeration in Lake Dalbang (South Korea). Becker et al. (2006) found a decline of cyanobacteria and the increase of green algae and diatoms in Bleiloch reservoir (Germany) when treated with aeration.However, these studies did not provide insight into the turbulence on competition among various phytoplankton. In addition, Huisman et al. (2004)showed that turbulence would aff ect the competitive relationship between cyanobacteria and green algae and indicated that cyanobacteria dominated under the condition of low turbulence intensity, but the eff ects of sediment were not considered.
Artif icial mixing would inevitably cause the suspension of sediment (Schallenberg and Burns,2004; Matisoff et al., 2017). Suspended sediment directly aff ects the light intensity in water, thus aff ecting the growth and the competition of phytoplankton. Watermann et al. (1999) found that sediment in the range of 63-200 μm promoted the growth ofMicrocoleuschthonoplasteswhile the sediment smaller than 63 μm showed an inhibitory eff ect. Brasil et al. (2018) found thatCylindrospermopsisraciborskiicould grow in the suspension of kaolinite and bentonite with particle sizes of 7.5 μm and 10.3 μm, respectively, and the growth rate in the kaolinite suspension was higher than that in the bentonite suspension. Since both kaolinite and bentonite are similar to the solid particles of sediment, it is assumed that sediment particle size may have comparable inf luence on algae. However,our knowledge about the eff ects of sediment particle size on the growth of phytoplankton is still limited.
The aim of this study was to understand the eff ects of turbulence on the growth and the competition of phytoplankton in the presence of sediments with varying particle sizes. In this study,MicrocystisaeruginosaandScenedesmusobliquuswere selected as the model organisms. Sediments with varying particle sizes were added into mono and mixed cultures of these organisms. The results may provide eff ective theoretical guidance to use the aeration for cyanobacteria management.
2 MATERIAL AND METHOD
2.1 Organisms and sediment
Unicellular strains ofMicrocystisaeruginosa(FACHB-469) andScenedesmusobliquus(FACHB-416) were purchased from the Institute of Hydrobiology (FACHB collection), Wuhan, Hubei Province, China. Both strains were axenically cultured in BG-11 medium (NaNO31 500 mg/L, K2HPO3·3H2O 40 mg/L, MgSO4·7H2O 75 mg/L, CaCl2·2H2O 36 mg/L,Na2CO320 mg/L, Ferric citrate 6 mg/L,Na2EDTA·2H2O 1 mg/L, ammonium ferric citrate 6 mg/L, Trace mental solution 1 mg/L, and pH 7) for three months prior to the experiments. The use of BG-11 medium to cultivate cyanobacteria and green algae has been recognized as the most popular method, and the nutrient concentration in BG-11 medium is suitable for the growth of cyanobacteria and green algae. In the experiment, the algae in the maximum growth phase (logarithmic growth period) were selected for inoculation.
Sediment was collected from the Weihe River, in Yangling section of Shaanxi Province, in November 2018. Sediments in the surface layer were collected using Petersen Grab Sampler and were then transferred to the laboratory for our experiments. The chemical properties of sediment are shown in Table 1. The distribution of the sediment particle size was in the range of 0.9-851 μm. Sediment was divided into three groups: small particle size (10-38.5 μm), medium particle size (38.5-75 μm), and large particle size(75-150 μm), which was dried prior to the experiments. The sediments larger than 150 μm were removed using sieves because they were very diffi cult to be suspended when treated with mixing. The small size sediment was obtained using a settling column and the others groups were obtained using sieving method. A laser particle size analyzer (2000E,Mastersizer, UK) was used to determine the size distribution of the sediment and the result was shown in Fig.1a.
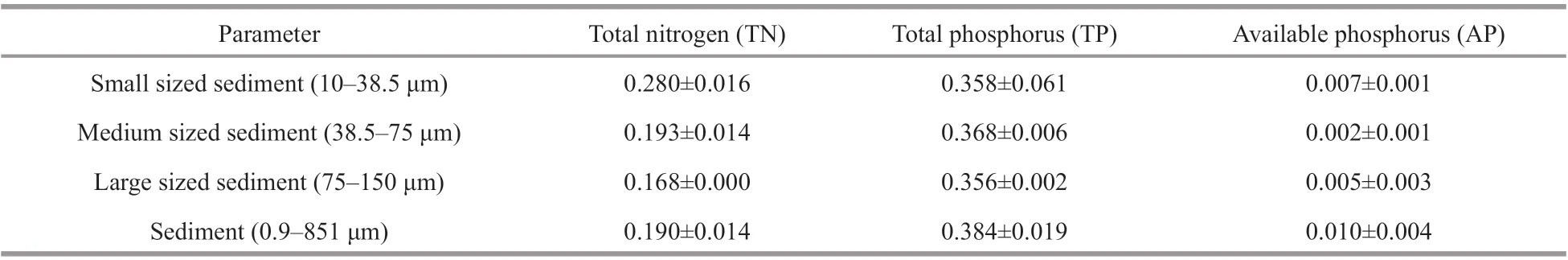
Table 1 The chemical properties (g/kg (mean±SD)) of sediment samples
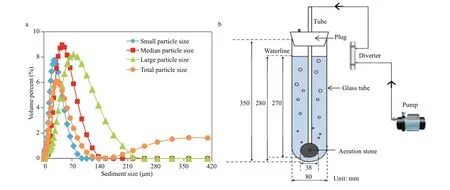
Fig.1 Particle size distribution of the sediment (a) used in the current study and the schematic diagram of the experimental apparatus (b)
2.2 Experimental design and culture conditions
A cylindrical reactor equipped with an aeration device was used for algae cultivation in this study.The cylindrical reactor was a glass tube with an inner diameter of 8 cm and a volume of 1.5 L (Fig.1b). Both mono and mixed cultures in BG-11 culture medium were treated with aeration and sediment addition.Aeration treatments were carried out in both daytime and nighttime which were mimicked in an incubator and the aeration intensity was divided into lowintensity (0.16 L/min) and high-intensity (1.2 L/min).For every batch experiment, aeration was carried out for 45 min, afterwards, the samples were rested for 15 min. This time interval was allowed for the aerator pump to dissipate heat. For each aeration treatment,sediments in diff erent particle sizes (small, medium,and large) were added. The concentration of dried sediment was adjusted to 500 mg/L.
Both mono and mixed cultures were carried out at 25 °C under a 12-h꞉12-h light-dark cycle in BG-11 medium. Light intensity was 50 μmol photons/(m2·s).The initial cell densities ofM.aeruginosaandS.obliquuswere 10×104cells/mL. Experiments were conducted for 12 days. Experiments on each treatment were conducted in triplicate.
2.3 Chemical analysis
Zeaxanthin (Zeax) is usually considered as the characteristic pigment ofM.aeruginosa. Lutein (Lut)and chlorophyllb(Chlb) are the characteristic pigments ofS.obliquus. Additionally, chlorophylla(Chla) is the common pigment forM.aeruginosaandS.obliquus. They were analyzed daily via high performance liquid chromatography (HPLC,Agilent-1100 series, USA) to indicate the biomass ofM.aeruginosaandS.obliquus, respectively. In this study, Zeax, Lut, Chlband Chlawere purchased from Sigma-Aldrich for pigment analysis. Except for the concentration of standard substance Zeax which is 2 mg/L, the concentration of other standard substance pigments is 20 mg/L.
Algae culture of 10 mL was f irstly f iltrated through a glass f iber membrane (GF/F, Whatman, UK). Then,the f iltered membrane was transferred into a 25-mL test tube with a stopper. Injected with 5-mL acetone solution (99.5%), the tube was treated with ultrasound at 4 °C for 10 min. The tube was then frozen at -20 °C in darkness. Afterwards, the extract liquid was injected into a 1.5-mL brown chromatographic bottle for HPLC analysis. The packing material of chromatographic column is YMC-Triart C18/S-5 μC/12 nm, and the f low rate is 1.00 mL/min. The HPLC elution was conducted as per the procedure suggested by Tian et al. (2019).
2.4 Assessment of turbulence intensity
The turbulent dissipation rate is a direct indicator to evaluate the intensity of turbulence, which can avoid the inf luence of the reactors on the test results.Signif icantly diff erent turbulent intensities could be induced by the same aeration intensity when the reactors are diff erent. Computational f luid dynamics(CFD) model was used to calculate the turbulent dissipation rate in the reactors treated with diff erent aeration intensities. The CFD model was constructed according to the method described by Feng et al.(2020). In this study, the turbulent dissipation rates of the experimental apparatus were 1.60×10-6and 1.16×10-5m2/s3corresponding to the low-intensity and high-intensity aerations.
2.5 Statistical analysis
The inhibition rate caused by sediment in monoculture was calculated according to Eq.1:
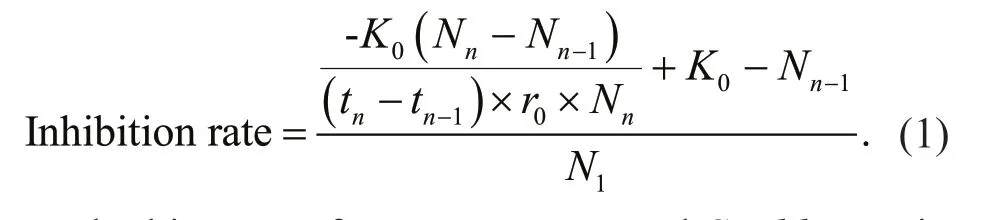
The biomass ofM.aeruginosaandS.obliquusin the monoculture, without sediment addition, aerated during the daytime was selected as the control for each treatment. In Eq.1,K0andr0represent the carrying capacity and the intrinsic growth rate of control, respectively.Nn,Nn-1, andN1represent the abundances of algal biomass with other treatment attn,tn-1, andt1(days), respectively.
A Lotka-Volterra’s competition model was conducted to assess the eff ects of sediment and aeration on the competition betweenM.aeruginosaandS.obliquus. The method was described in our previous work (Zhao et al., 2021). The signif icance was assessed using one-way ANOVA (P<0.05).
3 RESULT
3.1 Growth curves of M. aeruginosa and S. obliquus in monoculture
In the absence of sediment, the biomass ofM.aeruginosaunder low-intensity aeration was higher than that under high-intensity aeration (Fig.2).However, there was no signif icant diff erence in the biomass ofS.obliquusunder diff erent aeration intensities (Fig.3).
Under the conditions of small, medium, and large size and sediments low-intensity aeration during the daytime, the biomass ofM.aeruginosawas the largest, with 2.00, 2.42, and 2.65 mg/L, respectively.The biomass ofM.aeruginosaunder low-intensity aeration was higher than that under high-intensity aeration for all sizes of sediment. However, there was no signif icant diff erence in the biomass ofS.obliquusunder diff erent aeration modes. Compared with the absence of sediment, the biomasses ofM.aeruginosaandS.obliquuswere the lowest under the aeration mode with small size sediment.
3.2 Growth curves of M. aeruginosa and S. obliquus in mixed culture
In the absence of sediment, there was no signif icant diff erence in the biomass ofM.aeruginosaunder diff erent aeration intensities. The biomass ofS.obliquusunder low-intensity aeration was greater than that of high-intensity aeration in the daytime(Fig.4), while the biomass under high-intensity aeration was higher than that of low-intensity aeration in the nighttime (Fig.5).
In the presence of small, medium, and large size sediments, there was no signif icant diff erence in the biomass ofM.aeruginosaunder diff erent aeration modes. However, the biomass ofS.obliquusunder low-intensity aeration was greater than that of highintensity aeration in the daytime. The results were just the reversed in the nighttime. The biomass ofM.aeruginosawas much lower than that ofS.obliquusunder diff erent aeration modes.
3.3 Inhibition rates of M. aeruginosa and S. obliquus in monoculture

Fig.2 Growth curves of M. aeruginosa in monoculture under diff erent aeration intensities during daytime/nighttime
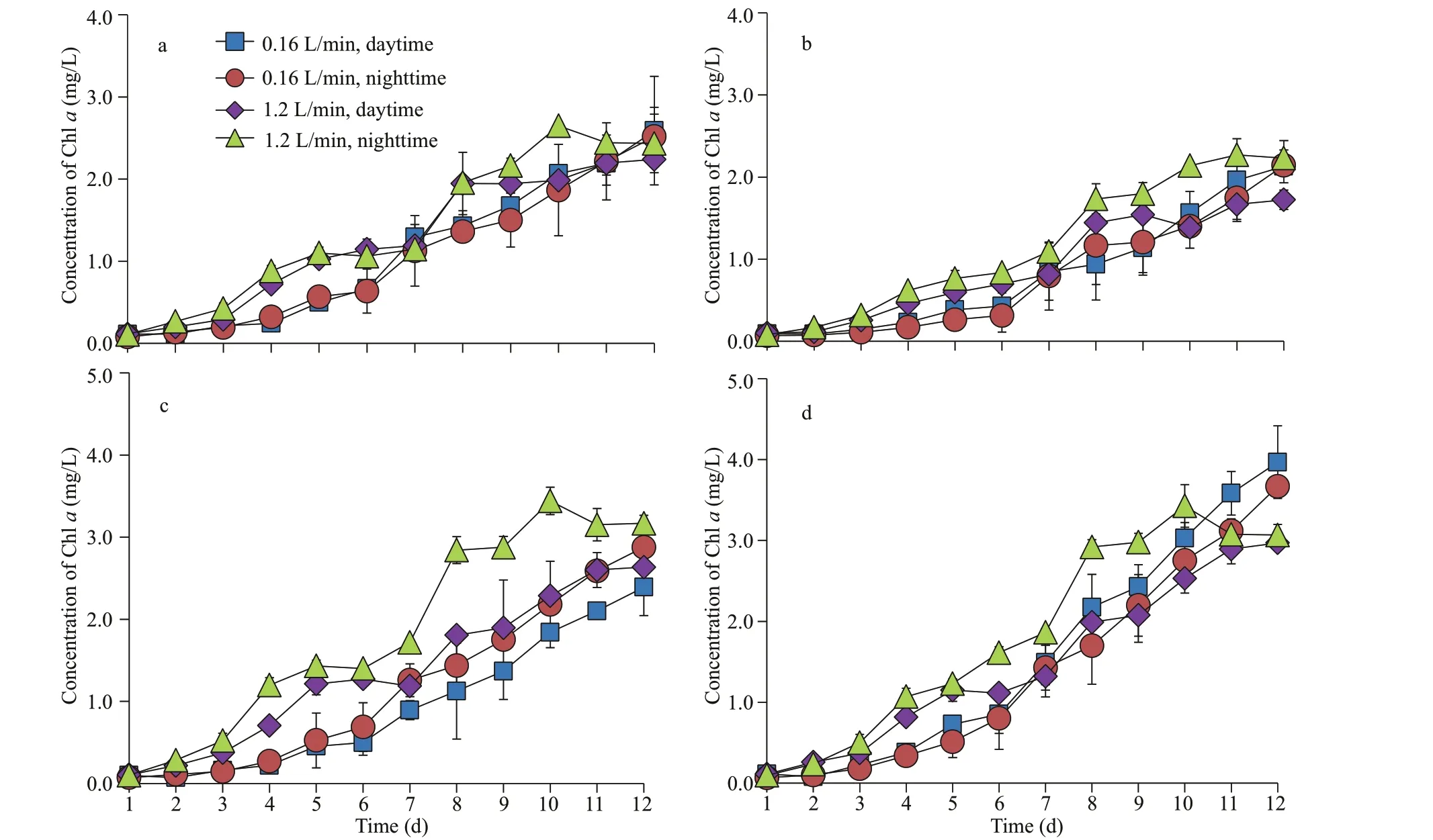
Fig.3 Growth curves of S. obliquus in monoculture under diff erent aeration intensities during daytime/nighttime
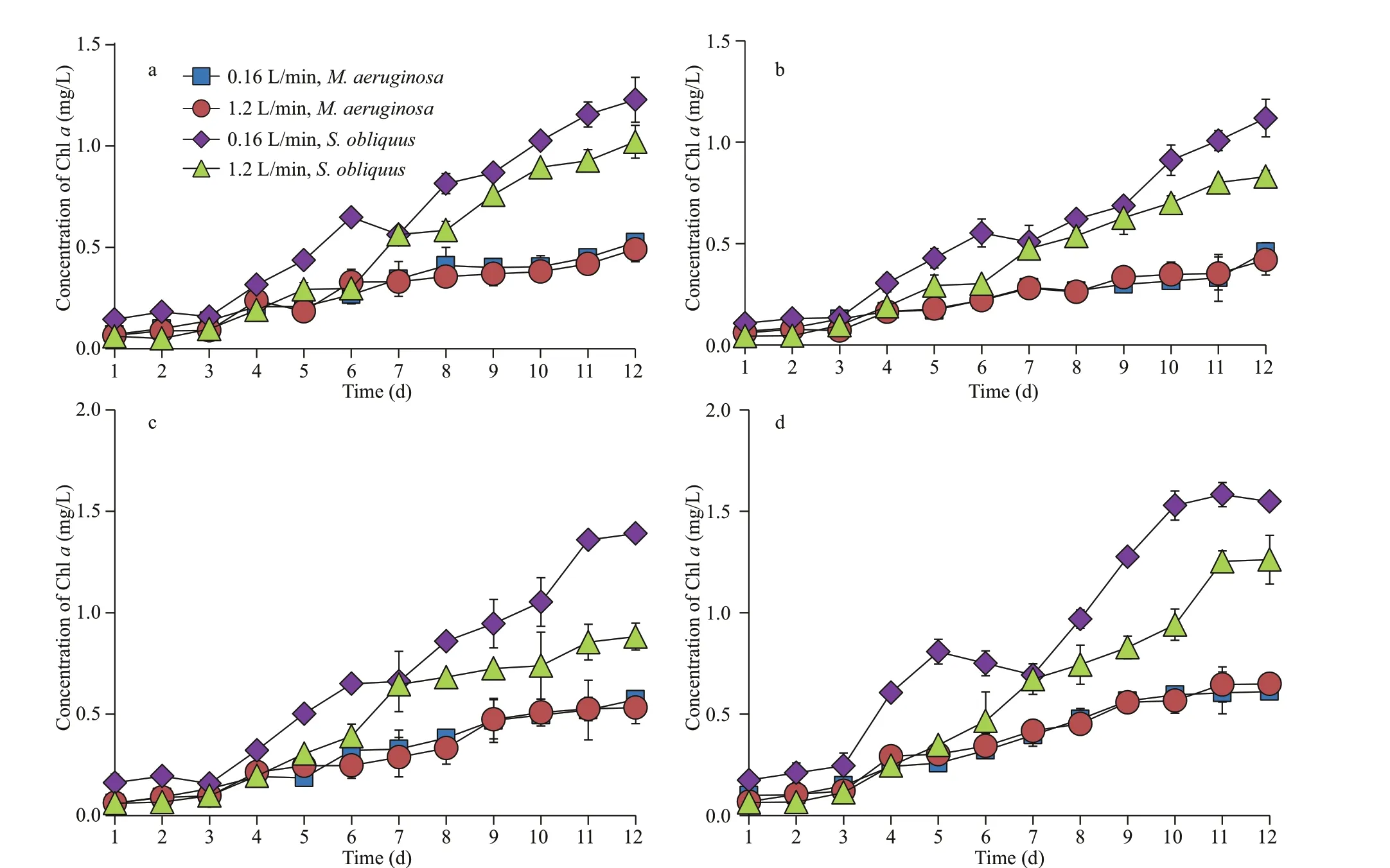
Fig.4 Growth curves of M. aeruginosa and S. obliquus under diff erent aeration intensities during daytime in mixed culture
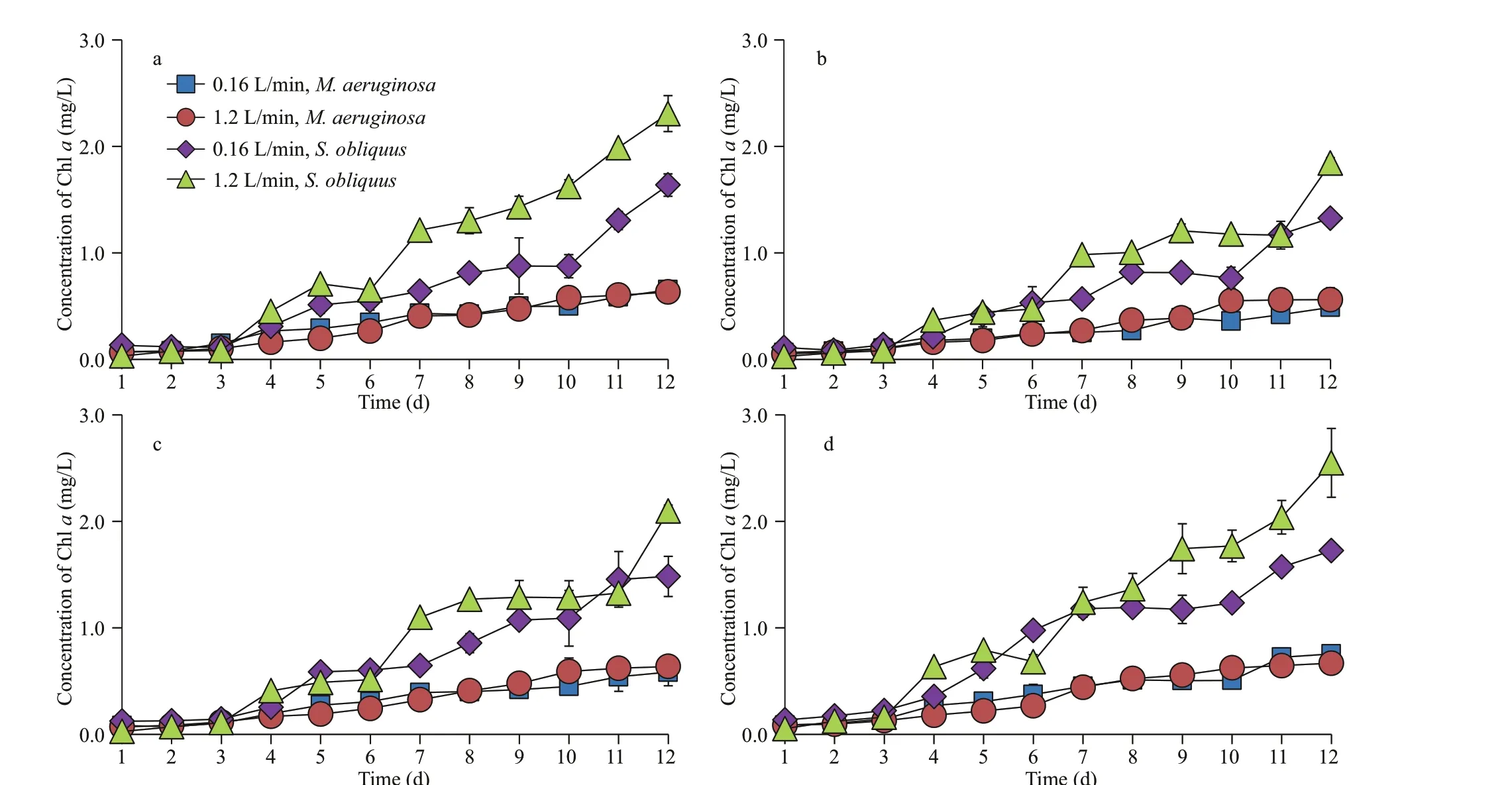
Fig.5 Growth curves of M. aeruginosa and S. obliquus under diff erent aeration intensities during nighttime in mixed culture
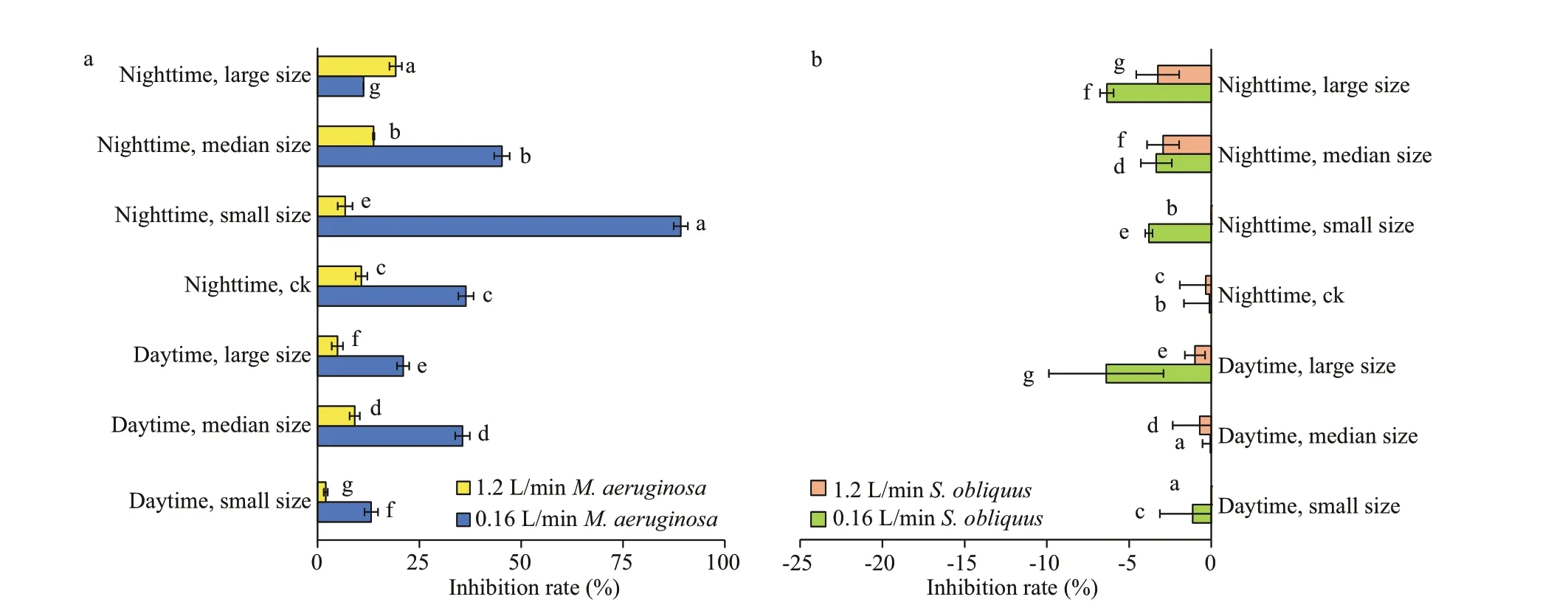
Fig.6 Inhibition rates of M. aeruginosa (a) and S. obliquus (b) in monoculture under diff erent aeration intensities during daytime/nighttime
Regardless of the presence of sediment, the inhibition rate ofM.aeruginosaunder low-intensity aeration was greater than that under high-intensity aeration. With the small size sediment addition, the inhibition rate ofM.aeruginosawas the highest with the low-intensity aeration in the nighttime, which was 89.2% (Fig.6a). The inhibition rate ofS.obliquuswas negative under various treatments, indicating a certain promotion eff ect (Fig.6b).
3.4 Competition coeffi cient of M. aeruginosa and S. obliquus in mixed culture
In the absence of sediment, competition coeffi cientα(S.obliquusagainstM.aeruginosa) decreased with the increase of aeration intensity, butβ(M.aeruginosaagainstS.obliquus) did not change with the increase of aeration intensity. The growth ofM.aeruginosawas inhibited byS.obliquusunder low-intensity aeration, but there was no inhibition eff ect betweenM.aeruginosaandS.obliquusunder high-intensity aeration (Fig.7).
In the presence of small, medium, and large size sediment, the growth ofM.aeruginosawas signif icantly inhibited byS.obliquusunder lowintensity aeration, which was higher than that cultured without sediment. The maximum competition coeffi cient was 12.1 when the small size sediment was aerated in the daytime. There was no inhibition eff ect betweenM.aeruginosaandS.obliquusunder high-intensity aeration.
4 DISCUSSION
Our results show that aeration inhibited the growth ofM.aeruginosabut promoted the growth ofS.obliquus. This phenomenon indicated that the tolerance ofScenedesmusto the turbulence was higher than that ofMicrocystis. Species-specif ic tolerance to turbulence was also reported by Hondzo and Wüest (2009). Thomas and Gibson (1990)reported that cyanobacteria was more sensitive to turbulence than other algae. Yu et al. (2018) found that the green algaeChlorellawas superior to the cyanobacteriaMicrocystisin highly turbulent water environments. Mitsuhashi et al. (1995) showed that the diff erences in size and shape of algal cells would lead to variations in algal nutrient transport in turbulent water and thus aff ecting the algal growth.Algae with larger cell size may have higher tolerance to turbulence (Cross et al., 2014). The cell size ofMicrocystiswas much lower than that ofScenedesmusandChlorella. This could be the main reason thatMicrocystiswas less resistant to turbulence compared toScenedesmus.
Turbulence generated by aeration can directly aff ect algal growth by producing shear stress but can also indirectly inf luence algal growth by changing the light and nutrient resources available to the algae(Chengala et al., 2013; Wilkinson et al., 2016).Moderate intensity turbulence promoted algal growth principally because turbulence motivated the exchange of the upper and lower layers of the water body, so that algae can get more light and uptake more nutrient (Warnaars and Hondzo, 2006). Moreover, the locally higher concentration of metabolites around the algae was also reduced (Grobbelaar, 1994).However, high intensity turbulence produced shear stress which may cause mechanical damage to algae and thus inhibit algal growth (Hondzo and Lyn, 1999;Barbosa et al., 2004; Michels et al., 2016).
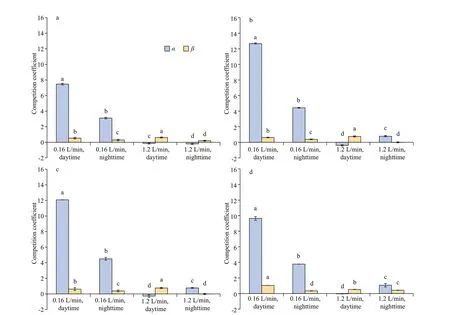
Fig.7 Competition coeffi cient of M. aeruginosa and S. obliquus in mixed culture under diff erent aeration intensities during daytime/nighttime
On the other hand, aeration also provided more carbon dioxide which was essential for algal photosynthesis (Gao et al., 1999). In the daytime, the carbon dioxide provided by aeration could be used directly for algal photosynthesis (Qiu and Gao, 2002),but the carbon dioxide was unlikely to be used in the nighttime (Harvey and Macko, 1997). Compared with daytime, the inhibitory eff ect of aeration in the nighttime onMicrocystisand the promotion toScenedesmusin the monoculture were stronger.Nevertheless, the growth ofMicrocystiswas also inhibited by aeration in the daytime. At this time,carbon dioxide provided by aeration may promote the growth ofMicrocystisto a certain degree, but the overall eff ect was inhibition. The inhibition rate of aeration in the nighttime toScenedesmuswas greater than that in the daytime. This may be due to the facultative heterotrophic ability ofScenedesmus,which meansScenedesmuscan grow well using organic carbon sources in darkness (Liang et al.,2009; Heredia-Arroyo et al., 2011).
Several studies have reported that sediment in water environments could aff ect algal biomass by means of inf luencing light attenuation and adsorbing nutrients (Guenther and Bozelli, 2004; Schallenberg and Burns, 2004; Effl er et al., 2012). He et al. (2017)demonstrated that sediment aff ected algal growth by adsorbing or releasing nutrients. Kang et al. (2019)found that sediment could inhibit algal photosynthesis by reducing light intensity. In the current study, there was no obvious diff erence in the growth of bothM.aeruginosaandS.obliquusbetween the daytime and nighttime treatments with sediment addition. This phenomenon indicated that sediments merely reduced the light intensity, and subsequently aff ected algal growth in the current study. In monocultures, the inhibition rate ofM.aeruginosadecreased with the increase of sediment particle size when treated with low-intensity aeration in the nighttime. The reason could be that the sediments with larger particle size were more prone to attenuate turbulence intensity at microscale.
Some studies indicated that turbulence aff ected the phytoplankton communities. The dominant species changed from cyanobacteria to green algae or diatoms when a lake was treated with aeration (Visser et al.,1996; Qu et al., 2018). This was mainly attributed to the fact that the turbulence reduced the f loating advantage of cyanobacteria (Oliver, 1994; Huisman et al., 2004). The current study found that the growth ofM.aeruginosawas inhibited byS.obliquuswhen treated with aeration, which was consistent with the previous studies (Zhou et al., 2016).
5 CONCLUSION
Our results demonstrate thatScenedesmuswas more resistant to the turbulence thanMicrocystisin monocultures. In the presence of sediment,S.obliquuswas promoted, whileM.aeruginosawas inhibited under all aeration conditions. The highest inhibition rate ofM.aeruginosawas identif ied under the condition of low-intensity aeration in the nighttime with small sediment addition, which was 89.2%. Our results indicated that aeration induced turbulence could be used to mitigate the harmful algal blooms,particularly under the small sediment particle size condition. Furthermore, our results indicated that sediments had insignif icant eff ect on the algal growth by reducing the light intensity. Artif icial mixing could be an eff ective measure to promote a shift in phytoplankton composition from cyanobacteria to green algae. Our results provided a theoretical guidance to use the aeration for cyanobacteria management. It is also suggested that the f ield study of variation in phytoplankton community caused by the artif icial mixing should be undertaken in the future.
6 DATA AVAILABILITY STATEMENT
Data are available upon reasonable request from the authors.
 Journal of Oceanology and Limnology2022年1期
Journal of Oceanology and Limnology2022年1期
- Journal of Oceanology and Limnology的其它文章
- The adjoint-based Two Oceans One Sea State Estimate(TOOSSE)*
- Structure and formation of the South Yellow Sea water mass in the spring of 2007*
- Lagrangian eddies in the Northwestern Pacif ic Ocean*
- Seasonal variability in dissolved oxygen in the Bohai Sea,China*
- In-situ experiments reveal mineralization details of porphyry copper deposits
- Chemical composition and Pb(II) binding of dissolved organic matter in a hypersaline lake in China*
