生物质热解过程中氮迁移转化机理研究进展
刘 亮,郑 扬,黄思彪,肖庭熠,田 红,卿梦霞
生物质热解过程中氮迁移转化机理研究进展
刘 亮,郑 扬,黄思彪,肖庭熠,田 红,卿梦霞※
(长沙理工大学能源与动力工程学院,长沙 410114)
生物质热解产物中热解气和热解油具有较高能源利用价值,可作为替代燃料或化工原料,但伴随热解过程迁移至热解气/油中的氮元素不仅会影响其品质,热解气/油进一步利用后也会污染大气环境。该研究围绕生物质资源制备清洁能源的总目标,系统分析生物质热解过程中氮迁移转化机理,重点论述气相氮、液相氮和焦炭氮的生成与转化机理。通过总结前人研究,得出生物质热解气中的含氮物质主要为HCN、NH3等,其中NH3主要来源于氨基酸热解释放的氨基以及HCN在焦炭表面的水解转化;HCN主要来源于腈、含氮杂环等一次热解产物的二次裂解;热解油中的含氮物质主要为含氮杂环、腈与酰胺,其中含氮杂环主要由部分氨基酸片段或氨基酸间的脱水缩合反应产生;腈主要来源于氨基酸分子脱H2反应以及酰胺脱H2O反应;酰胺主要来源于NH3与羧基的置换反应。不同生物质种类与热解工况下氮的迁移转化特性复杂多样,生物质种类以及热解过程中的压力、停留时间、升温速率、温度、热解气氛、粒径、催化剂等因素均会影响热解过程中氮的迁移转化路径,最终影响生物质热解气/油中含氮物质的组成及分布。进一步提出生物质热解过程中氮排放控制未来研究方向,以期为实现农村生物质资源高效清洁利用提供参考。
生物质;热解;气相氮;液相氮;焦炭氮;NO
0 引 言
生物质具备分布广泛、易于存储运输、近零排放等优点,在世界能源战略中的地位日益凸显[1]。生物质废弃物转化为生物能源主要有热化学和生物化学2种途径,其中,热解技术可以减少土地资源占用且适用性广,是生物质资源化利用中最有前景的技术之一[2]。生物质热解产物中的热解气与热解油可作为能源燃料或化工原料,其中热解气可直接作为高热值可燃气;热解油可直接作为液体燃料,其中含有吲哚、酰胺等也是重要的化工原料[3]。
生物质中蕴含丰富的氮元素[4],其在热解过程中会迁移至热解气/油等产物中,转化为相应的NO前驱物或其他含氮物质。NO前驱物存在于热解气中,主要为HCN、NH3和HNCO,其中NH3被吸入肺后容易通过肺泡进入血液,与血红蛋白结合,破坏运氧功能;HCN是一种剧毒气体,严重危害人体健康。NO前驱物在空气中燃烧又会转化为NO,严重影响人体健康与环境安全[5]。而含氮物质主要以含氮杂环、腈与酰胺等形式存在于热解油中,其含量过高时会影响热解油品质[6]。部分含氮杂环高温下易分解生成HCN与HNCO,腈具有生物毒性,且高温下易分解生成HCN[7]。
生物质作为未来重要的清洁能源,掌握其热解过程中N的迁移转化路径与规律,有助于控制其热解过程中控制含氮物质向热解气/油中的转化,对生物质能源的高效化、清洁化利用具有重要意义。
1 生物质中氮的赋存形态
生物质中氮元素主要赋存于蛋白质中,约占生物质中氮总量的60%~80%;有5%的氮以游离形态的氨基酸形式存在;其余则存在于核酸、叶绿素、酶、维生素和激素等物质中[8]。不同生物质中蛋白质氮含量略有差异,如:稻壳与豆萁中蛋白质氮含量高达92%~97%,木质生物质中为70%~77%,而麦秸、烟草中蛋白质氮仅含约62%[9]。农业废弃物(麦秆、水稻秸秆、玉米秸秆)中的蛋白质含量低于食品废物(猪肉、牛肉、大豆),其热解气/油中含氮物质相对更少[10-12]。
生物质中蛋白质由多种氨基酸组成,不同生物质中各氨基酸含量显著不同,但相对占比基本相当[10-12],可为生物质含氮物质模型中氨基酸种类与占比的初步确定提供依据。总体而言,谷氨酸(Glu)与天冬氨酸(Asp)为各生物质中主要的氨基酸物质,而半胱氨酸(Cys)、组氨酸(His)、色氨酸(Trp)与甲硫氨酸(Met)在生物质中的含量相对较低[10-12]。
生物质中各氨基酸由于R基的不同,其化学性质也有所不同,导致热解过程中反应产物差异显著。表1为常见生物质中氨基酸的分类,主要可分为极性氨基酸、非极性氨基酸、芳香类氨基酸和杂环亚氨基酸4类[13]。极性氨基酸中谷氨酸(Glu)和天门冬氨酸(Asp)的含量较高,这些氨基酸具有长侧链,易发生脱水缩合反应而生成多种杂环化合物[14];而非极性氨基酸中亮氨酸(Leu)与丙氨酸(Ala)含量较高,这些氨基酸易发生脱水缩合反应生成哌嗪二酮类化合物(Diketopiperazines,DKPs)[15];芳香类氨基酸中苯丙氨酸(Phe)热解易产生芳香族化合物[16];杂环亚氨基酸中酪氨酸(Tyr)热解主要产生酚类物质[17]。不同氨基酸热解过程中的反应路径及产物均有不同,生物质的热解反应机理可通过选择相应的氨基酸构建含氮模型化合物开展试验与模拟。

表1 常见生物质中各氨基酸的分类[13]
2 生物质热解过程氮转化机理
2.1 NOx前驱物的生成机理
生物质热解过程中氮元素会在气相、液相与固相产物中迁移,转变为相应的气相氮、液相氮与焦炭氮。NO前驱物一方面直接来源于气相氮,也可由液相氮与焦炭氮的进一步转化生成。
2.1.1 气相氮转化为NO前驱物
气相氮主要有2个来源:一是生物质中的蛋白质等含氮物质直接一次裂解生成;二是蛋白质等一次裂解后形成的杂环化合物随着温度升高后继续分解产生[18-19],其中NH3、HNCO、HCN是NO前驱物[20-21]。NO前驱物进一步反应后主要转化为NO,但是在一定条件下也可以转化为N2。气相氮对NO的产生具有直接影响[22],因此,了解气相氮的生成机理对生物质的清洁利用具有重要意义。
气相氮在生物质热解转化过程中的来源略有不同。如图1所示,NH3主要有2个来源[23]:一是生物质中氨基酸热解释放的氨基(路径1),这是热解初期NH3的主要来源;二是HCN与H2O在焦炭的催化作用下反应生成NH3与CO[24-25](路径6)。温度的升高会促进NH3的析出,导致停留在焦炭表面参与反应的NH3减少[26],因此通过转化的HCN也随之减少。HCN主要有3个来源:一是生物质一次热解中生成的各种含氮杂环随着温度进一步分解生成[24](路径3);二是高温下热解油中腈类物质二次裂解生成[7](路径2);三是热解油中的环酰胺进一步分解生成[27]。随热解温度升高,NH3和HCN的生成量均增加[18]。但不同升温速率下其生成特性有所不同,低升温速率下NH3是主要产物,而高升温速率下HCN是唯一产物,在高温环境下,生成NH3的主要途径来自气态HCN在焦炭催化作用下与氢自由基的反应,因此当温度逐渐升高时HCN会快速减少而NH3则快速增加[28]。HNCO化学性质不稳定、具有毒性、且缺少标准标定光谱,因此难以分析其生成路径以及生成量。有研究认为,热解生成的HNCO含量非常少,且易还原成HCN,所以对NO生成影响较小[19]。
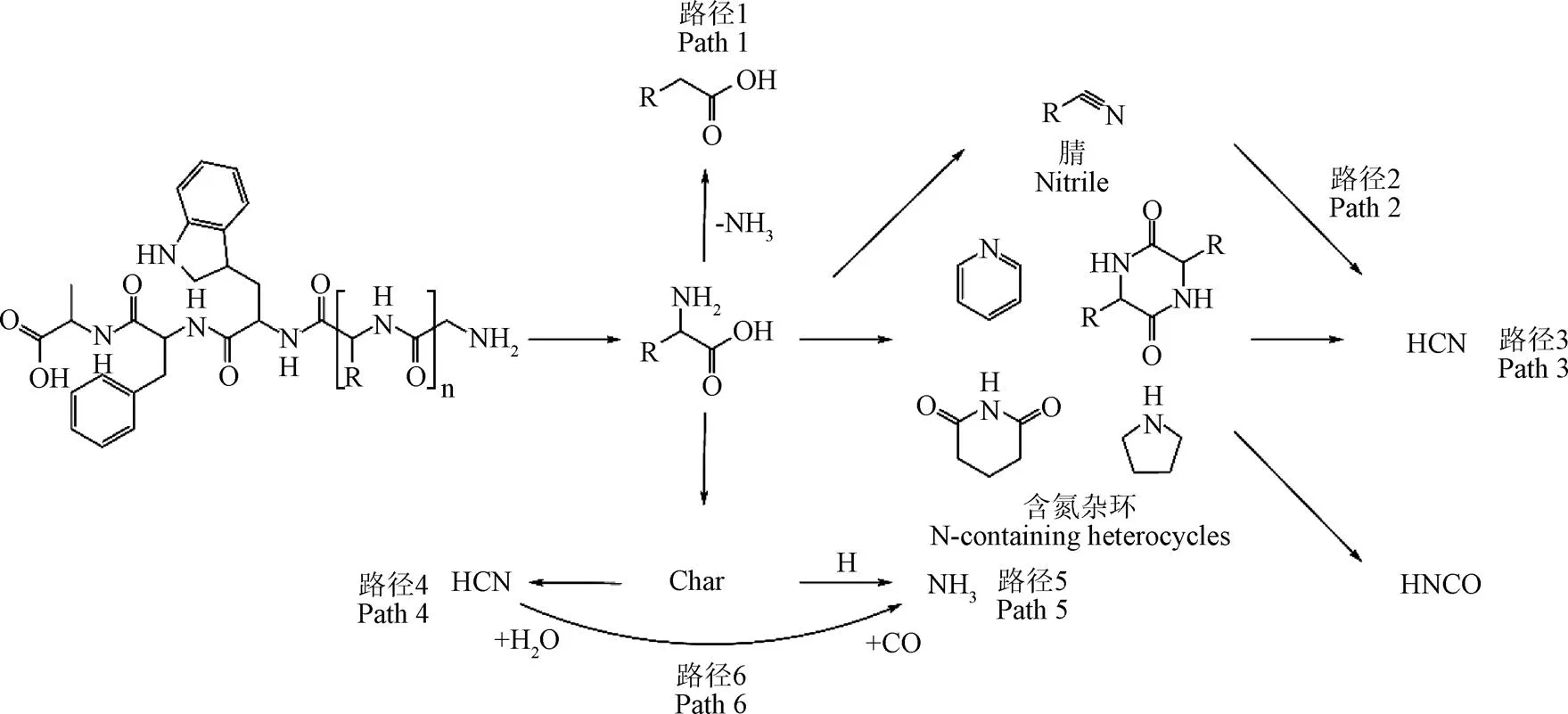
图1 NOx前驱物的生成路径[18-19]
2.1.2 液相氮转化为NO前驱物
液相氮中含量较高的物质有五元环氮、六元环氮、甲基吡啶、喹啉和多环含氮化合物,这些物质主要来源于氨基酸热解[29]。液相氮含量与组分特性受生物质成分影响,如纤维素会促进氮元素向液相氮中的迁移。此外,热解条件也会改变液相氮的迁移转化,如反应温度的升高则会导致液相氮向气相氮的转化,但不同温度条件下其转化产物差异明显[30]。在400~800 ℃温度范围内,温度升高会促进生物油进一步裂解生成HCN和NH3,但当温度超过600 ℃时,HCN的产率高于NH3[31]。当反应温度超过1 000 ℃时,热稳定性强的含氮物质易生成NH3,热稳定性弱的则生成HCN[32]。多环含氮化合物比单环含氮化合物具有更好的热稳定性,更易转变为NH3[33]。此外,热解气氛也会影响液相氮向气相氮的转化特性,如在较低气体温度及高水蒸汽条件下易生成NH3,而高气体温度和低水蒸汽条件下则易生成HCN[34]。
2.1.3 焦炭氮转化为NOx前驱物
焦炭氮中的N元素主要以N-C、N-H键的形式存在[35]。当热解温度达到900 ℃时,生物质中仍有30%的N存在于焦炭中[18]。焦炭氮主要来源于氨基酸的分子内脱水成环反应、分子间脱水成环反应[36-37]和蛋白质侧链间的相互反应[38]。随着热解反应的进行,焦炭氮在高温下会继续裂解,转化为气相氮,此时,若反应气氛中有足够的H自由基,焦炭氮会与H自由基反应生成NH3[39](路径5),反之则更倾向于生成HCN[40](路径4)。在热解过程中加入适量的H2O可生成H自由基,从而有助于降低HCN的生成[41]。
2.2 热解油中含氮物质的生成机理
生物质中氮元素向热解油中的迁移转化机理如图2所示,其主要产物为含氮杂环、腈与酰胺等物质[6]。
含氮杂环主要有吡啶、吡咯、DKPs、吲哚以及多种五、六元环化合物[26]。含氮杂环直接来源于蛋白质的裂解,其中吡咯主要由脯氨酸和赖氨酸片段组成[42];DKPs通常由蛋白质的2个氨基酸之间的脱水缩合反应生成[15];吡咯、吡啶和哌啶类化合物也来源于蛋白质片段的缩合反应[42];吲哚主要由色氨酸和苯丙氨酸转化而来[17](路径1、2),其中色氨酸通过R基的断裂可以直接生成吲哚,苯丙氨酸通过氨基与苯环环化反应以及脱羧基反应生成吲哚;含氮五元环或六元环主要通过部分具有长侧链的氨基酸如谷氨酸、天冬氨酸等发生脱水缩合反应生成[14](路径6、7)。随着热解反应的进行,部分含氮杂环会随温度升高而裂解,而超过6个环的多环化合物,因具有较好的热稳定性,高温时不易裂解[17]。
腈类物质主要通过氨基酸分子脱H2反应以及酰胺脱H2O反应生成(路径9、10)[43-44]。其中,脱H2反应能垒较高,反应要吸收大量的热,温度升高会促进腈类物质的生成。但高温下腈类物质自身会发生裂解反应[21],导致其最终生成量逐渐下降。
酰胺类物质主要由NH3与羧基通过置换反应生成[43-44](路径8)。生物质热解油中的酰胺多具有长链结构,长链酰胺随着温度的升高会裂解成短链酰胺[14]。高温环境会促进酰胺发生脱H2O反应生成腈类物质[43]。此外,高温会促使热解气中NH3直接析出而不与羧基反应,降低酰胺类物质生成[26]。
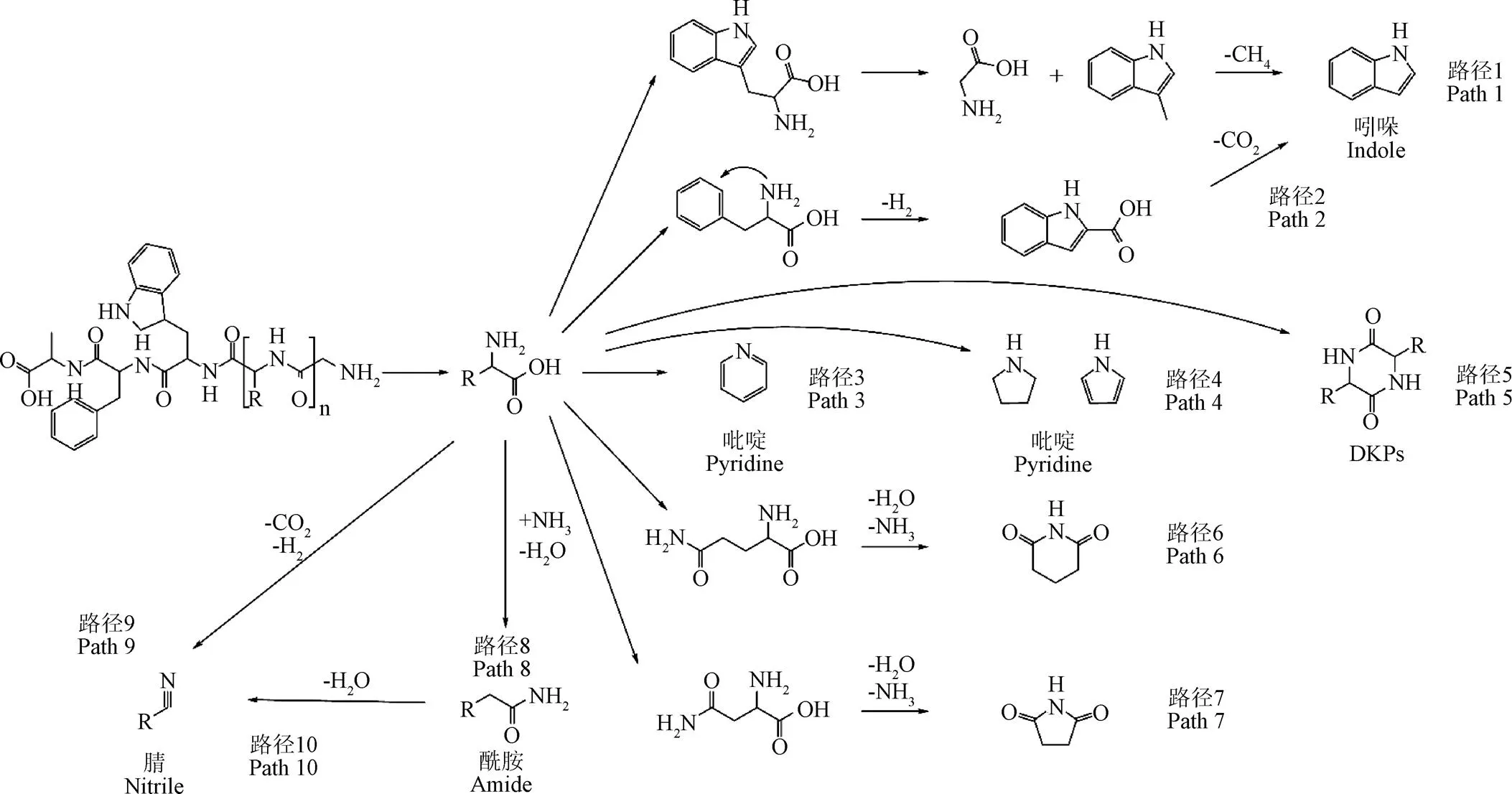
图2 生物质热解油中含氮组分的生成路径[18-19]
3 影响氮迁移路径的因素
不同生物质组分及其中的氮含量与分布均有所不同,这必将导致不同生物质热解过程中含氮物质的迁移转化路径不同。此外,热解过程中的压力、停留时间、升温速率、热解温度、热解气氛、粒径与催化剂等因素也会影响相应的氮的迁移转化特性[45]。表2给出了上述因素对N转化的影响。
3.1 生物质种类
生物质主要由木质素、纤维素、半纤维素、蛋白质与淀粉等组分组成,其在热解过程中极易发生相互反应而影响N的迁移转化,如糖类物质易与蛋白质或氨基酸发生美拉德反应[46],促进各种含氮化合物的生成,木质素易与蛋白质或氨基酸发生聚合反应[47],促进N在热解油及热解焦中的固定,这些相互作用均会影响氮的迁移路径。由于不同生物质中的各组分含量占比不同,相应的热解产物组成特性也存在差异。如餐厨垃圾热解生成的气相氮与液相氮高于农林废弃生物质[4];农林废弃生物质中棉秆热解产生的焦炭氮含量低于水稻秸秆,但气相氮含量高于玉米秆[48]。

表2 不同因素对N转化的影响
生物质中氨基酸与木质素和纤维素等其他组分间的反应也会影响热解产物的组成与分布特性。天冬氨酸与纤维素、半纤维素或木质素共热解过程中,产生的NH3和HCN高于天冬氨酸单独热解;脯氨酸与纤维素、半纤维素或木质素共热解过程中,产生的HCN高于脯氨酸单独热解[47];木质素、纤维素与氨基酸共热解时发生的美拉德反应减少了氮向生物油的转化,从而提高热解油的品质[49];葡萄糖与苯丙氨酸、谷氨酸共热解时均以聚合反应为主,生成大量的含氮杂环[46];天冬酰胺与葡萄糖共热解过程中,生成的HCN高于天冬酰胺单独热解,而生成的NH3和HNCO则会下降[50]。葡萄糖、果糖与甘氨酸共热解时生成的HCN低于甘氨酸单独热解[51]。不同氨基酸与生物质中其他典型组分间相互作用对控制HCN或NH3的排放以及提高热解油的品质具有一定作用。
生物质蛋白质中不同的氨基酸间也存在相互作用,从而影响含氮物质的迁移转化过程。如谷氨酸与甘氨酸共热解时焦炭氮增加;谷氨酸与亮氨酸共热解时气相氮减少,且主要为NH3;谷氨酸与脯氨酸共热解时气相氮中HCN含量高于NH3;谷氨酸与苯丙氨酸共热解时HCN生成温度降低且生成量显著下降[52]。不同氨基酸间的相互作用各不相同,生物质中蛋白质的热解反应过程复杂,单一采用1种或几种氨基酸作为含氮模型化合物并不能充分研究N的迁移转化路径。直接选用蛋白质作为含氮模型化合物可探究各氨基酸在热解过程中的相互作用,减小实际生物质与模型化合物热解特性间的差异。
此外,生物质中存在少量具有催化作用的矿物质,其对生物质热解过程中氮的迁移转化也会产生影响[53-54]。生物质中许多矿物元素都可以促进焦炭氮向HCN的转化[55];钾、钙或铁可以改变氨基酸的初级分解反应途径,进而改变DKPs的二级裂解途径[55];钾离子还可以促进大分子液相氮发生重聚反应,增加气相氮与焦炭氮的生成量[56]。
3.2 升温速率
升温速率的变化会改变生物质热解初始反应路径,从而影响热解产物的组成及分布特性[57]。低升温速率下,气相氮的生成量较低,液相氮的生成量较高。升温速率提高会促进液相氮的裂解而导致气相氮生成量增加[58],其中丙氨酸、亮氨酸、异亮氨酸、脯氨酸等热解生成HCN的量均随着升温速率的提高而增加[59]。高升温速率下焦炭表面孔隙更为丰富,有助于气相氮快速析出,但热解过程焦炭产率基本不变[60]。此外,升温速率对生物质不同典型组分热解特性的影响存在差异,如较低的升温速率下木质素会促进气相氮的生成,而纤维素则促进了液相氮的生成[61]。总体而言,较高的升温速率会促进生物油裂解生成NO前驱物,低升温速率条件下有助于热解油的生成。
3.3 热解温度
生物质热解过程中热解温度的升高会促进热解油与热解焦的二次裂解,促进气相氮的生成,但不同温度下液相氮与焦炭氮的生成转化特性显著不同[62-63]。
当温度低于500 ℃时,温度升高促进了液相氮的生成;当温度高于500 ℃时,温度升高反而抑制了其生成[33]。低温下,温度升高促进热解反应过程,一次热解生成的液相氮含量不断增加;当温度超过500 ℃时,生物油及其中的液相氮自身开始分解,且随着温度的升高其分解速率不断加快[64-65]。由上述可知,高温会促进气相氮的生成并减少生物油的产量,而低温则会导致热解不完全。因此,选择合适的热解温度对提高热解油产量以及减少气相氮的产生具有重要意义。
焦炭氮的生成量随着温度的升高也呈现出先上升后下降的趋势,高温会使焦炭氮转化为气相氮[66],但随温度升高焦炭中剩余的焦炭氮热稳定性逐渐上升[67]。焦炭中的N大多以吡咯啉-N、吡啶-N、季铵盐-N和吡啶-N-O的形式存在。这些物质的热稳定性排序为:吡咯啉-N<吡啶-N<季铵盐-N<吡啶-N-O[66]。
3.4 热解气氛
热解气氛会影响生物质热解反应过程,热解过程中加入水蒸气加入提高了H自由基含量而促进NH3的产生,且提高了热解气中氢气产率[68]。热解气氛中O2的加入与H2O具有相似的作用,在O2与H2O气氛下,生物质热解过程中会生成大量的OH、O、H自由基,从而促进HCN向NH3的转化[69]。在麦秆的热解过程中,发现CO2可促进氮析出,增加N2的产量,同时减少焦炭氮与气相氮的生成[70]。热解过程中加入H2能够促进液相氮的裂解[71],而NH3的加入则会导致液相氮生成量大幅上升[72]。不同的反应气氛对生物质热解过程影响不同,合适的调控反应气氛可实现生物质热解过程中氮的选择性控制。
3.5 停留时间与压力
热解反应的停留时间对氮元素迁移转化产生的影响可以分为2种:固相反应物停留时间与气相产物停留时间。在相同的工作温度和加热频率下,随着固相反应物停留时间的缩短,焦炭氮含量降低,气相氮含量增加。气相产物停留时间一般不影响生物质的一次热解,但是会影响焦油氮的二次裂解。气相产物停留时间越长,焦油氮的二次裂解反应越剧烈,导致焦油氮向气相氮发生显著转化[73-74]。
压力的大小通过影响气相产物停留时间来影响热解产物的二次裂解,压力越大气相氮与液相氮的停留时间越长,导致相应的液相氮二次裂解反应时间更长。此外,当压力逐渐升高时,生物质热解反应活化能逐渐减小,热解反应会更剧烈[75]。
3.6 粒 径
生物质粒径越大,热解过程挥发分析出越慢,气相氮的生成量大幅度增加,液相氮与焦炭氮的生成量减少[62]。生物质的粒径是作用于热解过程中的升温速率以及传热传质从而改变热解产物[76]。较小的粒径会促进热解反应的进行,增加气相氮的生成。较大的粒径会增加生物质的加热时间,导致含氮化合物长时间在低温下热解,生成大量的焦炭氮和不可冷凝气体[77]。因此,适当增大生物质的粒径有利于减少热解过程中气相氮的生成。
3.7 催化剂
催化剂的添加可以减少热解所需时间,降低热解所需温度,对热解过程中氮元素的迁移转化具有显著的影响[78]。
Fe2O3、Co3O4和NiO均可以促进液相氮的生成,其中Co3O4的促进效果更显著[79]。赤泥中的Fe3O4能够降低生物质热解油中含氮化合物的含量[80]。CaCO3可减少热解后剩余焦炭氮含量,增加气相氮和液相氮的生成量[81]。CaO能促进焦炭中氮的固定,降低反应活化能并强烈抑制NH3和HCN的析出[82],相比于HCN,CaO对NH3的抑制作用更大[83]。铁盐类催化剂能够促进生物质的热解,增加焦炭氮的含量[84]。KCl能够促进焦炭氮的生成[56]。此外,近些年一些新型催化剂逐渐被广泛使用,如生物炭以及分子筛催化剂。松木锯末焦炭(PSC)催化剂可以有效地抑制高温下焦油中大分子化合物的缩聚反应,从而减少液相氮的产生[71]。HZSN-5分子筛催化剂能够促进大分子液相氮的裂解,减少液相氮的生成[80]。
不同催化剂的催化效果差异较大,选择合适的催化剂对提高热解油的质量与产量,减少NO前驱物的生成具有重要作用。
4 结 论
本文针对生物质热解过程中氮元素的迁移转化机理对前人的研究进行了归纳与总结,对热解过程中NO前驱物以及热解油中典型含氮物质的产生机制进行了分析并结合相应的机理图进行解释说明;对生物质种类、升温速率、热解温度、热解气氛、停留时间、压力、粒径与催化剂等外部因素对热解过程中氮元素迁移的影响进行了归纳与总结,具体结论如下。
1)生物质热解过程中氮元素会迁移至三相产物中。其中,热解气中的含氮物质主要是NH3与HCN;热解油中的含氮物质主要是含氮杂环、腈与酰胺。
2)气相氮中NH3主要来源于氨基酸热解释放的氨基以及HCN在焦炭表面的水解转化,HCN主要来源于腈、含氮杂环等一次热解产物的二次裂解。
3)液相氮中含氮杂环主要由部分氨基酸的片段以及氨基酸间的脱水缩合生成。腈主要来源于氨基酸分子脱H2反应以及酰胺的脱H2O反应。酰胺主要由NH3与羧基的置换反应生成。
4)生物质种类以及热解反应参数如升温速率、热解温度、热解气氛、停留时间、压力、粒径、催化剂等均会影响氮的迁移路径,最终改变热解产物中氮的组成与分布特性。
5)有效控制生物质中氮元素向热解气与热解油中的迁移转化是实现其高效清洁燃烧的重要问题之一。但现阶段烟气中的NO常采用尾部脱除技术,无法从根源上减少污染物的排放;通过总结现有研究发现热解油中含氮组分多样,且不同含氮物质的迁移转化特性存在差异,难以通过单一控制某一参数实现液相氮的控制。因此,探究生物质热解过程中气相氮的迁移转化路径以减少NO前驱物的生成,通过控制热解参数、生物质掺混热解等调控减少热解油中含氮物质的生成是未来生物质热解技术需关注的重点方向。
[1] 单明. 生物质能开发利用现状及挑战[J]. 可持续发展经济导刊,2022(4):48-49.
Shan Ming. Current situation and challenge of biomass energy development and utilization[J]. China Sustainability Tribune, 2022(4): 48-49.(in Chinese with English abstract)
[2] 王芳,刘晓风,陈伦刚,等. 生物质资源能源化与高值利用研究现状及发展前景[J]. 农业工程学报,2021,37(18):219-231.
Wang Fang, Liu Xiaofeng, Chen Lungang, et al. Research status and development prospect of energy and high value utilization of biomass resources[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2021, 37(18): 219-231. (in Chinese with English abstract)
[3] 李志成,王辉辉,买文鹏,等. 酰胺的合成方法综述[J]. 广东化工,2013,40(3):62-63,90.
Li Zhicheng, Wang Huihui, Mai Wengpeng, et al. Synthetic methods of the amide[J]. Guangdong Chemical Industry, 2013, 40(3): 62-63, 90. (in Chinese with English abstract)
[4] Qing M X, Long Y T, Luo Y D, et al. Insights into the slagging process during the utilization of food waste: Ash-making temperature and additives[J]. Chemical Engineering Science, 2022, 255: 117680.
[5] 仉利,姚宗路,赵立欣,等. 生物质热解制备高品质生物油研究进展[J]. 化工进展,2021,40(1):139-150.
Zhang Li, Yao Zonglu, Zhao Lixin, et al. Research progress on preparation of high quality bio-oil by pyrolysis of biomass[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 139-150. (in Chinese with English abstract)
[6] 董长青,杨勇平,高攀,等. 生物质热解过程中含氮模型化合物研究进展[J]. 环境污染与防治,2019,41(1):110-117.
Dong Changqin, Yang Yongping, Gao Pan, et al. The mechanism of nitrogen conversion during the pyrolysis of biomass: A review of model compounds study[J]. Environmental Pollution and Control, 2019, 41(1): 110-117. (in Chinese with English abstract)
[7] 龚千代. 甘氨酸与DKP高温热解含氮产物生成机理研究[D]. 长沙:长沙理工大学,2017.
Gong Qiandai. Mechanistic study on the generation of nitrogen-containing products from high-temperature pyrolysis of glycine and DKP[D]. Changsha: Changsha University of Technology, 2017. (in Chinese with English abstract)
[8] Yuan S, Chen X, Li W, et al. Nitrogen conversion under rapid pyrolysis of two types of aquatic biomass and corresponding blends with coal[J]. Bioresource Technology, 2011, 102(21): 10124-10130.
[9] 王鹏,朱立军,尚军,等. 国内外不同卷烟总氮、蛋白质氮、总植物碱氮的差异分析[J]. 湖北农业科学,2011,50(7):1406-1407,1410.
Wang Peng, Zhu Lijun, Shang Jun, et al. The variation analysis of total nitrogen, proteinnitrogen, nicotine nitrogen contents in different cigarettes[J]. Hubei Agricultural Science, 2011, 50(7): 1406-1407, 1410. (in Chinese with English abstract)
[10] 陈超,唐洁,姜涛,等. 不同类型玉米氨基酸组成及含量的比较分析[J]. 粮食储藏,2020,49(4):38-41.
Chen Chao, Tang Jie, Jiang Tao, et al. Comparative analysis of amino acid composition and content in different maize[J]. Grain Storage, 2020, 49(4): 38-41. (in Chinese with English abstract)
[11] 欧行奇, 李新华, 朱彬. 甘薯茎尖与常见叶菜类蔬菜氨基酸含量及组成的比较分析[J]. 氨基酸和生物资源,2007,29(3):5.
Ou Xingqi, Li Xinhua, Zhu Bin. Comparison of amino acid content and composition between vegetable sweet potato tips and leaf vegetables[J]. Amino Acids & Biological Resources, 2007, 29(3): 5. (in Chinese with English abstract)
[12] Dhyani V, Bhaskar T. A comprehensive review on the pyrolysis of lignocellulosic biomass[J]. Renewable Energy, 2018, 129: 695-716.
[13] 陈德民. 生物质中氮赋存形式与热解过程氮迁移转化实验研究[D]. 武汉:华中科技大学,2016.
Chen Demin. Experimental Study on Nitrogen Fugitive Forms in Biomass and Nitrogen Migration Transformation during Pyrolysis[D]. Wuhan: Huazhong University of Science and Technology, 2016. (in Chinese with English abstract)
[14] Kang P, Qin W, Fu Z Q, et al. Generation mechanism of NOand N2O precursors (NH3and HCN) from aspartic acid pyrolysis: A DFT study[J]. International Journal of Agricultural and Biological Engineering, 2017, 99: 169-176.
[15] Fabbri D, Adamiano A, Falini G, et al. Analytical pyrolysis of dipeptides containing proline and amino acids with polar side chains. Novel 2,5-diketopiperazine markers in the pyrolysates of proteins[J]. Journal of Analytical & Applied Pyrolysis, 2012, 95: 145-155.
[16] 田红,何正文,刘亮,等. 基于量子化学理论的苯丙氨酸热解过程中NO前驱体生成机理研究[J]. 太阳能学报,2021, 42(10): 317-323.
Tian Hong, He Zhengwen, Liu Liang, et al. Study on formation mechanism of NOprecursor in pyrolysis process of phenylalanine based on quantum chemidtry theory[J]. Acta Energiae Solaris Sinica, 2021, 42(10): 317-323. (in Chinese with English abstract)
[17] Kim Y M, Han T U, Lee B, et al. Analytical pyrolysis reaction characteristics of Porphyratenera[J]. Algal Research, 2018, 32: 60-69.
[18] 柏继松. 生物质燃烧过程氮和硫的迁移、转化特性研究[D]. 杭州:浙江大学,2012:49.
Bai Jisong. Migration and Transformation Characteristics of Nitrogen and Sulfur During Biomass Combustion[D]. Hangzhou: Zhejiang University, 2012: 49. (in Chinese with English abstract)
[19] Zhou J Q, Gao P, Dong C Q, et al. TG-FTIR analysis of nitrogen conversion during straw pyrolysis: A model compound study[J]. Journal of Fuel Chemistry and Technology, 2015, 43(12): 1427-1432.
[20] Wang X, Tang X H, Yang X Y. Pyrolysis mechanism of microalgae Nannochloropsis sp. based on model compounds and their interaction[J]. Energy Conversion and Management, 2017, 140: 203-210.
[21] 刘亮,龙雨田,卿梦霞,等. 餐厨垃圾典型组分的热解产物分布特性[J]. 可再生能源,2022,41(3):11-16.
Liu Liang, Long Yutian, Qing Mengxia, et al. Distribution characteristics of pyrolysis products of typical kitchen waste fractions[J]. Renewable Energy, 2022, 41(3): 11-16. (in Chinese with English abstract)
[22] Li D, Gao S Q, Song W L, et al. NO reduction in decoupling combustion of biomass and biomass-coal blend[J]. Energy & Fuels, 2009, 23(1): 224-228.
[23] Ren Q Q, Zhao C S. NOand N2O precursors from biomass pyrolysis: Role of cellulose, hemicellulose and lignin[J]. Environmental Science & Technology, 2013, 47(15): 8955-8961.
[24] Kang P, Qin W, Fu Z Q, et al. Generation mechanism of NOand N2O precursors (NH3and HCN) from aspartic acid pyrolysis: A DFT study[J]. International Journal of Agricultural and Biological Engineering, 2017, 99: 169-176.
[25] 周建强,高攀,董长青,等. 固体生物质燃烧中氮氧化物产生机理综述[J]. 热力发电,2018,47(12):1-9,16.
Zhou Jianqiang, Gao Pan, Dong Changqin, et al. Formation mechanism of nitrogen oxides during solid biomass fuel burning:A review[J]. Thermoelectric Power Generation, 2018, 47(12): 1-9, 16. (in Chinese with English abstract)
[26] 展新,吴文广,崔国民. 生物质气化过程中热解焦油的生成及其均相转化机理[J]. 能源研究与信息,2019,35(3):125-133.
Zhan Xin, Wu Wenguang, Cui Guoming. Formation and homogeneous conversion mechanism of biomass pyrolysis tar[J]. Energy Research and Information, 2019, 35(3): 125-133. (in Chinese with English abstract)
[27] Hansson K M, Samuelsson J, Tullin C, et al. Formation of HNCO, HCN, and NH3from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combustion and Flame, 2004, 137(3): 265-277.
[28] Gong Q D, Liu L, Tian H, et al. Theoretic and experiment study on nitrogen-containing products of glycine during high temperature pyrolysis[J]. Acta Energiae Solaris Sinica, 2019, 4: 1107-1113.
[29] Chen H F, Tomoaki N, Kunio Y. Characteristics of tar, NOprecursors and their absorption performance with different scrubbing solvents during the pyrolysis of sewage sludge[J]. Applied Energy, 2011, 88(12): 5032-5041.
[30] 李小华,焦丽华,樊永胜,等. 纤维素木聚糖和木质素含量对生物质热解特性及产物的影响[J]. 农业工程学报,2015,31(13):236-243.
Li Xiaohua, Jiao Lihua, Fan Yongshen, et al. Effects of cellulose, xylan and lignin content on biomass pyrolysis characteristics and product distribution[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2015, 31(13): 236-243. (in Chinese with English abstract)
[31] Zhao C, Yan Z Z, Yang S, et al. Affecting the migration of nitrogen elements during coal pyrolgsis[J]. Applied Chemical Industry, 2018, 47(4): 208-211.
[32] Wei F, Cao J P, Zhao X Y, et al. Formation of aromatics and removal of nitrogen in catalytic fast pyrolysis of sewage sludge: A study of sewage sludge and model amino acids[J]. Fuel, 2018, 218: 148-154.
[33] Neves D, Thunman H, Matos A, et al. Characterization and prediction of biomass pyrolysis products[J]. Progress in Energy & Combustion Science, 2011, 37(5): 611-630.
[34] Archan G, Scharier R, Leonhard P, et al. Detailed NOprecursor measurements within the reduction zone of a novel small-scale fuel flexible biomass combustion technology[J]. Fuel, 2021, 302(15): 113-121.
[35] 周赛,刘虎,于鹏飞,等. 基于密度泛函理论的CO2氧化含氮焦炭的机理研究[J]. 燃料化学学报,2022,50(1):19-27.
Zhou Sai, Liu Hu, Yu Pengfei, et al. Study on the mechanism of oxidation of nitrogen-containing char by CO2based on density functional theory[J]. Journal of Fuel Chemistry and Technology, 2022, 50(1): 19-27. (in Chinese with English abstract)
[36] Kibet J K, Khachatryan L, Dellinger B. Molecular products from the thermal degradation of glutamic acid[J]. Journal of Agricultural and Food Chemistry, 2013, 61(32): 7696-7704.
[37] Hao J, Guo J, Ding L, et al. TG-FTIR, Py-two-dimensional GC-MS with heart-cutting and LC-MS/MS to reveal hydrocyanic acid formation mechanisms during glycine pyrolysis[J]. Journal of Thermal Analysis and Calorimetry 2014. 115(1): 667-673.
[38] 涂德浴,潘庆民,张传佳,等. 生物质成型燃料热解半焦产率及理化特性[J]. 农业工程学报,2019,35(21):229-234.
Tu Deyu, Pan Qingmin, Zhang Chuanjia, et al. Yield and physicochemical properties of pyrolysis char of biomass briquetting pellets[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(21): 229-234. (in Chinese with English abstract)
[39] Li C Z, Tan L L. Formation of NOand SOprecursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3during pyrolysis[J]. Fuel, 2000, 79(15): 1899-1906.
[40] Chen H, Wang Y, Xu G, et al. Fuel-N evolution during the pyrolysis of industrial biomass wastes with high nitrogen content[J]. Energies, 2012, 5(12): 5418-5438.
[41] Tian F J, Yu J, Mckenzie L J, et al. Conversion of fuel-N into HCN and NH3during the pyrolysis and gasification in steam: A comparative study of coal and biomassextdagger[J]. Energy and Fuels, 2007, 21(2): 517-521.
[42] Debono O, Villot A. Nitrogen products and reaction pathway of nitrogen compounds during the pyrolysis of various organic wastes[J]. Journal of Analytical & Applied Pyrolysis, 2015, 114: 222-234.
[43] Kim S W, Koo B S, Lee D H. A comparative study of bio-oils from pyrolysis of microalgae and oil seed waste in a fluidized bed[J]. Bioresource Technology, 2014, 162: 96-102.
[44] 米铁,徐玲娜,袁羽书,等. 生物质热解过程中焦油形成机理的研究[J]. 华中师范大学学报(自然科学版),2013,47(5):671-675.
Mi Tie, Xu Linna, Yuan Yushu, et al. Study of tar formation and variation mechanism for biomass pyrolysis gasification[J]. Journal of Huazhong Normal University (Natural Science Edition), 2013, 47(5): 671-675. (in Chinese with English abstract)
[45] 胡二峰,赵立欣,吴娟,等. 生物质热解影响因素及技术研究进展[J]. 农业工程学报,2018,34(14):212-220.
Hu Erfeng, Zhao Lixin, Wu Juan, et al. Research advance on influence factors and technologies of biomass pyrolysis[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(14): 212-220. (in Chinese with English abstract)
[46] 宫梦,方阳,陈伟,等. 纤维素组分对氨基酸热解的影响[J]. 化工学报,2020,71(5):2312-2319.
Gong Meng, Fang Yang, Chen Wei, et al. Effect of cellulose composition on amino acids pyrolysis[J]. Journal of Chemical Engineering, 2020, 71(5): 2312-2319. (in Chinese with English abstract)
[47] 吴丹焱,辛善志,刘标,等. 基于木质素部分脱除及其含量对生物质热解特性的影响[J]. 农业工程学报,2018,34(1):193-197.
Wu Danyan, Xin Shanzhi, Liu Biao, et al. Influence of lignin content on pyrolysis characteristics of biomass based on part of lignin removal[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(1): 193-197. (in Chinese with English abstract)
[48] 于雪斐,伊松林,冯小江,等. 热解条件对农作物秸秆热解产物得率的影响[J]. 北京林业大学学报,2009,31(S1):174-177.
Yu Xuefei, Yi Songlin, Feng Xiaojiang, et al. Effects of pyrolytic conditions on pyrolysis yield of crop stalks[J]. Journal of Beijing Forestry University, 2009, 31(S1): 174-177. (in Chinese with English abstract)
[49] Chen H, Xie Y, Chen W, et al. Investigation on co-pyrolysis of lignocellulosic biomass and amino acids using TG-FTIR and Py-GC/MS[J]. Energy Conversion and Management, 2019, 196: 320-329.
[50] Tian H, Wei Y Y, Huang Z J, et al. The nitrogen transformation and controlling mechanism of NH3and HCN conversion during the catalytic pyrolysis of amino acid[J]. Fuel, 2023, 333(1): 126215.
[51] Hao J, Guo J, Xie F, et al. Correlation of hydrogen cyanideformation with 2, 5-diketopiperazine and nitrogen heterocycliccompounds from co-pyrolysis of glycine and glucose/fructose[J]. Energy& Fuels, 2013, 27(8): 4723-4728.
[52] 周建强. 麦秆热解过程中有机氮转化机理研究[D]. 北京:华北电力大学,2019.
Zhou Jianqiang. Study on the Mechanism of Organic Nitrogen Conversion During Straw Pyrolysis[D]. Beijing: North China University of Electric Power, 2019. (in Chinese with English abstract)
[53] Liu Z, Balasubramanian R. A comparative study of nitrogen conversion during pyrolysis of coconut fiber, its corresponding biochar and their blends with lignite[J]. Bioresour Technol, 2014, 151: 85-90.
[54] Ohshima Y, Tsubouchi N, Ohtsuka Y. Iron-catalyzed nitrogen removal as N2from PAN-derived activated carbon[J]. Applied Catalysis B: Environmental, 2012, 111: 614-620.
[55] Ren Q Q, Zhao C S. NOand N2O precursors from biomass pyrolysis: Role of cellulose, hemicellulose and lignin.[J]. Environmental Science & Technology, 2013, 47(15): 170-174.
[56] 谭洪,王树荣,骆仲泱,等. 金属盐对生物质热解特性影响试验研究[J]. 工程热物理学报,2005,26(5):742-744.
Tan Hong, Wang Shurong, Luo Zhongyang, et al. Influence of metallic salt on biomass flash pyrolysis characteristics[J]. Journal of Engineering Thermophysics, 2005, 26(5): 742-744. (in Chinese with English abstract)
[57] Li J, Liu Y W, Shi J Y, et al. The investigation of thermal decomposition pathways of phenylalanine and tyrosine by TG-FTIR[J]. Thermochimica Acta, 2007, 467(1): 20-29.
[58] Tan L L, Li C Z. Formation of NOand SOprecursors during the pyrolysis of coal and biomass. Part II. Effects of experimental conditions on the yields of NOand SOprecursors from the pyrolysis of a Victorian brown coal[J]. Fuel, 2000, 79(15): 1891-1897.
[59] 郝菊芳,王洪波,曹得坡,等. 升温速率对氨基酸裂解生成含氮气体的影响研究[J]. 化学研究与应用,2013,25(7):981-986.
Hao Jufang, Wang Hongbo, Cao Depo, et al. Effect of heating rate on the formation of nitrogenous gases from the pyrolysis of amino acids[J]. Chemical Research and Applications, 2013, 25(7): 981-986. (in Chinese with English abstract)
[60] 李永玲,吴占松. 秸秆热解特性及热解动力学研究[J]. 热力发电,2008,37(7):1-5.
Li Yongling, Wu Zhansong. Study on charicters and dynamics concerning pyrolysis of corn stalks[J]. Thermal Power Generation, 2008, 37(7): 1-5. (in Chinese with English abstract)
[61] Chen H P, Si Y H, Chen Y Q, et al. NOprecursors from biomass pyrolysis: Distribution of amino acids in biomass and Tar-N during devolatilization using model compounds[J]. Fuel, 2017, 187: 367-375.
[62] 刘啸天,于洁,孙路石. 温度与粒径对生物质热解特性影响实验研究[J]. 能源研究与管理,2022(1):57-64.
Liu Xiaotian, Yu Jie, Sun Lushi. Experimental study on effects of temperature and particle size on biomass pyrolysis characteristics[J]. Energy Research and Management, 2022(1): 57-64. (in Chinese with English abstract)
[63] 杨选民,王雅君,邱凌,等. 温度对生物质三组分热解制备生物炭理化特性的影响[J]. 农业机械学报,2017,48(4):284-290.
Yang Xuanming, Wang Yajun, Qiu Ling, et al. Effect of temperature on physicochemical properties of biochar prepared by pyrolysis of three components of biomass[J]. Transactions of the Chinese Society for Agricultural Machinery, 2017, 48(4): 284-290. (in Chinese with English abstract)
[64] Kathirvel B, Susaimanickam A, Eldon R, et al. Effect of reaction temperature on the conversion of algal biomass to bio-oil and biochar through pyrolysis and hydrothermal liquefaction - science direct[J]. Fuel, 2021, 285(1): 119106.
[65] Li J, Tian Y Y, Zong P J, et al. Thermal cracking behavior, products distribution and char/steam gasification kinetics of seawater spirulina by TG-FTIR and Py-GC/MS[J]. Renewable Energy, 2020, 145: 1761-1771.
[66] Xu, S Y, Chen, J F, Peng, H Y, et al. Effect of biomass type and pyrolysis temperature on nitrogen in biochar, and the comparison with hydrochar[J]. Fuel, 2021, 291(1): 120128.
[67] 李飞跃,汪建飞,谢越,等. 热解温度对生物质炭碳保留量及稳定性的影响[J]. 农业工程学报,2015,31(4):266-271.
Li Feiyue, Wang Jianfei, Xie Yue, et al. Effects of pyrolysis temperature on carbon retention and stability of biochar[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2015, 31(4): 266-271. (in Chinese with English abstract)
[68] 付鹏. 生物质热解气化气相产物释放特性和焦结构演化行为研究[D]. 武汉:华中科技大学,2010.
Fu Peng. Study on the Release Characteristics and Coke Structure Evolution Behavior of Gas Phase Products From Biomass Pyrolysis Gasification[D]. Wuhan: Huazhong University of Science and Technology, 2010. (in Chinese with English abstract)
[69] 詹昊,张晓鸿,阴秀丽,等. 生物质热化学转化过程含N污染物形成研究[J]. 化学进展,2016,12:1880-1890.
Zhan Hao, Zhang Xiaohong, Yin Xiuli, et al. Formation of nitrogenous pollutants during biomass thermo-chemical conversion[J]. Progress in Chemistry, 2016, 12: 1880-1890. (in Chinese with English abstract)
[70] 洪文鹏,张钰,姜海峰,等. CO2气氛耦合粉煤灰催化生物质热解生油特性分析[J]. 农业工程学报,2022,38(4):235-241.
Hong Wenpeng, Zhang Yu, Jiang Haifeng, et al. Characteristics of bio-oil generated from biomass pyrolysis catalyzed by coal fly ash under CO2atmosphere[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(4): 235-241. (in Chinese with English abstract)
[71] Li J, Tian Y Y, Qiao Y Y, et al. Synergistic effect of hydrogen atmosphere and biochar catalyst on tar decomposition and methane-rich gas production during biomass pyrolysis[J]. Fuel, 2022, 330(15): 125680.
[72] Zheng Y W, Li D H, Wang J D, et al. Ammonia (NH3)/ nitrogen (N2) torrefaction pretreatment of waste biomass for the production of renewable nitrogen-containing chemicals via catalytic ammonization pyrolysis: Evolution of fuel-N under a N2/NH3-rich atmosphere[J]. Journal of the Energy Institute. 2022, 102: 143-159.
[73] 马承荣,肖波,杨家宽,等. 生物质热解影响因素研究[J]. 环境技术,2005,5:16-18,41.
Ma Chengrong, Xiao Bo, Yang Jiakuan, et al. Study on the effects of operating conditions of biomass pyrolysis[J]. Environmental Technology, 2005, 5: 16-18, 41. (in Chinese with English abstract)
[74] Rolando Z, Kridter S, Emilia B. Rapid pyrolysis of agricultural residues at high temperature[J]. Biomass and Bioenergy, 2002, 23(5): 357-366.
[75] Cetin E, Gupta R, Moghtaderi B. Effect of pyrolysis pressure and heating rate on radiata pine char structure and apparent gasification reactivity[J]. Fuel, 2004, 84(10): l328-l334.
[76] 马培勇,孙亚栋,邢献军,等. 粒径对棉秆成型颗粒热解动力学特性的影响[J]. 太阳能学报,2016,37(5):1308-1314.
Ma Peiyong, Sun Yadong, Xing Xianjun, et al. Effect of particle size on pyrolysis kinetic characteristics of cotton stalk briquette[J]. Acta Energiae Solaris Sinica, 2016, 37(5): 1308-1314. (in Chinese with English abstract)
[77] 何明明. 生物质快速热解生物油产率和组分的影响因素[J].木材加工机械,2010,21(1):38-41.
He Mingming. Influnce factors of biomass fast pyrolysis bio-oil' production rate and composition[J]. Wood Processing Machinery, 2010, 21(1): 38-41. (in Chinese with English abstract)
[78] 杨延璐,许成君,仝坤,等. 污泥热解催化剂的研究进展[J]. 化工环保,2020,40(6):580-585.
Yang Yanlu, Xu Chengjun, Tong Kun, et al. Research progresses on catalysts for sludge pyrolysis[J]. Environmental Protection of Chemical Industry, 2020, 40(6): 580-585. (in Chinese with English abstract)
[79] 何正文. 碱(土)金属对生物质热解过程中含氮化合物转化过程的影响[D]. 长沙:长沙理工大学,2021.
He Zhengwen. Effect of Alkali (earth) Metals on The Conversion Process of Nitrogenous Compounds in Biomass Pyrolysis[D]. Changsha: Changsha University of Science and Technology, 2021. (in Chinese with English abstract)
[80] Mohamed H A, Nuuo B, Zeid A A, et al. Transforming red mud into an efficient acid-base catalyst by hybridization with mesoporous ZSM-5 for co-pyrolysis of biomass and plastics[J]. Chemical Engineering Journal, 2022, 430: 132965.
[81] 孙振杰,黄思思,时号,等. 生物质炭负载镍钙催化剂催化裂解/重整生物质热解气研究[J]. 农业工程学报,2021,37(17):211-217.
Sun Zhenjie, Huang Sisi, Shi Hao, et al. Investigation into the catalytic cracking/reforming of biomass pyrolysis gas by biochar supported Ni-Ca catalyst[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2021, 37(17): 211-217. (in Chinese with English abstract)
[82] 惠吉成. CaO对污泥中蛋白质热解生成NO前驱体的脱除机理研究[D]. 吉林:东北电力大学,2019.
Hui Jicheng. Study on the Removal Mechanism of CaO to Protein Pyrolysis to NOPrecursors in Sludge[D]. Jilin: Northeast Dianli University, 2019.
[83] Guo S, Liu T C, Hui J C, et al. Effects of calcium oxide on nitrogen oxide precursor formation during sludge protein pyrolysis[J]. Energy, 2019, 189: 213-217.
[84] Xia S W, Yang H P, Lei S S, et al. Iron salt catalytic pyrolysis of biomass: Influence of iron salt type[J]. Energy, 2022, 262: 125415.
Review of nitrogen migration and transformation during biomass pyrolysis
Liu Liang, Zheng Yang, Huang Sibiao, Xiao Tingyi, Tian Hong, Qing Mengxia※
(,,410114,)
Biomass pyrolysis can generate tar and gas products with high industrial value. But the nitrogen (N) element in the biomass can inevitably migrate to the products along with the pyrolysis process, thus possibly polluting the environment. Focusing on the overall goal of preparing clean energy from biomass resources, this study systematically analyzes nitrogen migration and conversion mechanism during biomass pyrolysis, focusing on the research progress of the generation and conversion mechanism of gas nitrogen, tar nitrogen and char nitrogen. The NOprecursors can be the HCN and NH3in the biomass pyrolysis gas. Specifically, the NH3comes from the amino acids that are released from the amino acid pyrolysis and hydrolysis of HCN on the surface of char, while the HCN is from the secondary cracking of primary pyrolysis products, such as nitrile and N-containing heterocycle. The N-containing substances in the pyrolysis oil include the N-containing heterocycles, nitrile, and amide. Furthermore, the N-containing heterocycles can be produced by the fragmentation of some amino acids and by dehydration condensation between the amino acids. The nitrile is derived from the de-H2reaction of amino acid molecules and the de-H2O reaction of amides. The substitution reactions can also be used to form amides from NH3and carboxyl groups. More importantly, the biomass varies greatly in the different pyrolysis characteristics and products, due to the composition during the reaction. The higher heating rates can promote tar cracking for higher NOprecursor production during biomass pyrolysis, while the lower heating rates can contribute to tar production for better quality. The pyrolysis temperature and atmospheres of biomass can pose a large effect on the yield and composition of the pyrolysis products. The pyrolysis in the O2and H2O atmosphere can enhance the conversion of HCN to NH3, while the pyrolysis in the CO2atmosphere can reduce the production of NOprecursors. In terms of the pyrolysis pressure, the gas-N residence time can facilitate the reaction path of the secondary pyrolysis for the migration path of nitrogen. The larger particle sizes of the biomass can increase the NOprecursors but less the tar production, whereas, the smaller particle sizes can promote the N fixation in the char. The catalysts can reduce the pyrolysis time and the temperature for the N migration and conversion during biomass pyrolysis. The mineral elements (such as K, Ca, and Fe) in the biomass can promote the conversion of nitrogenous substances in the coke into the HCN. By contrast, the metal oxides (such as Fe2O3, Co3O4, and NiO) can be used to enhance the production of Tar-N, where Co3O4has the best performance. The KOH can reduce the types of hydrocarbon compounds in the pyrolysis oil, but for less NH3and HCN production. The current NOtreatments are the catalytic, plasma, microbial, absorption, and adsorption methods. All tail-end treatments cannot reduce the emission of pollutants with low efficiency and high energy consumption. Anyway, the N migration and transformation mechanism in the pyrolysis of biomass can reduce the emission of N-containing pollutants at the source during the pyrolysis process.
biomass; pyrolysis; gas phase nitrogen; tar nitrogen; char nitrogen; NO
10.11975/j.issn.1002-6819.2022.19.025
TK6
A
1002-6819(2022)-19-0227-10
刘亮,郑扬,黄思彪,等. 生物质热解过程中氮迁移转化机理研究进展[J]. 农业工程学报,2022,38(19):227-236.doi:10.11975/j.issn.1002-6819.2022.19.025 http://www.tcsae.org
Liu Liang, Zheng Yang, Huang Sibiao, et al. Review of nitrogen migration and transformation during biomass pyrolysis[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(19): 227-236. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2022.19.025 http://www.tcsae.org
2022-07-11
2022-09-30
湖南省教育厅重点项目(21A0201);湖南省自然科学基金项目(2022JJ40489)
刘亮,博士,教授,研究方向为煤与生物质高效清洁利用。Email:liuliang_hn@163.com
卿梦霞,博士,讲师,研究方向为煤与生物质高效清洁利用。Email:qingmx@csust.edu.cn

