An Overview of Metal–Organic Frameworks for Green Chemical Engineering
Xiang-Jing Kong,Jian-Rong Li
Beijing Key Laboratory for Green Catalysis and Separation &Department of Environmental Chemical Engineering,Beijing University of Technology,Beijing 100124,China
ABSTRACT Given the current global energy and environmental issues resulting from the fast pace of industrialization,the discovery of new functional materials has become increasingly imperative in order to advance science and technology and address the associated challenges.The boom in metal–organic frameworks(MOFs)and MOF-derived materials in recent years has stimulated profound interest in exploring their structures and applications.The preparation,characterization,and processing of MOF materials are the basis of their full engagement in industrial implementation.With intensive research in these topics,it is time to promote the practical utilization of MOFs on an industrial scale,such as for green chemical engineering,by taking advantage of their superior functions.Many famous MOFs have already demonstrated superiority over traditional materials in solving real-world problems.This review starts with the basic concept of MOF chemistry and ends with a discussion of the industrial production and exploitation of MOFs in several fields.Its goal is to provide a general scope of application to inspire MOF researchers to convert their focus on academic research to one on practical applications.After the obstacles of cost,scale-up preparation,processability,and stability have been overcome,MOFs and MOF-based devices will gradually enter the factory,become a part of our daily lives,and help to create a future based on green production and green living.
Keywords:Metal-organic frameworks(MOFs)Application Green chemical engineering
1.Introduction
The beginning of the 21st century has been markedly characterized by immense pressure to implement energy conservation and emission reduction,and by a continual growth in demotic environmental awareness.Toward the alleviation of critical environmental concerns,a relatively new field of industry—namely,green chemical engineering—has gained tremendous impetus,as it promotes chemical products and processes that mitigate and/or eliminate the use and generation of hazardous substances[1,2].Such sought-after technological innovations require competitive targeted products in order to meet both market demand for remarkable nano-and micro-scale end-use properties,and environmental demand for sustainable meso- and macro-scale industrial processes.The ascendance of green chemical engineering underlines the necessity of discovering novel and versatile functionalized materials that can serve as platforms for multiple applications.
Metal–organic frameworks(MOFs),which are constructed from inorganic nodes(metal ions or clusters)and organic linkers,have aroused enormous attention due to their structural diversity,property uniqueness,and functional tailorability,particularly for the applications in green chemical engineering[3,4].The past decades have witnessed rapid growth in the design,synthesis,characterization,property,and application exploration of MOFs[5,6].Unlike traditional inorganic materials,MOFs permit close control of their composition,morphology,pore property,and function by the careful selection of building units and the incorporation of smart functionalities;this greatly widens the scope of their applications and improves their efficiency in given applications[7].
This review is not intended to be an exhaustive literature collection that minutely describes the basic compositions,structural features,and all inherent properties of MOFs.Several dedicated reviews have already been published for this purpose[2–13].Herein,we intend to provide a brief introduction to MOFs from the perspective of their synthesis and applications in green chemical engineering.This article is organized into the following sections:①methodologies of MOF synthesis and scale-up preparation;②general methods for MOF structural characterization and stability testing,strategies to enhance stability,and processing approaches;③several representative and cutting-edge domains in which many state-of-the-art MOFs would serve as good candidates for potential industrial applications,which would in turn instruct the exploration of new materials;④viability analysis of MOFs in commercialization and industrial implementation;and⑤outlooks for both academic research and practical applications,as illustrated in Fig.1.Overall,our aim is to show that MOFs can be much more than just intellectual exercises;they can make a difference in the course of changing the world.We hope that this review will catch the attention of both chemists and engineers,and will stimulate more research interest in the design and application exploration of MOF materials to push forward green chemical engineering.It should be pointed out that MOF composites and MOF derivatives also play a significant part in many research fields related to green chemical engineering,in which they may show improved performance in specific applications[14–16].Such materials always function through complex mechanisms,which totally differ from those of pristine MOFs,thus escaping discussion.
2.Synthesis
The synthesis of MOFs has been attracting close attention throughout their rapid development[5,8,17].The main objective in MOF synthesis is to establish appropriate synthesis conditions under which the desired compound is able to nucleate and grow.The concepts of miniaturization and parallelization for largescale screening conditions dramatically accelerate the discovery of new MOFs,and promote synthesis method optimization.For green and industrially sustainable MOF production,however,different criteria should be taken into consideration,namely:①choosing cheaper,safer,and/or biocompatible building units;②decreasing energy input;③using innocuous reaction media;④easy activation;and ⑤continuous manufacturing[18].
2.1.General synthesis
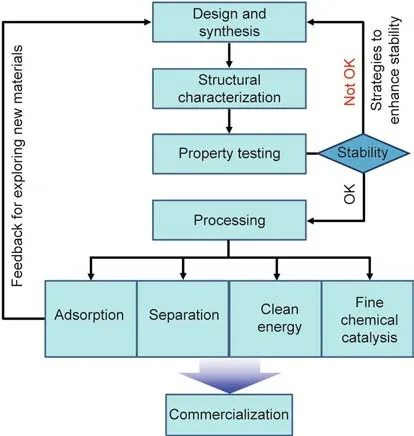
Fig.1.Flow diagram of MOF evaluation routes in green chemical engineering from foundation to application.
To date,numerous prominently varied synthetic methodologies have been developed for synthesizing MOFs[5,8,17].In general,appropriate MOF synthesis conditions should allow coordination bonds to be formed,broken,and reformed for error correction and extensive propagation during crystallization.Solvothermal synthesis is the most effective and widely adopted method;in this method,a metal species and a multi-topic organic ligand are mixed with a high-boiling-point solvent(e.g.,dimethylformamide(DMF),dimethylacetamide(DMA),or dimethyl sulfoxide(DMSO)),and then heated[8].Parameters including the reagent ratio,temperature,solvent,pH,reaction time,and so forth,can be systematically varied and optimized.These parameters are likely to influence not only the obtained structure,but also the crystal morphology,phase purity,and performance of the material.In some cases,particularly with strong coordination bonds such as those in zirconium(Zr)-MOFs,a modulator must be employed to help harvest crystalline products by preventing the rapid formation and precipitation of amorphous materials[19].High-throughput(HT)methods,which are a powerful tool to speed up the building of new MOFs and to modify synthesis conditions,are closely related to the concepts of miniaturization,parallelization,and automation[20,21].Alternative methods including electrochemical[22],mechanochemical[23],microwave-assisted[24],and sonochemical synthesis[25]have been established for specific purposes in MOF synthesis,such as morphology/size control,speeding up the reaction,and scale-up preparation[8].Various post-synthetic methods have also been developed to create new MOFs and,particularly,to functionalize given MOFs;these methods include solventassisted linker incorporation,post-synthetic modification,and transmetalation[26,27].
In the process of MOF synthesis,guest molecules(i.e.,solvents and residual starting materials,in a few cases)are inevitably trapped in the voids of the host network.In order to determine the porosity and surface area,the trapped guest molecules must be removed through an activation process.Heating the assynthesized MOF samples directly under vacuum is sometimes satisfactory,but is only suitable for certain stable MOFs synthesized in low-boiling-point solvents[28,29].In most cases,MOF activation through straightforward heating and degassing results in incomplete guest-molecule removal or even framework collapse.The most convenient approach to solve this issue is to replace highboiling-point or high-surface-tension solvents with lower species(e.g.,ethanol or acetone)before heating the MOF samples in a vacuum[5].Activation with supercritical carbon dioxide(scCO2)is an extension of traditional MOF activation methods.Milder scCO2activation,which eliminates strong capillary forces and surface tension through a supercritical phase,avoids phase changes in the removal of guest molecules[30].
2.2.Green synthesis
The principal aspect of researching MOFs for green chemical engineering is to find a method for greening MOF synthesis.In general,harmless reactants,an innocent solvent,mild conditions,and fewer byproducts are key factors in the green synthesis of MOFs.At least some of these considerations have been fulfilled in the synthesis of popularly explored MOFs such as Hong Kong University of Science and Technology(HKUST)-1,zeolitic imidazolate framework(ZIF)-8,and MOF-74[31,32].
To avoid possible byproducts,such as corrosive acids(mainly HCl and HNO3)generated by the reaction of metal salts and protonated ligands in an aqueous solution,metal salts containing benign anions or linker salts with innocuous metal cations are preferred.Thus,metal hydroxides and oxides are usually chosen,not only to ensure that water is the only byproduct,but also to achieve high atomic efficiency.In this regard,mechanochemical synthesis methods are always favored due to the insolubility of metal hydroxides/oxides.Other common approaches employ metal ions with carefully selected counter anions,such as acetates[32].
On the other hand,the choice of organic ligands has a strong impact on the design and utilization of MOFs,as it affects not only the final topology,but also specific functions.This selection criterion will be advantageous for the large-scale production of materials,based on simple,commercially available ligands or on those that can be readily obtained while producing less toxic byproducts.The Matériaux de l’Institut Lavoisier(MIL)-53(Al)series and MOF-74 were successfully synthesized with organic salts as the linker sources,thereby avoiding the generation of corrosive acids and environmental hazards[33,34].
The use of an innocuous solvent is also important for green synthesis.MOF materials are popularly synthesized using solvothermal methods,in which solvents are needed to dissolve the organic ligand and metal salt.DMF is a commonly used solvent with good solubility,albeit with toxicity,as it can decompose into harmful dimethylamine upon heating to high temperature.For green MOF synthesis,DMF should be omitted or replaced by an innocuous solvent[32].
From the standpoint of sustainability and environmental impact,solvent-free conditions are the best choice for the difficult MOF synthesis,as they involve safer and cleaner,albeit less soluble,sulfates or oxides salts.At present,three principal routes for the solvent-free synthesis of MOFs have been explored:mechanochemical,thermochemical,and diffusion-controlled‘‘accelerated aging” reactions.As reported by Cliffe et al.[35],ZIFs can be synthesized by the ‘‘accelerated aging” method.
Of course,water is the best alternative solvent because sufficient reserves are available and it is environmentally friendly,which facilitates subsequent purification and recycling.A large number of MOFs have been prepared in water,including HKUST-1[36],the MIL series[37],and the Universitetet i Oslo(UiO)series[38].Cyrene(dihydrolevoglucosenone),a green solvent with almost no eco-toxicity of mutagenicity,has been investigated in the preparation of several representative MOFs[39].Ionothermal synthesis,which uses ionic liquid as both a solvent and a template,has also been considered as a green method to prepare MOFs[40,41].
In addition to solvent-free and green-solvent-based strategies,microwave-assisted synthesis is an eco-friendly approach for the preparation of MOFs in aqueous conditions,and can significantly accelerate the self-assembly of inorganic and organic components[42].
2.3.Scale-up preparation
MOFs are generally prepared in milligram scales in the lab by heating in expensive organic solvents for several hours to days.The evaluative criteria for industrial MOF synthesis are commonly set to resolve the safety,toxicity,availability,and cost of all reagents,as well as the operability and productivity in amplified production[18,43–46].The huge disparity between the conditions required for laboratory preparation and those for commercialization has created a strong demand for the development of highefficiency and low-cost methods for the large-scale production of MOFs.
The majority of promising scale-up approaches present one or more of the following challenges:
(1)Use of organic solvents.In a scale-up approach,the corrosivity,toxicity,cost,recyclability,and flammability of organic solvents present intractable problems in some cases.
(2)Anion accumulation.Nitrates present an explosion hazard,and chlorides can give rise to corrosive byproducts.Oxides and hydroxide metal species are thus favored.
(3)Ligand availability.Customized and complicated organic ligands are needed for many MOFs.
(4)Particle-size control.Thin film applications require nanosized particles,while for storage applications,larger particles are needed in order to preserve their stationary state.
(5)Activation.Non-volatile solvents and unreacted raw materials in MOF pores must be removed before utilization.
(6)Shaping.For practical industrial applications,the shaping of a MOF into different forms is necessary[45].
Each MOF presents its own specific challenges due to its unique composition,structure,and properties,making the synthesis of these materials more complicated than that of zeolites.Various strategies have recently been evaluated to cope with these challenges,including electrochemical,mechanochemical,and microwave-assisted synthesis,as well as continuous-flow production.BASF was the first to demonstrate the scale-up synthesis of MOFs via an electrochemical process for the industrial preparation of HKUST-1,by using metal electrodes directly as a metal source in order to exclude metal anions[46].Microwave-assisted synthesis,spray drying,and flow chemistry permit crystallization to occur at a faster rate and result in the production of MOF crystals with a smaller size.Mechanochemical synthesis,in which extra solvent or heating is not required,avoids post-synthetic washing and activation,and thus exhibits great potential for the scale-up production of MOFs.For the time being,plenty of archetypal MOFs are being produced using scale-up approaches,and are then shaped into different bodies,including ZIF,MIL,the UiO series,HKUST-1,MOF-5,MOF-74,and so forth(Fig.2)[43–46].
3.Structural characterization and stability
3.1.Structural and morphologic characterization

Fig.2.(a)Differently shaped bodies of MOF materials;(b)large-scale production facilities of MOFs at BASF in Germany.(a)Reproduced from Ref.[43]with permission of the Royal Society of Chemistry,©2009;(b)reproduced from Ref.[46]with permission of the Royal Society of Chemistry,©2015.
Single crystal X-ray diffraction(SXRD)or Rietveld refinement of powder X-ray diffraction(PXRD)data give absolute structural information on MOFs.Basic MOF characterization data contain a PXRD pattern to determine the crystallinity and phase purity of the material,and a Brunauer–Emmett–Teller(BET)measurement to confirm the porosity.Additional characterization protocols and techniques include:chemical stability testing of MOFs in aqueous solutions at varied pH;thermogravimetric analysis(TGA)to indicate the framework thermal stability;scanning electron microscopy(SEM)for particle size and morphology,in combination with energy-dispersive X-ray spectroscopy(EDS)for elemental composition and content;nuclear magnetic resonance(NMR)spectroscopy to quantify the linker ratios in mixed linker-based structures;inductively coupled plasma optical emission spectroscopy(ICP-OES)for elemental ratios;and diffuse reflectance infrared Fourier-transform spectroscopy(DRIFTS),as well as Fourier-transform infrared(FT-IR)spectroscopy,to confirm the existence or non-existence of infrared(IR)-responsive functional groups in the target MOF material.All characterization methods should be used for the correct purpose in order to identify the precise composition,structure,and morphology traits of target MOF materials,thus laying a foundation for subsequent property prediction and application exploration[5].
3.2.Stability
Good stability of MOFs,including their mechanical,thermal,and/or chemical stability,is the prerequisite for their application in an expansive scope.The stability of a MOF is influenced by various factors,including the species of metal ions,size and configuration of organic ligands,coordination geometry between metals and ligands,operating environment,and hydrophobicity of pore surfaces.Coordination bond strength between inorganic nodes and organic ligands—as such bonds have a weaker linkage than covalent bonds—always plays a crucial role in determining MOF stabilities[47].Here,we overview the specific influencing factors in MOF stability in different forms,along with associated enhancing strategies.
3.2.1.Mechanical/framework stability
MOFs are well known for their high porosity,which inescapably reduces their mechanical strength in resisting pressure or a vacuum.Under mechanical stress or a vacuum,this instability contributes to phase transitions,partial collapse,or even amorphization of the framework structure.To fully activate MOFs while avoiding structural collapse,solvent-exchange methods are commonly adopted before evacuation[5].Compared with MOFs built with low-valent metal ions,Zr-MOFs typically have better mechanical stability due to their high connectivity of Zr-based clusters and strong Zr–O coordination bonds in the framework[47].Framework geometry,structural defects,and network interpenetration are also relevant to the mechanical stability of MOFs[48].Furthermore,an interesting subset of MOFs have been rationally designed with reversibly flexible structures,and exhibit superior mechanical compliance[49].
3.2.2.Thermal stability
In most cases,the thermal decomposition of MOF materials is a consequence of the cleavage of coordination bonds,which is accompanied or followed by the dehydration of the metal nodes,the dehydrogenation,carbonization,or even burning of the linker,and the amorphization or melting of the MOF.The thermal stability of MOFs is normally determined by the strength and connectivity of the coordination bond.
Coordination bond strength can be increased by replacing lowvalent metal ions connected to the carboxylate linkers with highvalent species(Al3+,Zr4+,and Ti4+),thus increasing the thermal stability of MOFs[50].Another way to increase the coordination bond strength in order to increase the framework thermal stability is to vary the functional groups attached on the organic ligand.The interpenetration or interweaving of networks can enhance the stability through favorable framework–framework interaction[47].In addition,intentional mechanical stress during preparation sometimes prompts the MOF phase to transform from a metastable state to a more stable one[51].
3.2.3.Chemical stability
Chemical stability refers to the ability of MOFs to retain their framework structural integrity under specific chemical conditions[47].Two main factors that determine the chemical stability of MOFs are the MOF structure(i.e.,internal factors)and the operating environment(i.e.,external factors).
Water stability is a significant quality for industrial applications of MOFs due to the presence of a large amount of water in processes such as preparation,storage,and application.The decomposition of MOFs in liquid water or water vapor can be considered as a series of substitution reactions in which the coordinated linkers with metal nodes are replaced by OH-or H2O entities.Hence,a direct way to withstand such substitution is to increase the robustness of the coordinate bonds between the metal nodes and organic linkers,or to introduce hydrophobic functional groups to prohibit water molecules from attacking[52,53].
Compared with neutral H2O molecules,the OH-and H+in basic and acidic aqueous solutions are much more destructive to MOFs.As the chemical environments in alkaline and acidic solutions are different,MOF stability would be affected in different ways by bases and acids.Various MOFs composed of carboxylate-based ligands and high-valent metal cations show great stability in acidic solutions,but are more vulnerable in basic conditions.On the other hand,MOFs composed of azolate-based ligands and low-valent metal ions generally exhibit better stability in basic conditions,whereas their robustness in acidic solutions is much weaker[54].
Besides OH-and H+,other coordinating anions(e.g.,F-,CO32-,and PO43-,to name a few)can also destroy the framework structure of MOFs.Carboxylate ligands are easily displaced when such coordinating anions are present as competitors in the solution,especially for MOFs built with high-valent cations,such as Zr4+,Fe3+,and Al3+[55].The high binding constants between the highvalent metal ions and these competitive anions indicate a strong interaction.An effective strategy to prevent the destruction of frameworks by these anions is to construct MOFs with azolatebased linkers and low-valent metal cations[56].
3.2.4.Strategy for enhancing stability
As discussed above,the crucial issue in enhancing MOF stability is improving the strength of the coordinate bond between the inorganic nodes and organic linkers.Based on Pearson’s hard/soft acid/base theory,the affinity between soft Lewis bases and acids(or hard Lewis bases and acids)is much stronger than that between soft bases and hard acids(or hard bases and soft acids).Therefore,researchers choose to build networks with carboxylate-based ligands(hard Lewis bases)and high-valent metal ions(hard Lewis acids),or with azolate-based ligands(soft Lewis bases)and lowvalent transition metal ions(soft Lewis acids)for robust MOFs.The viability of this strategy has been demonstrated by many examples of stable MOFs[54].Selecting a suitable operating environment,creating framework catenation,and incorporating hydrophobic functional groups into pores are also valid means of strengthening MOF stability[47,54].
4.Processing
With the inherent properties retained or improved,processing MOFs into different forms provides obvious advantages in allowing them to be readily stored,transported,and used.Systematic control over shape and size is favorable for advancing MOF materials for industrial application,achieving their great potential in practice,and eventually placing them on the market[57].MOFs can usually be shaped into different forms with either anin situ(direct method)or post-synthesis(indirect method)procedure.In the former method,crystalline powders are directly arranged into desired forms such as membranes or hollow superstructures during the formation of MOFs.In the latter method,processing is a secondary process executed on preformed crystalline powders.Among the most common shapes that MOF crystallites can be fabricated into are granules,pellets,membranes,foams,gels,paper sheets,and hollow structures(Fig.3)[58–66].However,the specific shape of a MOF is usually determined by the requirements of real-life applications,given the processing feasibility and performance availability.In this section,we highlight popular processing methods that have been adopted to engineer MOF powders into various shapes.
4.1.Granules
Granulation is the process of agglomerating powder materials into granules without altering their chemical composition and architecture.This can be executed using wet or dry approaches according to the processing technique.In the wet approach,in order to adhere the powder particles into granule forms,a volatile solvent is generally needed,which is removed by drying afterwards.The properties of the formative granules rely strongly on those of the original MOF powder and the viscosity of the binder,as well as on the interactions between them;thus,binder selection is critical.Poly(vinyl alcohol),graphite,and cellulose ester are widely chosen as binders in the shaping of MOFs.When a material is vulnerable to solvents or heating,a dry granulation approach is applied,which uses high stress(compression).Practical granulation processes for MOFs often involve a centrifugal granulator,a high shear mixer,or an extruder[58].
Ren et al.[59]shaped UiO-66 powder into granules(with diameters ranging from 0.5 to 15 mm)(Fig.3(a))using a granulator,with 10% mass fraction sucrose as the binder.This approach produced UiO-66 granules in a kilogram batch.The results of the drop test and simulated tumbler drum test showed zero breakage of the granules after 70 consecutive drops at a height of 0.5 m,and 5%breakage after one hour of tumbling time at a speed of 25 r·min-1;thus,these granules demonstrated better mechanical strength in resisting abrasion in a real hydrogen storage environment than those made using a mechanical pressing method.
4.2.Pellets
As the most conventional and effective method of shaping MOFs,pellet formation follows similar rules to granulation.Particle agglomerates are formed by imposing pressure on the powder materials,with or without the addition of a binder(i.e.,according to a wet or dry method).The shapes of the resulting agglomerates differ,with granulation resulting in spherical agglomerates and pellet formation resulting in cylindrical agglomerates.Such a difference in the shapes of MOF materials can affect their performance in certain industrial applications.The addition of binders can also influence the MOF properties.For example,an organic polymer binder can improve the mechanical stability of the MOF pellets,while a graphite binder can enhance their thermal conductivity.On the other hand,the binder molecules may block the pores of the source MOFs,causing a decrease in the surface area.Mechanochemistry is another extensively employed method to shape MOF powders into pellets.Several classical MOFs,such as ZIF-8,Al(fumarate)(OH)(AlFu),HKUST-1,and UiO-66,have been shaped into pellets(Fig.3(b))with an extrusion approach that involves imposing pressure on precursor powders[60,67].
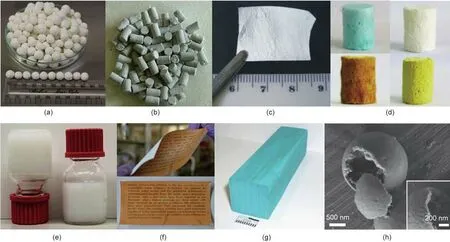
Fig.3.MOFs shaped in the forms of(a)granules,(b)pellets,(c)a thin film,(d)foams,(e)gels,(f)a paper sheet,(g)a monolith,and(h)a hollow structure.(a)Reproduced from Ref.[59]with permission of Hydrogen Energy Publications,©2015;(b)reproduced from Ref.[60]with permission of the American Chemical Society,©2012;(c)reproduced from Ref.[61]with permission of the Royal Society of Chemistry,©2012;(d)reproduced from Ref.[62]with permission of the American Chemical Society,©2016;(e)reproduced from Ref.[63]with permission of the Royal Society of Chemistry,©2017;(f)reproduced from Ref.[64]with permission of the Royal Society of Chemistry,©2016;(g)reproduced from Ref.[65]with permission of the John Wiley and Sons,©2010;(h)reproduced from Ref.[66]with permission of Nature,©2013.
In industry,adsorption beds such as fluidized and fixed beds are optimal choices for the separation of liquid and gas mixtures.Granules and pellets are ideal stationary phases for separation in these beds,due to the close packing and high density of MOFs in a small volume[58].In a study by Kim et al.[68],MIL-100(Fe)granules(1.18–1.70 mm in size)were fabricated and then packed into an adsorption column for the separation of SF6from a nitrogen(N2)stream.With a higher working capacity(1.45 mmol·g-1)than Zeolite 13X(0.97 mmol·g-1)at 1 bar(1 bar=105Pa),as well as easier regeneration,the MIL-100(Fe)granule has great potential for SF6/N2separation.
4.3.Membranes
Processing MOF powders into membranes is critical for many industrial applications such as toxic gas filters and water purification.Pure MOF membranes(including most MOF thin films)are prepared by continuous growth methods,and their chemical properties merely depend on those of the MOF materials themselves.A bottom-up approach is adopted to form MOF thin films on different substrates,employing methods such as crystal growth,layer-bylayer(LBL),liquid-phase epitaxy(LPE),seeding,electrochemical deposition,and Langmuir–Blodgett deposition.The fabrication of a series of free-standing MOF membranes well-integrated on electrospun nanofibrous supports has been realized via a seeded secondary growth approach(Fig.3(c))[61].The design principles of MOF-based thin films for high-efficiency applications emphasize‘‘design for purpose”;that is,suitable MOF materials and synthesis methods are chosen according to the targeted component or process.Numerous MOF thin films have been prepared based on different MOF materials for gas or liquid separation,which will be discussed in detail in Section 6.3.However,due to the inherent fragility of MOF thin films and the intricate methods that are used to make them,much research on MOF thin films is still necessary to achieve further technical breakthrough in their actual separating application[58].
4.4.Foams and gels
Unlike efforts to shape MOFs into granules,pellets,and thin films,the formation of a standalone MOF foam has rarely been reported.The majority of relevant investigations in this area concentrate on growing MOF crystals on foam-like structures[69].For this purpose,Chen et al.[62]proposed a continuous-phase transformation processing strategy.Several MOFs were fabricated and shaped into processable fluids,shaped bodies,and even MOF foams,which could realize a reversible conversion among these forms(Fig.3(d)).To be specific,HKUST-1@Fe3O4nanoparticles were dispersed into a carboxymethylcellulose solution to form a magnetic fluid,which could be patterned into different shapes and transformed into a foam or a gel form using different methods.In addition,the robust and hierarchically porous HKUST-1@Fe3O4foam showed excellent catalytic C–H oxidation performance.
Xerogels and aerogels are technically regarded as foams.A xerogel can be obtained by removing the liquid in a gel,and an aerogel is obtained by replacing the liquid with a gas,while the structure of the gel has tiny or no changes[70].Many solid materials can be readily molded into different shapes by using porous gels.MOF foams and sponges are extraordinary candidates for energy carrier storage and transportation due to their features of light density and high porosity.Nevertheless,MOF-based gel materials(Fig.3(e))have scarcely been reported as yet,primarily because of their high fragility[63].
4.5.Paper sheets,hollow structures,and other shapes
Previous studies have reported on MOFs in forms such as paper sheets(Fig.3(f))[64],monoliths(Fig.3(g))[65],and hollow structures(Fig.3(h))[66].MOFs shaped as filter papers and sheets can easily be prepared by means of crystal growth,pulp processing,coating,and inkjet printing onto polymer,pulp,and cotton fibers.Another way to process MOFs is to grow MOF crystals into hollow structures using methods such as templating,Ostwald ripening,etching,and spray drying.Interfacial synthesis and spray drying are prospective methods for shaping MOF powders in scale-up processing for their fast,facile,and continuous operation procedures.
To achieve better performance in industrial applications,MOFs need to be produced on a large scale,be industrially tested,and be shaped into different forms.By carefully controlling the MOF chemistry,various methodologies have been established for processing MOFs.However,strategies for shaping MOFs require further optimization.For example,an appropriate pressure is requisite to prevent the MOF framework from structurally collapsing during compression,and a suitable binder is necessary to avoid pore blocking and ensure optimum void fractions between primary powders in order to relieve the diffusion limitations of shaped bodies.Although the network fragility and complex fabrication procedures introduce significant difficulties,there is an excellent chance of developing applicable device-processing methods with greater feasibility and efficiency,in order to access the outstanding potential of MOFs,as promised by their elaborate structures and prominent properties in practical implementation.
5.Adsorption applicatio n
The growing scarcity of fossil fuels—accompanied by increasingly severe environmental problems and climate change,attributed to rapid civilization and industrialization—has driven an initiative to develop alternative green fuels and pollutantmanagement methods.Adsorption-based technology has demonstrated some particular advantages for these purposes,including low energy input,high storage capacity and selectivity,ease of operation,and good regeneration of adsorbents.Thanks to their tunable pore size and structure,high porosity,rich active open metal sites(OMSs),and wide range of modifiable functional groups on the framework,MOFs are an attractive category of materials for adsorption applications.The use of MOF adsorbents for industrial processes aligns with the demands of green chemical engineering,to some extent.It can be forecasted that,in the near future,the discovery of advanced porous MOF materials for efficient fuel storage,pollutant removal,water harvesting,and other adsorption-based technologies will significantly contribute to clean energy exploitation and environmental protection.
5.1.Gas storage
Among diverse possible fuels,hydrogen(H2)and methane(CH4)are considered to be predominant candidates for clean energy source carriers.However,their volumetric energy densities under ambient pressure are relatively low,which seriously impedes their widespread utilization,especially in mobile/transportation applications.As an alternative to compression and liquefaction,adsorption technology based on MOF adsorbents offers a prospective solution to the continual challenges of safely storing and efficiently using H2and CH4[71,72].A desired MOF adsorbent—as a fuel storage material for vehicular application—should have not only a high fuel uptake capacity,but also(and even more importantly)a high deliverable capacity.Various strategies for improving the fuel storage capacity of MOFs have been investigated,including tuning pore chemistry,embedding OMSs,and linker or node functionalization[73].
H2is the most ideal green energy carrier for sustainable development because of its high thermal efficiency and pollution-free combustion.To achieve a target driving range of 483 km for fuel-cell-based lightweight vehicles,the US Department of Energy(DOE)has confirmed specific targets for H2storage systems:a gravimetric capacity of 5.5%mass fraction and a volumetric capacity of 40 g·L-1based on the mass and volume of the entire system,respectively.These targets must be available in the temperature range of 40–60°C with a maximal pressure of 100 bar.In 2018,Kapelewski et al.[74]revealed that the modified MOF-74 type M2(m-dobdc)(M=Co,Ni;dobdc4-=2,5-dioxido-1,4-benzenedicarboxylate)was corroborated to be the best-performing physisorptive storage material,with a working volumetric capacity of 11.0 g·L-1at 25°C between 5 and 100 bar,and of 23.0 g·L-1with a temperature swing between -75 and 25°C at 100 bar(Fig.4(a)).Fig.4(b)[71]shows the correlation between excess gravimetric H2uptake and enthalpy of H2adsorption for various porous materials,which can instruct the better designing and screening of MOF sorbents for H2storage.
CH4,as the major constituent of natural gas,is also regarded as a preferable clean fuel.For onboard CH4storage systems,the gravimetric capacities of adsorbents should reach 0.5 g·g-1(700 cm3·g-1)at 298 K,while the volumetric capacities should be 263 cm3methane per cubic centimeter sorbent,which is equivalent to the density of compressed natural gas at 298 K and 250 bar[75].Yan et al.[76]reported the CH4storage properties of a series of isostructural MOFs—that is,Manchester framework material(MFM)-112a,MFM-115a,and MFM-132a—among which MFM-115a showed an astonishingly high deliverable CH4capacity of 208 volume fraction at room temperature between 5 and 80 bar,rendering it among the top-performing MOFs for CH4storage(Figs.4(c)and(d)).
Acetylene(C2H2)is an indispensable gaseous chemical in modern industry that is commonly used as a gas fuel for oxy-acetylene welding and metal cutting,and as a crucial starting material to produce multiple fine chemicals and electronic materials.The secure storage and transport of C2H2is greatly impeded by its explosiveness when compressed under a pressure over 2 bar at ambient temperature,even in the absence of oxygen[72].Matsuda et al.[77]have reported that a MOF,Cu2(pzdc)2(pyz)(pzdc=pyrazine-2,3-dicarboxylate;pyz=pyrazine),can accommodate C2H2with an adsorbed density of about 0.434 g·cm-3,which is 200 times higher than the compression limit for the safe usage of C2H2at room temperature.
5.2.Pollutant removal
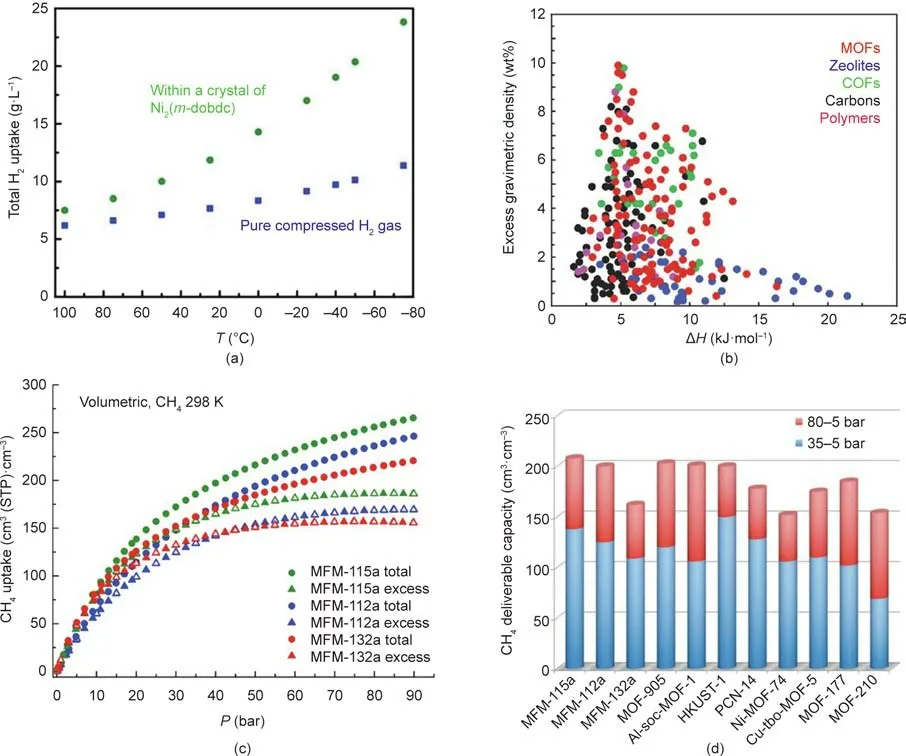
Fig.4.(a)Comparison of the total volumetric capacities of Ni2(m-dobdc)and pure compressed H2 at 100 bar;(b)excess gravimetric H2 uptake versus enthalpy of H2 adsorption for various classes of hydrogen storage materials(COF:covalent organic frameworks);(c)total and excess volumetric CH4 adsorption isotherms for MFM-115a,MFM-112a,and MFM-132a in the pressure range 0–90 bar at 298 K(STP:standard temperature and pressure);(d)comparison of the deliverable CH4 capacities in some MOFs at 298 K(soc:square–octahedral;tbo:twisted boracite).(a)Reproduced from Ref.[74]with permission of the American Chemical Society,©2018;(b)reproduced from Ref.[71]with permission of the Royal Society of Chemistry,©2018;(c,d)reproduced from Ref.[76]with permission of the American Chemical Society,©2017.
In recent years,the excessive burning of fossil fuels worldwide has had a permanent effect on habitats due to the discharge or emission of massive amounts of hazardous compounds into the air or water.The removal of such chemicals has conventionally posed a challenge due to their extensive utilization,fine water solubility,and poor biodegradability.Therefore,adsorption technology has experienced rapid growth as an effective decontamination tool for applications such as air treatment,water purification,and fuel upgrading,as it avoids the need for intensive energy consumption and offers good adsorbing capacity[2,78–81].Many MOFs are potential sorbent materials due to their inherent merits in terms of structure and property,which can be tailored to achieve stronger or more selective binding affinity toward targeted adsorbates.
5.2.1.Air treatment
Various airborne pollutants,including toxic industrial chemicals(TICs),volatile organic compounds(VOCs),and fine particulate matter(PM),have caused severe air contamination and deteriorated human living conditions.To mitigate these detriments,abundant MOFs have been found to be promising adsorbents for the adsorptive capture of harmful gases in the atmosphere[2,78,79].
The TICs(e.g.,NH3,H2S,SOx,NOx,and CO)are a notorious family of air pollutants.Ammonia(NH3)is widely employed in the production of fertilizers,detergents,and pharmaceuticals,and has an odor threshold for humans as low as 5 ppm.Through hydrogen bonding,NH3can strongly interact with the inorganic nodes or organic linkers of MOFs.Certain functional groups,such as–OH and–NH2,typically show strong affinities to the NH3molecule in hydrogen bonds,in addition to–COOM(M=Cu,Ag,Na,or K)groups in chemisorption mechanisms.NH3can connect to OMSs through coordinate bonds,as observed in MOF-74 and HKUST-1.Furthermore,it is a critical ability of MOFs to bind the adsorbate with priority over water,because most ambient airstreams include moisture.Hydrophobic MOFs have been suggested to interact with NH3preferentially over water[78].
Hydrogen sulfide(H2S)is a hypertoxic gas that is generally viewed as an impurity in the natural gas and petroleum industry,and is a byproduct of anaerobic decomposition.Humans are extremely sensitive to the pungent rotten-egg odor of H2S,which can be perceivable at levels as low as 4.7 ppb.Bhatt et al.[82]built three isoreticular rare earth(RE)-fcu-MOFs(fcu=face-centred cubic)displaying remarkable performance in the removal of H2S from CO2-containing gases and CH4-containing gases such as biogas,natural gas,and landfill gas.Their high H2S/CO2selectivity even outperformed those of the benchmark materials activated carbon and zeolites.
Sulfur and nitrogen oxides(SOxand NOx)are atmospheric pollutants that are byproducts of fossil fuel combustion and are known to cause acid rain.Tan et al.[83]minutely investigated the interaction between the acid gases SO2and NO2and M-MOF-74(M=Zn,Co,Ni,Mg)with abundant OMSs,and found that SO2can be molecularly adsorbed into Zn-MOF-74 and Mg-MOF-74 with high binding energy.In contrast,for NO2,a strong Zn–NO2binding significantly weakens the N–O bond,accelerating the divergent degradation of NO2molecules into NO and NO3-.These results indicate that denitrification and desulfurization processes using MOF-74 as the adsorbing material in flue gas decontamination are viable.
Yang et al.[84]recently reported that MIL-100(Cr)has the supreme N2O-capture capacities of 8.25 mmol·g-1at 273 K and 5.78 mmol·g-1at 298 K,and thus exhibits an extremely high N2O/N2simulated gas separation selectivity of up to 1000.
Statistics from the Centers for Disease Control and Prevention reveal that roughly 500 people die from carbon monoxide(CO)-incurred asphyxia in the United States annually.MOFs bearing rich OMSs,such as HKUST-1 and MOF-74,have been studied for CO capture due to the electrostatic interaction and coordinative bonding between OMSs and CO molecules.In addition,CO removal can be realized through catalytic oxidation to CO2,in some cases[78].
VOCs(e.g.,benzene(Bz)and xylenes)are widely used as reagents in industrial processes;they also exist in the urban atmosphere due to vehicle emissions,which is gravely deleterious to our environment and to human health.Furthermore,because of their high reactivity with other gassy contaminants that exist in the outdoor atmosphere(e.g.,NOx),VOCs energetically participate in the generation of secondary pollutants(e.g.,ozone).As demonstrated by Xie et al.[85],[Zr6(μ3-O)4(μ3-OH)4(BDB)6](Beijing University of Technology(BUT)-66),which shows high hydrolytic stability and possesses small hydrophobic pores,demonstrates high volumetric Bz adsorption capacity even at high temperature and low pressure,outperforming the commercial benchmark adsorbents Carboxen 1000 and Mobil Composition of Matter No.41(MCM-41).BUT-66 can adsorb parts per million levels of Bz in air even with moisture,which implies great prospects for practical application.
Among the frequently encountered solid pollutants in daily life(e.g.,dust,PM,pollen),fine PM is the most detrimental species,as it creates serious and long-term damage to air quality and regional climates.Levels of particulates with an aerodynamic diameter below 2.5μm(PM2.5)and 10μm(PM10)have been increasing worldwide in recent years,reducing local air visibility and impairing human respiratory systems.In the research of Zhang et al.[86],nanocrystals of four MOFs(ZIF-8,Mg-MOF-74,MOF-199,and UiO-66-NH2)were fabricated into nanofibrous filters(known as‘‘MOFilters”)with high MOF loadings(up to a mass fraction of 60%).When tested in a hazy environment,high PM-removal efficiencies of up to 88.33%±1.52% and 89.67%±1.33% for PM2.5and PM10,respectively,were achieved by the MOFilters,which also demonstrated good durability.
5.2.2.Wastewater treatment
A broad scope of contaminants are relatively soluble in water,including heavy metal ions,dyes,pesticides,detergents,pharmaceuticals,phenols,and others;water pollution with such contaminants can severely impair the health of all life forms along related food chains and,ultimately,endanger human life.Pollutants in wastewater can be classified into inorganic and organic species according to their chemical composition.From the perspective of energy conservation and cost reduction,adsorption[53,80]or photocatalytic degradation induced by solar energy[87](see Section 7.2.1 for details)are promising routes for eliminating wastewater contaminants.MOFs with hydrolytic stability and specific functionality are good sorbent candidates for grafting these substances from water,through interactions such as ion exchange,hydrogen bonding,acid-base interaction,and electrostatic interaction.To this end,the incorporated OMSs,functionalized linkers,and loaded active species could be optimized to enhance the adsorption capacity and selectivity of MOF materials in wastewater treatment.
Inorganic contaminants in sewage principally include heavy metal ions,excessive fluoride ions,and radioactive substances.Massive efforts have been devoted to eliminating these pollutants from wastewater with MOFs.
The casual disposal of residual heavy metals from labs and factories causes extensive water pollution.Common heavy metal ions in wastewater are arsenic,cadmium,lead,mercury,chromium,copper,nickel,cobalt,and zinc ions.Based on the strong Zr–O bond,which has good chemical stability,Li et al.[88]inspected MOF-808 for the removal of arsenic(As5+)from water.They discovered that the adsorption capability for As5+of monodispersed MOF-808 octahedral nanoparticles was about 24.8 mg·g-1,with an original As5+level of 5 ppm.
Wang et al.[89]demonstrated that HKUST-1 functionalized with sulfonic acid is capable of removing cadmium ions(Cd2+)from aqueous solution,with a high Cd2+uptake of 88.7 mg·g-1that exceeds that of the benchmark adsorbents.Furthermore,this method has fast kinetics,high selectivity,and easy regeneration toward cadmium ion adsorption.
According to Pearson’s hard/soft acid/base theory,soft bases generally show a strong affinity to soft acids[54].Based on this premise,many intriguing examples have been reported for the capture of mercury(Hg2+)using MOFs based on sulfur-containing ligands[90].Liang et al.[91]have established a new methodology of employing NCS–-functionalized MOF FJI-H12 to remove Hg2+from wastewater.FJI-H12 can remove Hg2+from water completely and selectively with a high saturation(439.8 mg·g-1)and distribution coefficient(1.85×106mL·g-1)relative to other MOFs.Remarkably,rapid and continuous removal of Hg2+from water was also achievable using a column loaded with FJI-H12 microcrystals,indicating that this method holds promise for realistic pollutant control and sewage treatment application.
Luo et al.[92]proposed the so-called MOF+technique for toxic chromate(in the form of Cr2O72–)removal from aqueous solutions,which is based on a significant synergic effect between UTSA-74 and Fe2SO4.The findings suggest that relative to pristine MOFs(i.e.,those without obvious chromate adsorption),the MOF+approach presents a superior performance,with a chromate adsorption capacity of 796 mg·g-1—the highest value among all known porous adsorbents in this regard.
For the adsorptive removal of excessive fluoride ions(F–),material robustness in fluoride solution is a prerequisite for MOF performance.Preliminary studies revealed that a relatively inactive metal center with high connectivity and pores with suitable hydrophobicity contributed to good stability of MOFs in fluoride solution.Stable AlFu MOF demonstrates an ultrahigh fluoride uptake capacity of 600 mg·g-1at 293 K,as reported by Karmakar et al.[93].
Nowadays,investigations on the use of MOFs as adsorbents for the removal of radioactive matter in wastewater are primarily centered on133Ba,99Tc,129I,232Th,235U,and238U.Zheng et al.[94]recently synthesized two crystalline zirconium phosphonate frameworks,SZ-2 and SZ-3.Strikingly,these two complexes have ultrahigh stability and exhibit a high uranium uptake over a wide pH range of 3–7.This process also proceeds in a very fast adsorption rate by the ion-exchange mechanism.
Organic contaminants in wastewater involve a wider range of classifications than inorganic contaminants,and comprise dyes,detergents,pesticides,pharmaceuticals and personal care products(PPCPs),phenolics,and more.Adsorption removal by MOF materials has been demonstrated to be an efficient and economical means of treating organic pollutants in water[80].
Dyes are a category of ubiquitous organic contaminants in water.Their intense color weakens or prevents incident sunlight from entering water bodies,thereby interfering with the balance of aquatic ecosystems.Furthermore,most dyes are poisonous and can induce disorders,including cancer.Haque et al.[95]demonstrated that MOF-235 is capable of the adsorptive removal of hazardous dyes(i.e.,anionic methyl orange(MO)and cationic methylene blue(MB))with good efficiency from water.The adsorption capacities of MOF-235 toward MO and MB were found to be as high as 477 and 187 mg·g-1,respectively,under appropriate pH values,greatly outperforming activated carbon for the same use.
Studies with MOFs as the sorbent in the removal of toxic pesticides from water have been relatively scarce,and the target objects have been limited to 2,4-dichlorophenoxyacetic acid(2,4-D),methylchlorophenoxypropionic acid(MCPP),and diquat(DQ)to date[80].
As a new class of organic pollutant,PPCPs have appeared in diversified water systems,including surface water,groundwater,and even drinking water.Due to their undegradability,the accumulation of PPCPs in wastewater poses a terrible environmental threat.A series of MOFs have been exploited for the adsorptive removal of PPCPs from water,including the MIL family,UiO-66,and ZIF-67.Wang et al.[96]constructed two stable isostructural Zr-MOFs—BUT-12 and BUT-13—for sensing and adsorbing a wide range of antibiotics and organic explosives in aqueous solution.BUT-12 and BUT-13 are responsive to nitrofurazone(NZF)and 2,4,6-trinitrophenol(TNP)at parts per billion levels,and are thus among the top-performing MOF-based luminescent sensors.Both MOFs also show high adsorption abilities,and therefore serve as effective tools for pollutant removal.
Van de Voorde et al.[97]attempted to segregate phenolics from aqueous mixtures by means of liquid-phase adsorption over a family of MOFs(MIL-140(B/C/D)),which were found to outperform other kinds of porous solids.A breakthrough experiment with an MIL-140C column in aqueous solution confirmed its potential for the capture of pure hydroquinone.
5.2.3.Fuel upgrading
The major species of impurities in fossil fuels are sulfurcontaining and nitrogen-containing compounds(SCCs and NCCs,respectively),which naturally exist as byproducts in fossil fuels.Upon burning,these compounds give off toxic SOxand NOxgases,in addition to CO2,resulting in numerous environmental problems.Hence,the capture and isolation of hazardous materials—especially SCCs and NCCs—are extremely important prior to the use of fossil fuels.Although some strategies for the removal of these contaminants have been developed in the last few decades,the latest progress in adsorptive desulfurization(ADS)and denitrogenation(ADN)with MOF-based materials promises a more facile approach[81].
Studies show that specific functionalities such as active metal sites or pendent groups on ligands are much more important than porosity for ADS with MOFs.Yoon et al.[98]reported on the ADS capacity of diverse MOFs,and demonstrated that MOFs with OMSs are the most efficient adsorbents.MOF-74(Ni)and HKUST-1,which have sufficient OMSs in their structures,showed the maximum uptake of SCCs among the investigated MOFs.The polarity of the incorporated functional groups in a MOF is also responsible for its selective adsorptive performance toward these compounds.
In fact,the use of MOFs in ADN is more notable than their use in ADS,because of their preferred adsorption of NCCs over SCCs in most cases.The general trend in MOF adsorption selectivity is as follows:NCCs >SCCs >aromatics >aliphatics[99].Possible reasons for this trend are that the coordinative interactions between NCCs and the OMSs in MOFs are comparatively more exothermic than the interactions with SCCs,and the affinity of aromatics to MOF materials is greater than that of aliphatics.The adsorption behavior can also be explained using Pearson’s hard/soft acid/base theory.Van de Voorde et al.[100]applied a series of MIL-100 MOFs for the adsorption of contaminants in various model fuels,including indole(IND),thiophene(THP),and 1,2-dimethylindole(1,2-DMI).The results suggest that MIL-100(V)is an outstanding adsorbent with both high adsorption capacity and selectivity toward ADN.
5.3.Water adsorption
Water adsorption technologies have been extensively adopted in commercial applications,and are playing a significant part in our daily life.Ideal adsorbents should possess ①excellent chemical stability in water,②adjustable hydrophilicity,and ③a tailorable aperture for fine-tuning the adsorption profile and regulating the sorption kinetics.To improve the energy efficiency,water adsorbents should be developed to allow facile regeneration at or below 80°C;they should also utilize low-grade energy sources for further energy savings.Tuning the structure and function of MOFs makes it possible to modulate the strength of interactions with adsorbates accurately,thus facilitating the exploitation of solar energy or waste heat for sorption cycles.MOFs with high porosity and good hydrolytic stability are prominent candidates for industrial water sorbents in various fields,including fresh water production,adsorption-driven heat-reallocation systems,industrial or indoor dehumidification,humidity control,and more[52,53].
Atmospheric humidity is roughly estimated to equal 10%of the gross liquid freshwater reserves on earth.In many areas prone to drought around the world,atmospheric moisture is the most copious water resource.Therefore,the technology of adsorption-based water harvesting from the air is an energy-saving approach that is expected to relieve local water shortages[101].
Kim et al.[102]reported the assembly of a device based on porous Zr-based MOF-801 that can harvest water vapor from the air at ambient conditions and is simply driven by natural sunlight at a flux of less than 1 kW·m-2.This device is able to capture 2.8 L of water per kilogram of MOF each day at relative humidity(RH)levels as low as 20%,and requires only low-grade heat from sunlight with no additional energy input(Figs.5(a)and(b)).
In the following year,Fathieh et al.[103]reported a lab-todesert demonstration in which a prototype device using up to 1.2 kg of MOF-801 adsorbents was tested in the lab,and then tested later in the desert of Arizona,USA.Using only natural cooling and ambient sunlight as an energy source,this device can harvest 100 g of water per kilogram of MOF-801 in each dayand-night cycle.These researchers also reported an aluminum(Al)-based MOF-303,which can deliver more than twice the amount of water.The desert experiment revealed crucial parameters relating to the material,energy,and atmosphere requirements for the efficient capture of moisture from desert air,even at a subzero dew point.These efforts bring water production in desert climates one step closer to practical application(Figs.5(c)and(d)).
Efficient MOF water adsorbents that can be recycled at low temperatures are particularly desired for the development of green adsorption-driven heat pumps and chillers for refrigeration;however,the design of such MOFs presents significant difficulties to date.Wang et al.[104]provided the water sorption profiles of a porous Zr-MOF,MIP-200(MIP stands for the materials of the Institute of Porous Materials from Paris),which is characterized by a high water uptake of 0.39 g·g-1belowP/P0=0.25(whereP0is saturation vapor pressure)on S-shaped sorption isotherms,facile regeneration,and—strikingly—a remarkably high coefficient of performance of 0.78 for refrigeration at a low driving temperature(below 70°C).Given its outstanding water adsorption performance,MIP-200 may be a promising substitute for currently available adsorbents for the purposes of commercial refrigeration.
Seo et al.[105]reported that two hierarchically porous MOFs with good hydrothermal stability—MIL-100 and MIL-101—show high adsorption capacities even at 40°C in combination with fast desorption below 80°C.The enhanced performance of MIL-100 and MIL-101 indicates their feasibility for commercial applications such as freshwater production and energy-efficient desiccant dehumidification.
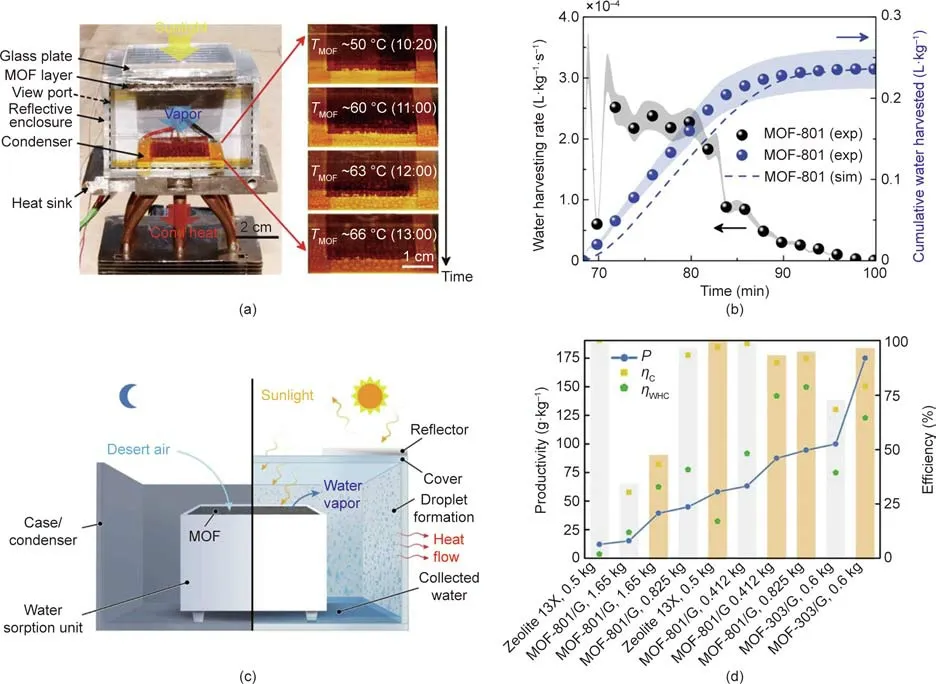
Fig.5.(a)A water-harvesting device based on MOF-801(left);formation and growth of water droplets as a function of temperature and local time(right)(Cond:condensed).(b)Experimentally characterized water-harvesting rate and cumulative water harvested during desorption(exp:experiment;sim:simulation).(c)Schematic of a water harvester prototype for producing water from desert air.(d)Comparison of parameters pertaining to the efficiency and productivity of the water harvester in the desert.Measurements under low and high fluxes are shown as gray and orange bars,respectively(P:productivity;ηC:collecting efficiency;ηWHC:harvesting efficiency of a water harvesting cycle).(a,b)Reproduced from Ref.[102]with permission of American Association for the Advancement of Science,©2017;(c,d)reproduced from Ref.[103]with permission from the authors,©2018.
Indoor RH levels for comfortable and healthy surroundings within residential structures range from 45% to 65%.Towsif Abtab et al.[106]recently reported that a highly stable MOF,Cr-soc-MOF-1(soc=square–octahedral),exhibits an exceptional water vapor uptake of 1.95 g·g-1at 70%RH,and that the adsorbed water can be fully desorbed by means of easy reduction.Cr-soc-MOF-1 showed a record performance in terms of gross and deliverable capacity,reversibility,and recyclability,and thus has great potential for use in indoor moisture control and dehumidification.
The use of MOFs for diversified adsorption-based applications—ranging from fuel storage to pollutant removal and water sorption—has been demonstrated in efforts to address energy and environmental issues.The performances of MOFs surpass those of many conventional materials used for adsorption and require far less energy supply.However,several problems must be resolved before the practical implementation of MOFs as superior materials.Much more intensive research is needed on starting material availability,fabrication and regeneration cost,and framework stability in processing and working,in order to sharpen the competitive edge of MOF materials and make them realistic options to advance green chemical engineering.
6.Separation application
Separation is a critical process in production-and living-related applications including the petrochemical industry,mining,fine chemical engineering,pharmaceuticals,environmental management,and more.A broad range of separation approaches have been established to satisfy widely varying separation requirements.Although much progress has been made,significant challenges still remain in separation science and technology,such as intensive energy consumption,complex equipment,and safety hazards under harsh conditions.Hence,there is a pressing need to develop alternative methods for separation with higher efficiency,fewer adverse impacts,and lower cost.In adsorptive processes,separation relies on differences in the adsorption and desorption behavior of the constituents of a mixture,which are closely connected to their absorbency in terms of adsorptive equilibria and kinetics.Therefore,advancements in high-performing separation materials can facilitate the sustainability of separation processes.The intrinsic properties of MOFs—especially their highly tailorable pore and surface chemistry,which permits high selectivity toward guest species—makes this library of materials particularly appropriate for this challenging task.Research on MOF separations spans a wide range of fields,including gas or vapor separation,liquidphase separation,chiral separation,and MOF membrane-based separation,which are categorized according to the properties of the analytes[107,108].
6.1.Adsorptive gas separation
6.1.1.Air separation
One of the most attractive separation applications of research in porous materials is the efficient separation of light gases(i.e.,H2,N2,CH4,O2,CO,and CO2)to address energy and environmental issues.MOF materials have recently demonstrated great promise in the sequestration of CO2from other gases,among which selective CO2sorption from gas mixtures(especially from CO2/N2and CO2/CH4)has gained the most attention.Several comprehensive reviews have already summarized research advances in this area,including basic concepts,simulative and experimental results,insights into interaction mechanisms,and strategies for target designs[73,109,110].Most of the reported selective adsorption performances can be explained as being due to molecular sieving and/or specific interactions between guest species and the host frameworks.
Although some MOFs have been researched for CO adsorption,most cannot adsorb CO molecules strongly enough to capture trace amounts or to selectively bind CO over various other gases.Other frameworks afford such strong CO binding that the adsorption process becomes irreversible,making regeneration difficult,which hinders their utilization in practical applications.As a result,the design of smart frameworks with appropriate interaction with CO molecules is of great importance in CO separation.Reed et al.[111]demonstrated the selective adsorption of CO in a family of MOFs—Fe2Cl2(bbta)(H2bbta=1H,5H-benzo(1,2-d:4,5-d′)bistriazole)and Fe2Cl2(btdd)(H2btdd=bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo[1,4]dioxin)—through the cooperative binding effect.These compounds present high CO separation capacities with tiny temperature variations and facile regeneration,and thus may enable more efficient extraction of CO from industrial waste feeds.
Due to similarities between the shape of the O2and N2molecules and their size with that of argon(Ar),it is not easy to discover a porous material that is suitable for efficiently separating O2from air.Some MOFs have exhibited selective adsorption of O2over N2and other gases via size exclusion.In contrast,the presence of active OMSs in MOFs always results in a stronger preference for chemisorbing O2relative to N2through charge transfer.Bloch et al.[112]discovered the ability of Fe2(dobdc)to selectively uptake O2over N2via an electron transfer interaction.This O2adsorption is completely reversible,and the capacity is as high as 18.2%in mass fraction at 211 K,which is estimated to be equivalent to one O2molecule being adsorbed per iron(Fe)center.These findings,together with ideal adsorbed solution theory(IAST)calculations,indicate that Fe2(dobdc)is a competent candidate for the separation of O2from air at temperatures much higher than those currently set in industry.
6.1.2.Light hydrocarbons separation
Although adsorptive separation is considered to be a more energy-efficient alternative to traditional separations,its use in light hydrocarbon separation remains challenging due to the similar physicochemical properties of gaseous olefin,paraffin,and their olefin and alkyne counterparts with the same number of carbon atoms.MOFs,which permit precise tuning of composition and structure,have recently been demonstrated to have an exceptional ability to discriminate between the minor variations among these molecules in light hydrocarbon separation.
(1)Separation of gaseous olefins and paraffins.In the preparation of pure C2H4and C3H6for the production of polymers and high-value fine chemicals,the key processing step is the separation of ethylene/ethane(C2H4/C2H6)and propylene/propane(C3H6/C3H8)mixtures.Although various separations with MOFs were reported based on the π-complexation interaction and kinetic separation,these methods have not yet been demonstrated to be fairly effective.
Most MOF adsorbents preferably trap C2H4over C2H6and can thus be used for the removal of C2H6byproducts from C2H4.Bao et al.[113]reported a series of MOFs—namely,the M-gallate series(M=Ni,Mg,Co)—whose aperture sizes(3.47–3.69 Å,1 Å=10-10m)are perfectly adaptable to the molecular sieving of C2H4(3.28 Å×4.18 Å×4.84 Å)and C2H6(3.81 Å×4.08 Å×4.82 Å)via molecular cross-section size variations.In particular,Co-gallate shows a record IAST selectivity of 52 for C2H4adsorption over C2H6,as well as a C2H4uptake of 3.37 mmol·g-1at 298 K and 1 bar.Breakthrough experiments with 50:50(volume ratio)C2H4/C2H6mixtures also evidenced M-gallate’s high selectivity toward ethylene.
In the separation of C2H4/C2H6mixtures,if C2H6is preferentially adsorbed,the desired C2H4product stream can be directly obtained from the adsorption cycle through a simpler process,with higher efficiency,selectivity,and productivity.Compared with C2H4-selective adsorbents,a preference for C2H6would result in a decrease in the energy input of approximately 40% for C2H4/C2H6separation.Li et al.[114]recently reported a microporous MOF—Fe2(O2)(dobdc),which has Fe-peroxo sites—that prefers C2H6over C2H4and thus shows highly selective separation of C2H6/C2H4.With the use of a fixed-bed column packed with this MOF,polymer-grade pure C2H4(99.99%)can be directly recovered from C2H6/C2H4mixtures during the first adsorption cycle.The potential of Fe2(O2)(dobdc)for C2H6/C2H4separation is thus confirmed,with low energy consumption under ambient conditions.
More recently,Qazvini et al.[115]reported that a MOF,Massey University framework(MUF)-15,can sequester C2H6from C2H6/C2H4mixtures.Its productivity in this separation is unprecedented:1 kg of MOF yields 14 L of polymer-grade C2H4gas in a single adsorption step from a 50:50 C2H6/C2H4feedstock.Multicomponent breakthrough curves suggest that this separation performance can be maintained over a wide range of feed compositions and operating pressures.Its attributes of framework robustness,synthesis from inexpensive precursors,constant performance in the presence of acetylene,and easy regeneration provide a MOFbased solution for challenging separations in chemical engineering(Fig.6).
The purity of C3H6mainly relies on the removal of C3H8,similar to the case of C2H4/C2H6.Cadiau et al.[116]used chemically stable fluorinated MOF King Abdullah University of Science and Technology(KAUST)-7 to separate C3H6from C3H8by fine-tuning the aperture size on a 0.2–1 Å scale.The restricted MOF window that was resulted from the incorporation of(NbOF5)2–leads to the selective molecular exclusion of C3H8from C3H6at ambient pressure.
(2)Separation of gaseous olefins and alkynes.The removal of a trace amount of alkyne impurity(i.e.,acetylene and propyne(C3H4))from an olefin stream is essential for the production of polymer-grade olefin feed gas.Adsorptive alkyne-selective capture using MOFs is more energy efficient and environmentally friendly than traditional methods,and the adsorbed alkynes can be further utilized in other applications;thus,this method has enormous appeal for researchers.
Cui et al.[117]developed control over pore chemistry and size in the hexafluorosilicate(SiF62-,SIFSIX)MOFs(SIFSIX-1-Cu,SIFSIX-2-Cu,SIFSIX-2-Cu-i,SIFSIX-3-Cu,SIFSIX-3-Zn,and SIFSIX-3-Ni)for the preferred capture of C2H2molecules over C2H4.Among these materials,SIFSIX-2-Cu-i exhibits a high adsorption capacity toward C2H2(2.1 mmol·g-1at 0.025 bar)and the highest C2H2/C2H4separation selectivity(39.7–44.8)under ambient conditions,as evidenced by the experimental breakthrough curves(0.73 mmol·g-1from 1:99 mixture).The absorbed C2H2molecules are assembled in an orderly manner within the MOF pores,by means of cooperative host–guest and guest–guest interactions.
Compared with C2H2/C2H4separation,C3H4/C3H6separation is notoriously more difficult due to the closer kinetic diameters of C3H4and C3H6.Li et al.[118]carried out a comprehensive screening of a series of MOFs with diverse structures,pore sizes,and functionalities,and identified UTSA-200 as the optimal separating material for the segregation of trace C3H4from C3H4/C3H6mixtures.This material has a record purification capacity for the removal of C3H4impurities from a 1:99(or 0.1:99.9)C3H4/C3H6mixture,and yields 99.9999%pure C3H6with a productivity of 62.0(or 142.8)mmol·g-1.
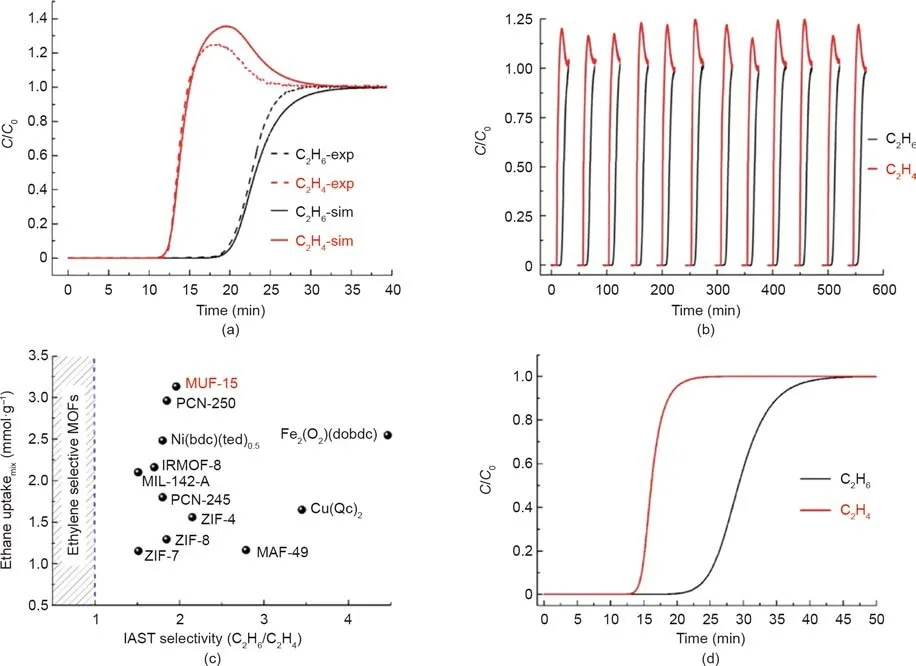
Fig.6.(a)Simulative and experimental breakthrough curves for an equimolar C2H6/C2H4 mixture with MUF-15.(b)Several C2H6/C2H4 separation cycles for a 25:75 C2H6/C2H4 mixture.(c)Ethane uptake in an equimolar C2H6/C2H4 mixture as a function of IAST selectivity among top-performing MOFs(IRMOF:isoreticular metal–organic framework;bdc:benzenedicarboxylate;ted:triethylenediamine;Qc:quinoline-5-carboxylate).(d)Simulative breakthrough curves for a 0.1:99.9 C2H6/C2H4 mixture.C/C0 represents the relative concentration.Reproduced from Ref.[115]with permission of the American Chemical Society,©2019.
A major barrier to the production of high-purity C2H2is the removal of coexisting CO2impurity,as C2H2and CO2are extremely close in size(3.32 Å×3.34 Å×5.70 Å vs 3.18 Å×3.33 Å×5.36 Å),shape,and boiling point(189.3 K vs 194.7 K).The separation of these compounds is indispensable but arduous.To address this issue,Peng et al.[119]employed two ultra-microporous MOFs with high hydrolytic stability,Nankai metal–organic framework(NKMOF)-1-M(M=Cu or Ni),to selectively adsorb acetylene versus several gases at ambient temperature.NKMOF-1-M shows better low-pressure uptake than current physisorbents and provides the highest selectivity reported to date for C2H2/CO2separation.
1,3-Butadiene(C4H6),which is a necessary raw material in rubber manufacturing,is usually the desired component in C4 hydrocarbon mixtures comprising butanes,butylenes,and butadiene from the steam cracker.The separation of C4 hydrocarbons is more challenging than the separation of C2 and C3 hydrocarbon mixtures,due to the coexistence of various structural and cis/trans isomers.Liao et al.[120]showed that a hydrophilic MOF,[Zn2(btm)2](H2btm=bis(5-methyl-1H-1,2,4-triazol-3-yl)methane),weakens 1,3-butadiene adsorption in a C4 hydrocarbon mixture.In a breakthrough measurement under ambient conditions,this adsorbent eluted the target 1,3-butadiene first,followed successively by butane,butene,and isobutene.Through this facile separation,the 1,3-butadiene purity was considerably improved(≥99.5%);meanwhile,side reactions under high temperature such as polymerization were avoided.
6.1.3.Isotopes separation
Although molecular sieving principles are widely applicable,they are not relevant for isotope separation,because isotopic molecules have almost identical adsorption properties.To separate isotopes,a quantum molecular sieving process may suffice,in which heavier isotopes are preferably adsorbed over lighter ones,based on the difference in the quantum energy levels of the atoms or molecules.The activity of several MOFs in H2/D2separation via quantum sieving was recently studied,including ZIF-7,ZIF-8,metal–organic framework Ulm-University(MFU)-4,and MOF-74(M).In this work,Cao et al.[121]selected an ultra-microporous MOF,[Fe(OH)(H2bta)]·H2O(H2bta=bis(tetrazolyl)amine),for experimental investigations of the ultralow-temperature separation of H2/D2through quantum sieving.They achieved a superior separation factor as high as 41.4±0.4 at 20 K.
6.1.4.Noble gases separation
Noble gases,including helium(He),neon(Ne),Ar,krypton(Kr),xenon(Xe),and radon(Rn),have very similar physical properties and occur naturally as mixtures with other gases.Thus,their separation is both important and troublesome.Of particular interest is the enrichment and separation of Xe and Kr from the off-gas stream from nuclear plants.The pore size of the adsorbents is crucial in determining Xe/Kr selectivity.In recent years,researchers from BASF have confirmed that with HKUST-1 as an adsorbent,a simple pressure-swing adsorption process is practicable in the preferable adsorption of Xe from Kr.The calculated adsorption capacity of HKUST-1 for Xe is greater than 60% in mass fraction—nearly twice the amount captured by commercial active carbon[122].
6.2.Liquid mixtures separation
As with gas-phase separations,porous materials have emerged as hopeful next-generation adsorbents for liquid-phase separations.Regrettably,fewer efforts have been devoted to MOFs for liquid-phase separations compared with gas-phase separations thus far.However,an increasing number of scientists have recently focused on the judicious design and selection of functional MOFs to separate target liquid mixtures[123,124].
6.2.1.Separation of linear/branched alkane hydrocarbons(C5–C6–C7)
The petroleum industry highly values the laborious separation of linear,monobranched,and dibranched isomers of alkanes,and particularly dibranched C5–C7 paraffins,which are the main components of gasoline with high research octane number(RON)values.Some MOFs can selectively accommodate linear alkanes while excluding their branched analogues in order to achieve effective separation.
In 2013,Herm et al.[125]published a report on Fe2(BDP)3(BDP2-=1,4-benzenedipyrazolate),a highly stable framework with triangular channels,which showed diverging behaviors toward different hexane isomers based on the degree of branching.A breakthrough experiment confirmed an adsorption selectivity trend ofn-hexane(nC6)>2-methylpentane(2MP)>3-methylpen tane(3MP)>2,3-dimethylbutane(23DMB)≈2,2-dimethylbutane(22DMB).Computational simulations indicate that Fe2(BDP)3can be similarly applied to separate pentane and heptane isomers based on the degree of branching.For pentane isomers,the adsorption strengths of Fe2(BDP)3show a decreasing order:n-pentane(nC5)>2-methylbutane(2MB)>neopentane(neo-P).For heptanes,they show the following order:n-heptane(nC7)>2-methylhexane(2MH)≈3-methylhexane(3MH)>2,2-dimethylpentane(22DMP)≈2,3-dimethylpentane(23DMP).
6.2.2.Separation of cyclic C6 isomers(benzene/cyclohexane)
The major difficulty in C6 hydrocarbon stream separation is segregating Bz from its azeotropic congener cyclohexane(Cy).Enabling the underlying Lewis acid/base interactions between the OMSs of an appropriate MOF and the target Bz molecules is expected to be a facile approach to manipulate the purification of Cy flow.Mukherjee et al.[126]exploited seven isostructural MOFs—M2(dobdc)(M=Ni,Mn,Zn,Mg,Cu,Co,Fe)—with abundant OMSs in structures for Bz adsorption in preference to Cy.IAST calculations and breakthrough simulations indicate that remarkably efficient separations of equimolar Bz/Cy mixtures are feasible over Mn2(dobdc).
Introducing favorable π–π stacking interactions between the πelectron-deficient MOF inner surface and π-electron-sufficient guest species(i.e.,Bz)can also accomplish Bz/Cy separation,as confirmed by Manna et al.[127].Following this concept,they then reported another MOF,diaminotriazine(DAT)-MOF-1,which is capable of sharp separations in a fixed bed,as evidenced by IAST calculations and breakthrough simulations.
6.2.3.Separation of cyclic C8 isomers(styrene/ethyl benzene and xylene isomers)
In the alkylation of a Bz reactor,the styrene(St)product stream usually contains 20%–40%unreacted ethyl benzene(Eb).Bulk production of polystyrene for commercialization demands large amounts of pure St as a primary monomer feedstock.A comparative performance assessment among HKUST-1,MIL-53(Al),and MIL-47(V)adsorbent beds indicated excellent St/Eb selectivity by MIL-47(V).Nevertheless,the more robust sorbent MIL-53(Al)is seemingly more appropriate for application in St/Eb separation on the required industrial scale,because during the exclusion of the undesirable Eb,higher St selectivity for other impurities(mainly toluene)in the crude St stream can also be achieved[128,129].
For the scale-up synthesis of polyethylene terephthalate(PET)in industry,p-xylene(pX),which is the well-known precursor,should have high purity.Nevertheless,discovering ap-selective sorbent is not an easy task,given the negligible differences between all of the critical physical parameters of xylene isomers,particularly from a pore and surface chemistry design standpoint.A series of MOFs have been developed as stationary phases forpX separation.In addition to the high performances provided by rigid MOF sorbents,such as the well-known MIL family,some MOFs with flexible pore windows(e.g.,ZIFs)exhibit a sorptionmediated ‘‘breathing” behavior that achieves the phenomenon of sorption-based separation of xylene isomers.As a significant benchmark,IAST calculations assessed the separation selectivity for breathable DynaMOF-100 and found them to be remarkably higher than those for BaX and metal–azolate framework(MAF)-X8,the best-performingpX-selective sorbents that have been evaluated for xylene isomer separation in the industrially recognized simulated moving-bed process[130].
6.2.4.Biofuel purification
Ethanol is the most common constituent of biofuel,and is also known as the popular concept ‘‘bioethanol.” The production of ethanol through the fermentation of molasses inevitably generates traces of methanol and water.Consequently,bioethanol purification is a current global focus in order to increase the deliverable capacity of this fuel.Zhang et al.[131]performed EtOH and water sorption experiments followed by molecular simulations involving six ZIFs(ZIF-8,ZIF-25,ZIF-71,ZIF-90,ZIF-96,and ZIF-97).Their study identified ZIF-8 as the most interesting candidate for practical use in ulterior biofuel purification.
6.2.5.Oil/water separation
The broad utilization of petroleum and diesel products as a major global energy source carries the perpetual risk of spillages and explosion during storage,transportation,and exploitation.Scavenging such spillages is urgent and costly,since the longer they were present,the more severe the destruction they wreak on aquatic biota would be,and the larger the amount of pollutants they would discharge.In this regard,MOFs have been verified as being suitable to contribute to some advances.
A family of highly hydrophobic MOFs,represented by fluorinated ultrahydrophobic metal–organic framework(UHMOF)-100,have been constructed on demand.Mukherjee et al.[132]further fabricated UHMOF-100 into a recyclable and low-cost membrane form namedUHMOF-100/polydimethylsiloxane(PDMS)/polypropylene(PP).This MOF composite shows high absorption capacity in an oil/water(1:1)mixture separation,which indicates that this composite is a potential solution to marine oil spillage and related environmental pollution.
6.2.6.Isomers separation
Isolating racemic mixtures to obtain enantiopure compounds is of great interest in pharmaceutical and fine chemical production.As racemates have almost identical chemical and physical properties,enantioselective separation is still a difficult task today.Many chiral MOFs have been constructed for enantioseparation,which serve as the chiral stationary phase for high-performance liquid chromatography(HPLC)and gas chromatography(GC),or as sorbents in liquid solutions and solid-phase extractions.Given their advantages of tunable pore chemistry and numerous chiral linkers,the use of homochiral MOFs as the chiral stationary phase could find promising applications in various molecular chiral resolutions and asymmetric catalysis[108].
The separation of cis and trans isomers is another difficult task,and little progress has been made with both conventional porous sorbents and MOFs in this field.MIL-96 was found to be capable of isolatingcis-piperylene andtrans-piperylene in the liquid phase,with an evaluated pore occupation ratio between the trans and cis isomers of 2:0.6.In contrast,Li et al.[107]reported a noticeable adsorption preference of HKUST-1 towardcis-olefins overtransolefins with different chain lengths.
6.3.Membrane separation
Compared with traditional separation methods,membrane separation processes are a very powerful tool for addressing energy and environmental issues,due to their inherent advantages including convenient operation,compact device,high efficiency,low energy consumption and secondary pollution,and excellent reliability.Although membrane-based separations have gradually been adopted in practical industrial production,MOFs hold the potential to tremendously enhance the performance and expand the possible application scope,due to their great structural diversity and their pore and functional tailorability.This section reviews research progress in separations and purifications using MOF-based membranes,with a primary focus on MOF thin films[133–135].
6.3.1.MOF thin films for gas separation
Among the numerous reports on gas separation using MOF thin films,hydrogen separation from other gases is the most common focus.HKUST-1-based,MOF-5-based,and ZIF-series-based thin films prepared via various growth strategies are widely employed.These versatile MOF films present good hydrogen separation abilities from binary mixtures of hydrogen with CO2,N2,and CH4at ambient temperature,as well as fine overall gas permeation performances,indicating their great potential for actual H2separation[133].
Research on CO2separation using MOF-based membranes is relatively limited.For example,Venna and Carreon[136]investigated CO2/CH4separation using ZIF-8 thin films.They inspected several ZIF-8 thin films with different thicknesses,and reported that the films exhibited high CO2permeances and high CO2/CH4separation selectivities ranging ~4 to 7 at 139.5 kPa and 295 K.
Pan and Lai[137]fabricated an intriguing ZIF-8 thin film via secondary growth in an aqueous solution near room temperature for C2/C3 hydrocarbon separation.High selectivities for C2/C3 hydrocarbon separation were obtained(for C2H6/C3H8mixtures,~80,for C2H4/C3H6mixtures,~10,and for C2H4/C3H8mixtures,~167).Moreover,the obtained thin films were much thinner(2.5μm)than those reported in the literature,resulting in permeances four-fold higher.
Aside from the aforementioned investigations,Demessence et al.[138]explored the adsorption and separation of water and organic solvent vapors by nanoZIF-8 thin films with a tunable thickness.The adsorption isotherms over these thin films indicated that only volatile organic molecules such as ethanol and tetrahydrofuran could be adsorbed,whereas water could not.These reusable ZIF-8 films are therefore potentially applicable for the vaporphase separation of organic solvents and water.
6.3.2.MOF thin films for liquid separation
Pervaporation,as a supplement to the filtration method for liquid separations,is commonly viewed as one of the most promising technologies in the bio-refinery,petrochemical,and pharmaceutical industries.The introduction of MOFs into continuous membranes has been proposed in order to enhance pervaporation performance by improving sorption,diffusion,and stability.A number of encouraging results regarding the employment of MOF membranes for pervaporation have been reported,although this investigation is still in its infancy.Recently,a wellintergrown UiO-66 MOFs membrane was applied for organic dehydration using pervaporation,and showed excellent performance[139].
The eruptive development of water-stable MOFs has helped in the evolution of water-treatment-related separation with MOFbased membranes.Recently,Liu et al.[140]prepared continuous UiO-66 polycrystalline membranes(pure-phase thin films)that exhibited an outstanding multivalent ion rejection with moderate permeance(0.14 L·m-2·h-1·bar-1)and good permeability(0.28 L·m-2·h-1·bar-1·μm).Their exceptional separation performance and high stability indicates the great potential of these fabricated UiO-66 thin films for use in practical industrial separations such as gas separation,pervaporation,and water desalination.
Organic solvent nanofiltration(OSN),also known as solvent resistant nanofiltration,is well known for extending membrane applications from aqueous systems(mainly for water/waterrelated treatment)to the filtration and concentration of organic solutions.The organic linkers in MOF structures favor a better affinity toward polymeric matrices,which makes it easier to control MOF-polymer interactions and allows the roughness and mechanical behavior of the thin film to be improved for solvent separation.Moreover,the tunable porosity and pore size of MOFs can be used to create selective cavities and paths to increase the solvent flux and maintain high rejection[135].
Considerable attention is also being paid to chiral resolution as an attractive application of MOF-based membranes in liquid separation.Recently,Kang et al.[141]facilely synthesized a homochiral Ni2(L-asp)2(bipy)(H2asp=aspartic acid;bipy=4,4′-bipyridine)film with chiral channels and fine thermal stability.A diol isomer mixture(2-methyl-2,4-pentane-diol)was employed to check the separation efficiency.For the first time,the researchers observed a higher penetration amount of R diols in combination with the temperature–pressure-relatedmembraneperformanceof homochiral MOF thin films.These parameters are of great significance in the optimization of chiral resolution using MOF-based membranes.
Adsorptive separation plays a key role in a wide range of industrial separation processes due to its energy efficiency,and therefore exerts tremendous influence on current social,economic,and environmental concerns.In comparison with other porous materials in use,MOFs are versatile platforms with excellent tailorability that allow precisely controlled crystal engineering and modification to meet specific separation requirements.During the past decade,research on separation with MOF materials has resulted in tremendous progress.Valuable insight into varying separation mechanisms has also been acquired,providing rewarding guidelines for appropriate material screening.For real applications in industrial implementation,certain important properties other than separation efficiency make a difference,as in MOF-based adsorption applications.Therefore,more factors must be taken into consideration during target material design and evaluation,such as separation kinetics,durability under industrial conditions,regeneration feasibility,expense of scale-up material synthesis and processing,and others.We look forward to employing MOF adsorbents in real-life separation processes after the necessary technical improvements have been made,which will be a great contribution to green chemical engineering.
7.Energy-related application
Global energy demand and consumption have been explosively increasing recently,leading to fuel shortages and environmental pollution.The development of clean,safe,and sustainable energy storage and conversion technologies has become an urgent focus in contemporary green chemical engineering.Among various energy storage and conversion systems,photochemical and electrochemical water splitting and CO2transformation are prime approaches to translate solar and electrical energy into the chemical bonds of simple species such as H2,O2and CH4that are easy to reserve and transport[142,143].Moreover,supercapacitors with high power densities and lithium(Li)-based batteries with high energy densities are promising energy storage devices for electronics,electrical vehicles,and grid electricity[144,145].
Due to their highly porous nanostructures and tunable semiconducting properties,research on MOFs has recently been thriving remarkably in the energy research community.The outstanding gas storage capacity of MOFs at room temperature has accelerated their progress in applications of fuel cells,vehicle gas tanks,and stationary power facilities used as ideal adsorbents[71,75].MOFs with photoactive or electroactive building units(i.e.,organic linkers or metal centers)exhibit potential for use in energy cycles,where they can serve as energy acceptors or catalysts for boosting energy-related chemical reactions[146–148].
7.1.Hydrogen storage
Clean energy storage can be classified into physical sorption,based on weaker physical interactions,and chemical storage in the form of chemical bonds.The former is more prevalent in fuel storage in research and industry,which has been discussed at length in Section 5.1.This section discusses chemical hydrogen storage as a complementary method.
In the past few decades,the chemical storage of hydrogen in liquid and solid phases has been broadly explored.Porous MOFs have the structural potency to confine chemical hydrides in their nanopores and liberate hydrogen under facile conditions with fewer undesired volatile byproducts.Chemical hydrides(e.g.,NaAlH4)can be integrated into MOF cavities via vapor-phase infiltration or through a liquid impregnation approach to generate chemical hydride@MOF composites,in which chemical hydrides are not only accommodated,but also—more importantly—nano-confined into the appropriate spatial pores in MOFs.Then,under suitable conditions such as elevated temperatures or with specific catalysts,hydrogen gas is liberated from liquid-phase chemical hydrides to produce hydrogen with high purity.HKUST-1 and MIL-101 are competent in nano-confining chemical hydrides,and then releasing hydrogen by heating at 70°C or being embedded with platinum(Pt)nanoparticles,respectively[148].
7.2.Solar energy conversi on
Sunlight is abundant and free;hence,solar energy storage and conversion is a brilliant channel for renewable and green energy exploitation.Photodegradation of water pollutants by semiconducting materials has been shown to be an efficient method to degrade wastewater contaminates into biocompatible or lesspoisonous molecules using solar energy.Artificial photosynthesis also represents a sustainable means of satisfying growing energy requirements and thus addressing serious environmental problems.In artificial photosynthesis,water or CO2is reduced to simple chemical fuels(e.g.,H2,CH4,or HCOOH)using harvested solar energy,and water is oxidized to O2.Due to the intrinsic advantages of MOFs,remarkable progress has been made in the design and use of MOFs to catalyze the reactions in photocatalytic systems[142,146–150].This section discusses a wide range of solarenergy-driven applications over photocatalytic MOFs,including water contamination degradation,water splitting,CO2reduction,and organic synthesis.
7.2.1.Photodegradation of water contaminants
The initial photocatalytic application of MOFs involved water contamination treatment using a photocatalytic MOF with a semiconducting property and chemical and optical stability[80,150].Aromatic compounds,which have a long half-life,have good chemical stability and are therefore highly detrimental pollutants.To date,very few pure MOF examples have been confirmed as applicable in the photocatalytic degradation of such stable chemicals.MOF-5(Zn)was employed to decompose phenol under visible light irradiation,and showed a photocatalytic efficiency comparable to that of TiO2[151].Furthermore,MOF-5(Zn)shows chemical selectivity for the photodegradation of 2,6-di-tert-butylphenol over phenol that exceeds the performance of traditional semiconductors[152].Guided by the successful example of MOF-5(Zn),other aromatic compounds,including chloroaromatic,nitroaromatic,and thiophene compounds,have been shown to undergo complete successful photodegradations using photocatalysis-active MOFs[150].
In the photodegradation of organic dyes,such as MB,MO,and Rhodamine B(RhB),the weak organic bonds of dyes are attacked by photo-induced active species,and the dyes then decompose into colorless compounds.Wastewater contamination in actual water environments also includes a great deal of cosmetic,medicinal,agricultural,and industrial inorganic wastes,which require increased efforts toward remediation.Representative chemicals include ethylenediaminetetraacetic acid(EDTA),triethanolamine(TEOA),and methanol.These three compounds are highly active in a range of photocatalytic reduction reactions,and are capable of being electron donors;thus,they could serve as the sacrificial agent in photocatalysis[153].Although plentiful new semiconducting MOFs have been developed,research on photocatalytic pollutant degradation merely focuses on classical MOF types,such as the ZIF,MIL,and UiO families[150].To increase the applications of MOFs in this field,the semiconducting properties and stabilities of new MOF materials should be further optimized and improved.
7.2.2.Photocatalytic water splitting
Through the photo-induced water-splitting process,the goal of light-to-fuel conversion can be achieved with the integration of two half reactions,offering a desirable solution to the contemporary energy issue[142,146–150].One of the half reactions of water splitting is the hydrogen evolution reaction(HER),which is the basis of solar-energy-driven hydrogen production.A photocatalytic system for hydrogen production consists of at least two basic elements:photosensitizers and catalysts.Typical strategies for developing photocatalytic MOF materials for hydrogen production principally center on the integration of photosensitive and catalytically active components into MOFs,with the goal of producing MOF catalysts with excellent stability over long-term operation,high visible-light harvesting efficiency,and desirable catalytic activity for hydrogen generation.
Porphyrins are an eminent class of photosensitizers due to their broad visible-light absorption.The use of two water-stable porphyrin-based metal–organic frameworks(PMOFs)—Al-PMOF and Al/Zn-PMOF—for visible-light-driven hydrogen generation from water was reported in 2012.Al/Zn-PMOF and Al-PMOF were observed to have H2production rates of 100 and 200μmol·g-1·h-1in two different hydrogen generation systems,respectively,both of which showed good reproducibility[154].
The water oxidative reaction—that is,the oxygen evolution reaction(OER)—is the other half reaction of water splitting.Investigations using MOF materials for photocatalytic water oxidation are relatively limited compared with those for photocatalytic hydrogen production,perhaps since most MOF networks have difficulty remaining intact under the rough conditions of water oxidation(e.g.,a strongly acidic electrolyte and strong oxidant).Furthermore,it is always necessary to incorporate other species,such as metal-based complexes,into MOFs,such as UiO-67 or MIL-101(Cr),to endow them with photocatalytic activity toward water oxidation under the necessary conditions[155,156].Nepal and Das[156]utilized MIL-101(Cr)to encage a highly reactive molecular catalyst(MnTD)for water oxidation.This simple yet efficient approach improved the total turnover number(TON)of the water oxidation catalyst(WOC)by more than 20 times at a sustained high initial rate.
7.2.3.Photocatalytic CO2 reduction
The effective capture and catalytic conversion of anthropogenic CO2is a prospective strategy to mitigate excessive CO2emission and global climate change caused by unrestrained fossil fuel burning.Imitating photosynthesis to convert CO2into other useful compounds by means of photocatalysis is a desired route for the production of CO and several organic chemicals(CH4,HCHO,CH3OH,and HCOOH).MOFs have demonstrated important advantages in selective CO2adsorption.When employed as photocatalysts for CO2reduction,MOFs can simultaneously accomplish the pre-concentration and conversion of CO2under visible-light irradiation,demonstrating their exceptional potential for use in solar-induced CO2utilization.Porphyrin moieties were used to prepare MOFs for efficient photocatalytic CO2reduction,and a high performance by stable MOF-525-Co in CO2photoreduction to CO and CH4was reported[157].
Chen et al.[158]successfully constructed a series of Zr-porphyrinic MOFs—ZrPP-n-M(n=1,2;M=H2,Zn,Cu,Fe,Co)—based on unusual phenolic porphyrins,which exhibited exceptional acid and base resistance.Strikingly,the cobalt(Co)-metallated isomorphic variant,ZrPP-1-Co,demonstrated not only high CO2sorption capacity(≈90 cm3·g-1at 1 atm(1 atm=101 325 Pa)and 273 K,which was among the top performances by Zr-MOFs),but also high photocatalytic activity for the reduction of CO2into CO(≈14 mmol·g-1·h-1)and high selectivity over CH4(>96.4%)with no need for a co-catalyst.This contribution offers a novel robust platform for effective CO2-to-CO photoreduction by uniformly isolating active centers in pristine MOFs.
7.2.4.Photocatalytic organic reaction
The inherent heterogeneous catalytic properties and flexible surface functionality of MOFs indicate their potential for rational design for the scaled-up production of certain industrial chemicals.To date,a few photocatalytic organic syntheses have been realized over MOFs,including Bz-to-phenol transformation,the oxygenation of phenol and sulfides,photoreduction,radical polymerization,aza-Henry reactions,and chiral synthesis.
As substitutes for TiO2,titanium(Ti)-oxo clusters and Ti-MOFs have been developed as semiconducting photocatalysts for energy and environmental applications.Keum et al.[159]recently constructed a Ti-carboxylate MOF—namely,Daegu Gyeongbuk Institute of Science and Technology(DGIST)-1—consisting of the Ti-oxo chain and a porphyrin ligand.Without co-catalysts,DGIST-1 presents a selectivity greater than 99.5%in the oxidation of benzyl alcohol to benzaldehyde,as well as a 93% conversion and a TON of 190μmol·mg-1.Its synchronous formation of singlet oxygen and superoxide species,as well as its high efficiency and selectivity toward the formation of benzaldehyde from benzyl alcohol,proves the visible-light-responsive photocatalytic activity of DGIST-1.
7.2.5.Photoelectric conversion
Solar cells,also known as photovoltaic cells,are electrical devices for the direct transformation of solar energy into electricity,and are another interesting application of MOFs.Semiconductive materials are crucial assembly units between the photosensitized anode and the electrolyte in solar cells.A number of studies have been conducted on MOF-based semiconductors,involving both theoretical calculations and experimental demonstrations.A surface-grafted metal–organic framework(SURMOF)thin film of porphyrin Zn-SURMOF2 was processed and assembled into a solar cell with a Pt cathode.When Pd2+ions were incorporated into the porphyrin cores of Zn-SURMOF2,the photophysical property of the modified photovoltaic solar cell was dramatically enhanced.MOFs’poor semiconducting properties have largely constrained their application in solar cells thus far;therefore,designing new MOF materials with regular arrangements of photoactive molecules will further advance their practical application in solar cells[160].
7.3.Electrical energy storage and conversion
7.3.1.Electrocatalysis
Photocatalytic processes are intrinsically dominated by the supply of sunlight.Electrocatalysis is an interesting alternative,as it does not necessarily depend on the constant input of sunlight as an energy source,and as the exact voltage can suffice to guarantee the occurrence of a target reaction[143,146–148,161].
The electrolysis of water splitting—including the HER and the OER—has been extensively explored in recent years,with the aim of providing clean and renewable fuels.Efficient catalysts are necessary to reduce the overpotential of these reactions and to impel them to proceed at the desired high catalytic current densities.The incorporation of HER catalytically active sites into MOFs offers a valid method for developing electrodes with high-performance catalysts,and thus realizing effective hydrogen evolution from electrolytic water splitting.Among various MOF-based catalysts,two-dimensional(2D)MOF films(metal–organic surface(MOS)1 and MOS 2)containing cobalt dithiolene catalytic units were designed for HER catalysis,and were found to exhibit significant HER catalytic activity[162].
With slow kinetics and high-performance catalysts,the OER involves a four-electron process(2H2O →O2+4H++4e–),which is the prerequisite to decrease the operational overpotential.Effective noble-metal oxide catalysts such as RuO2and IrO2usually have a high cost and a scarce reserve,which significantly hinders their widespread use in industry.Lu et al.[163]constructed an alkaline-stable MOF—MAF-X27-OH,which is functionalized with both OMSs and surface hydroxide ligands—using a post-synthetic ion-exchange strategy.This material resulted in drastically increased electrocatalytic activity for the OER(an overpotential of 292 mV at 10.0 mA·cm-2in 1.0 mol·L-1KOH solution),which was comparable to that of efficient inorganic metal and metal oxide/hydroxides in terms of both activity and durability.Such exceptional high performance is due to the distribution of hydroxide ligands on the framework surface,whose effect on accelerating the reaction is impossible for inorganic OER catalysts to imitate,since metal surfaces are generally vulnerable to oxidation under OER conditions.
Manna et al.[164]reported a three-dimensional(3D)MOF—namely,Co-WOC-1—which exhibits outstanding OER electrocatalytic activity in a basic medium.The {Co(H2O)4(DMF)2}2+cations are packed in the pores of the Co-WOC-1 network,and they act as the active sites for the OER in a ‘‘ship-in-a-bottle”-type host–guest system.A catalytic turnover frequency(TOF)of 0.05 s-1at an overpotential of 390 mV(vs normal hydrogen electrode(NHE))in 0.1 mol·L-1KOH was observed in this system,which also showed prolonged framework stability.
Hinogami et al.[165]used MOFs for electrochemical CO2reduction for the first time in 2012.They constructed a copper rubeanate MOF capable of reducing CO2to HCOOH in an electrochemical process,and found that it outperformed the benchmark copper(Cu)metal electrode.Two non-noble-metal-based porphyrinic MOFs—Fe-MOF-525 and Al2(OH)2TCPP-Co(H2TCPP=4,4′,4′′,4′′′-(porphyrin-5,10,15,20-tetrayl)tetrabenzoate)—were then processed into nano-sized thin films for electrocatalytic CO2reduction by Hod et al.[166]and Kornienko et al.[167],respectively.It was confirmed that Fe-MOF-525 can catalytically convert CO2to a mixture of CO and H2with an astonishing 100%total faradaic efficiency and a CO TON of 272(a CO TON of 1520 when trifluoroethanol(TFE)was added).In contrast,thin films of Al2(OH)2TCPP-Co are capable of generating CO selectively from CO2(76% faradaic efficiency and a TON of 1400).These findings verify the route to apply MOF chemistry to the fabrication of efficient electrochemical catalysts with competency for energy-relevant redox reactions.
The oxygen reduction reaction(ORR)typically proceeds through the four-electron transfer pathway in which the complete reduction(4e-+4H+)of O2produces H2O,which is the desired electrocatalytic reaction for energy applications,such as in fuel cells.Pure MOF materials always present poor performance for the ORR due to their inherent insulation and structural instability;therefore,many more efforts to design satisfactory MOF materials for this application are necessary[148].
7.3.2.Fuel cells
Fuel cells are electrochemical devices that transform fuels(e.g.,hydrogen,methanol,and methane)into electricity to power vehicles,portable appliances,and stationary facilities,which are key components of many energy storage and conversion technologies.At present,however,fuel cell development is still a certain distance away from the targets that have been set in terms of capacity,cost,and durability.This prompts researchers to optimize current electrode catalysts and electrolyte membranes further and to persistently improve the ancillary facilities of fuel cells.For fuel cell application,MOFs that exhibit the superior properties of redox,proton conduction,and catalysis hold the potential to become excellent electrolyte materials and electrode catalysts[148].
Designing stable electrolyte materials with high proton conductivity for applications in proton-exchange membrane fuel cells continues to be a challenging task.Some MOFs composed of phosphonate and sulfonate ligands can incorporate guest molecules within their void spaces for proton conduction.Most of these investigated materials have good conductivity at high RH,and dramatically lower conductivity at decreased RH.Yang et al.[168]constructed a new MOF with rich sulfonic acid(–SO3H)sites—namely,BUT-8(Cr)A—which showed good chemical stability and structural flexibility.This MOF not only has a record-breaking high proton conductivity of 1.27×10-1S·cm-1at 100% RH and 80°C,but can also retain moderately high conductivity at a wide range of RH and temperature.
Developing highly effective non-noble-metal catalysts for electrode reactions at a low cost and with an adequate supply is of prime significance for advancing fuel cells.Yang et al.[169]were the first to demonstrate the use of a noble-metal-free MOF catalyst—namely,[(HOC2H4)2dtoaCu](dtoa=dithiooxamide)—for ethanol electro-oxidation reactions.Although the ethanol was just partly oxidized to the acetaldehyde product under the tested conditions,the MOF’s performance in terms of oxidation potential and current density was comparable with that of expensive platinumbased catalysts.
7.3.3.Supercapacitors
It has been claimed in several works that MOFs can be used for supercapacitors applications in an aqueous electrolyte[170].In fact,MOF-derived materials(e.g.,functionalized nickel hydroxides or oxides)are the real active materials on the working electrode,rather than the pristine MOFs.To date,only a few MOFs can retain their integrity in traditional aqueous electrolytes(KOH,H2SO4),since MOF frameworks are always vulnerable in strong acidic and basic conditions.Therefore,organic electrolytes are preferable for pristine MOF-based supercapacitors.
Sheberla et al.[171]paved the way for the inherent conduction of MOFs for supercapacitors with MOF Ni3(HITP)2(HITP=2,3,6,7,10,11-hexaiminotriphenylene),whose bulk electrical conductivity is greater than 5000 S·m-1—much higher than those of activated carbons and graphite(≈1000 S·m-1).The conductive Ni3(HITP)2was then employed as an active material to fabricate a two-electrode symmetric device.The calculated areal capacitance was 18 μF·cm-2,which was superior to any carbonaceous materials aside from graphene.
7.3.4.Rechargeable batteries
MOF materials have been applied in various rechargeable batteries,such as Li-based batteries,Zn–air batteries,and sodiumion batteries(SIBs)[172].Among these emerging electrochemical energy storage devices,Li-based batteries,including Li-ion batteries(LIBs),Li–S batteries,and Li–O2batteries,are superior due to their high energy densities[173].Higher energy density is desired to power future electrical vehicles,so further progress is required in advanced anode,cathode,and electrolyte materials with satisfactory electrochemical performances[146–148].
Pristine MOFs have been developed as alternatives to the commonly exploited graphite anodes,since they provide permanent porosity and a high surface area for Li+storage and transfer during charge and discharge processes.Ogihara et al.[174]used the intercalated metal–organic framework(iMOF)—2,6-Naph(COOLi)2(Naph=naphthalene)—as an electrode material with a desirable operating potential of 0.5–1.0 V.Such an operating potential is inaccessible among other negative electrode materials for highvoltage bipolar LIBs.These results illustrate that the practical application of the iMOF in LIBs is favorable for the design and fabrication of high energy density batteries with intensified security.
Li–S batteries and Li–O2batteries,which possess much higher theoretical energy densities than LIBs,are considered to hold great promise as next-generation electrochemical energy storage devices,although numerous key issues remain to be addressed in the future.
The shortcomings of Li–S batteries include the exploitation of lithium metal as the anode,the generation of soluble polysulfides in the reduction process,and the necessary addition of conductive additives.Zheng et al.[175]reported that a novel Ni-MOF—namely,Ni6(BTB)4(BP)3(BTB=benzene-1,3,5-tribenzoate;BP=4,4′-bipyridyl)—significantly immobilized the formed polysulfides within the cathode material via chemical and physical interactions.Its capacity retention reached 89%after 100 cycles at 0.1 C,revealing the extraordinary cycling performance of the Ni-MOF/S composite.
In order to enhance the performance of Li–O2batteries,it is essential to alleviate the OER and ORR overpotentials and optimize the transfer kinetics of the mass and electrons.Wu et al.[176]showed that a Li–O2battery based on the stable Mn-MOF-74 realized a primary capacity of 9420 mA·h·g-1under 1 atm of O2,which is more than four times higher than that observed in a battery without using a MOF.These results imply that MOFs with OMSs are a feasible choice for cathode materials in Li–O2batteries.Furthermore,the variety of MOF structures and functions give them more advantages in crafting electrode materials(Fig.7).
As discussed above,the utilization of MOFs for a broad scope of energy-based applications provides a pathway for the development of more innovative energy technologies,which is expected to relieve the ever-growing demand for green energy from all sectors of the community.Various MOF materials have demonstrated great potential in photo- and electrochemical energy storage and conversion,in regards to green fuels reservation and generation,exoteric energy harvesting and cycling,reactivity promotion in elusive processes,and so on.Despite many current challenges,we have good reason to believe that the construction of MOFs with new chemistry and versatile functions will result in numerous MOF-based materials for more efficient,durable,economical,and sustainable energy storage and conversion applications.
Fig.7.(a)Schematic illustration of a Li–O2 battery with MOF-Super P composite as the O2 electrode.(b)Comparison of discharge profiles for Li–O2 batteries with MOFSuper P composites or only Super P,under an oxygen atmosphere.Reproduced from Ref.[176]with permission of Wiley-VCH Verlag GmbH &Co.KGaA,©2014.
8.Application in fine chemical catalysis
Increasing catalysis efficiency,reducing cost and waste,and employing eco-friendly reactants are crucial factors in industrial green catalytic chemistry.MOFs are emerging as versatile catalysts that are inherently capable of integrating the advantages of a‘‘homogeneous catalyst” with full usage of active sites and high reactivity,and the qualities of a ‘‘heterogeneous catalyst” with good recyclability.A large number of catalytic reactions over MOF-based materials have been reported[2,43,177,178].In this section,we discuss several classes of important reactions catalyzed by pristine MOFs,whose activities may be derived from metal centers,organic linkers,additional functional groups from postsynthetic modification,or synergistic effects between several of the abovementioned aspects.
8.1.Cycloaddition of CO2 to epoxides
CO2can be used as a starting material for the production of cyclic carbonates,which are precursors in the production of aprotic solvents and polymers,and are used as electrolytes in LIBs.This conversion is realized through the cycloaddition reaction,where CO2is added to an epoxide(mainly St oxide and propylene oxide),with a Lewis acid catalyst and a Lewis base co-catalyst,to form a cyclic carbonate.The OMSs in MOFs serve as available Lewis acid sites to excite the epoxides;meanwhile,the catalytic reactivity can be enhanced by the functional organic ligand with Lewis basic sites or other metal species.Cycloaddition reactions can be conducted in the presence of polar solvents such as acetonitrile and chlorobenzene,as well as in a solvent-free manner using liquid substrates,which is more favorable for the green catalytic process[179,180].
Increasing the OMS density may improve the catalytic reactivity of MOFs toward the cycloaddition of CO2.Target MOF materials with a high specific surface area and a large pore size can improve the accessibility of reactants to the OMSs and facilitate the effective diffusion of substrates and products,thereby contributing to reinforced catalytic performance.Examples of MOFs with catalytically active OMSs in their structure include HKUST-1,M-MOF-74,Fe-MIL-101,MOF-505,Hf-Northwestern University(NU)-1000,and Ni-TCPE(TCPE=1,1,2,2-tetra(4-carboxylphenyl)ethylene)),all of which have shown high efficiency in the cycloaddition of CO2to epoxides[179].
Functionalizing linkers with Lewis basic sites for the development of MOF catalysts may avoid the necessity of a co-catalyst.To achieve this goal,several MOFs with both OMSs and Lewis basic organic linkers were constructed for the CO2cycloaddition reaction.For example,-NH2-functionalized MIL-68(In),UiO-66,and University of Michigan crystalline material(UMCM)-1 demonstrated obvious catalytic efficiency enhancement in the synthesis of carbonates,compared with their pristine MOFs without functionalization.Among these–NH2-functionalized MOFs,UMCM-1-NH2outperformed most of the others due to its unique dual porous structure[181].
Defects in MOFs,which may result from crystal imperfections such as missing metals or linkers,can have an effect on catalyzing the reactions at active sites,as was found for MOF-5,ZIF-8,and ZIF-68[179].
8.2.Oxidation
In typical oxidation reactions,it is important to reduce the stoichiometric oxidants to a catalytic amount due to the environmental toxicity of the resulting waste.Although hydrogen peroxide and molecular oxygen are almost perfect oxidants due to their green nature and high oxygen content,these reagents lack selectivity,which can achieve with the assistance of a smart MOF catalyst.For conciseness,we focus here on the studies in which MOFs were used as ‘‘green” oxidants in catalytic oxidation.
8.2.1.Oxidation of alkanes and olefins
The aerobic oxidation of alkyl aromatics and benzylic compounds is important in the chemical engineering industry,and is typically facilitated by metal carboxylates in strong acid media.Given that most MOFs are porous networks consisting of metal nodes and carboxylate linkers,it is rational to expect good activity for this kind of reaction,even though the strong acidic environment poses a significant limitation on MOF stability.The aerobic oxidation of 1,2,3,4-tetrahydronaphthalene(tetralin)was accomplished with the MOFs MIL-101(Cr),Cu(2-pymo)2(2-pymo=2-hydroxypyrimidinolate),and Co(PhIM)2(PhIM=phenylimidazolate)as heterogeneous catalysts;they showed moderate to high conversion and selectivity[182].
Dhakshinamoorthy et al.[183]demonstrated that the aerobic oxidation of cyclooctane to its resulting ol/one mixture in the liquid phase can be efficiently promoted by MIL-53(Fe)(Fe-BTC;BTC=1,3,5-benzenetricarboxylate).A selectivity over 90% at a 28% conversion was achievable in several cases using this MIL-53(Fe)catalyst.This system was further expanded to other hydrocarbons,such as ethylbenzene and tetralin,and showed high selectivity(>85%).Considering the sustainable oxidant and the facile conditions that are employed in this method,these results reveal a novel catalyst generation for the oxyfunctionalization of hydrocarbon feedstock,and imply a good possibility of industrial application.
The oxidation of C=C bonds is involved in various significant reactions that can create a large collection of corresponding derivatives.Epoxidation is among these reactions.Farha et al.[184]reported an extended family of Zn(porphyrin)-MOFs in which a variety of metalloporphyrins(specifically Zn2+,Pd2+,Al3+,Fe3+,and Mn3+complexes)were incorporated directly.These MOFs featured stable frameworks,large pores,and readily accessible active sites.One Mn-containing MOF was shown to catalyze both the epoxidation of St and the hydroxylation of Cy with high efficiency.
Stubbs et al.[185]developed a MOF-5-based catalyst by the partial substitution of Zn2+with Mn2+.In the presence oft-BuSO2PhIO(PhIO=iodosylbenzene),it produces a high-spin Mn(IV)-oxo species,which catalyzes the oxidation of cyclic alkenes to form epoxides with greater than 99% selectivity.
8.2.2.Oxidation of alcohols
Since the chemical reactivity of aldehyde groups makes aldehydes appropriate starting materials in many synthetic routes,the selective oxidation of primary alcohols to aldehydes is of key importance in organic synthesis.[Pd(2-pymo)2]nhas been synthesized and used for the selective aerobic oxidation of cinnamyl alcohol with a selectivity toward the desired cinnamaldehyde product as high as 74%.This report demonstrated that the Pd(2-pymo)2catalyst can also work on Suzuki cross-coupling,as well as on the shape-selective hydrogenation of olefins[186].
Guo et al.[187]recently reported on the construction of a series of mixed-metal MOFs—namely,the CuPd-HKUST-1 family.With the doped Pd2+-OMS species as scattered single-active catalysts,these MOFs present strong catalytic activity and selectivity toward the aerobic oxidation of benzyl alcohol to benzaldehyde(up to 93%conversion and 89% selectivity after 22 h).
8.2.3.Oxidation of sulfides
Sulfides are valuable intermediates of various natural products,and their partial oxidation to sulfoxides is a crucial conversion in medicine and industrial processes.Perles et al.[188]synthesized threerare-earthmetal-basedMOFs—[Sc2(C4H4O4)2.5(OH)],[Y2(C4H4O4)3(H2O)2]·H2O,and[La2(C4H4O4)3(H2O)2]·H2O—which were inspected as redox catalysts in the oxidation of two sulfides:methylphenylsulfide and(2-ethylbutyl)phenyl sulfide.The results indicate that these catalysts have extremely high efficiency and high chemo-selectivity in the selective oxidation of thioether to sulfoxide(>70% and >90% conversion,respectively)under mild conditions via rational condition control.
8.3.Hydrogenation
8.3.1.Reduction of nitroaromatics
The heterogeneously catalyzed hydrogenation of aromatic nitro compounds is always the preferred method for the production of corresponding anilines.Noble metals supported on active carbon are traditionally chosen as catalysts for this reaction,although they carry obvious shortcomings such as functional group incompatibility in substrates.Gomez-Lor et al.[189]employed the MOF In2(OH)3[O4C8H4]1.5as a catalyst in the reduction of nitroaromatics(nitrobenzene and 2-methyl-1-nitronaphthalene),and found that it demonstrated complete conversion under mild conditions(with a TOF of 489 and 385 min-1,respectively).It is notable that this In3+-based network shows high efficiency at a metal/substrate ratio as low as 0.1%,which indicates that this MOF is a good candidate for a non-noble-metal catalyst in this reaction.
8.3.2.Hydrogenation of olefins
Although many industrial hydrogenation processes are still dependent on expensive and poisonous noble-metal catalysts,considerable attempts have been made to advance porous-materialssupported Fe- and Co-based heterogeneous hydrogenation catalysts,which offer better performance at a lower price.In particular,MOFs provide a versatile platform for engineering single-site solid catalysts for these hydrogenations.Manna et al.[190]reported a stable Zr-MOF—namely,sal-MOF—with the UiO topology.Fe- and Co-functionalized MOFs(sal-M-MOF,M=Fe,Co)were obtained via post-synthetic treatment,and were shown to be competent highly active heterogeneous catalysts in catalyzing alkene hydrogenation and to display excellent catalytic performance(with a very high TON of up to 145 000)and good reproducibility(>15 cycles).
8.4.Asymmetric organic catalysis
The significance of chirality in bioprocesses and pharmaceuticals has stimulated wide-scale explorations on the promotion of chiral materials for applications in enantioselective processes.Sensible selection of building units can produce chiral or achiral MOFs with active OMSs,which exhibit great potential for heterogeneous asymmetric catalysis via size/shape selection,such as addition to carbonyls.
Horike et al.[191]constructed a new MOF—Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2(H3BTT=1,3,5-benzenetristetrazol-5-yl)—that features a high density of Lewis acidic Mn2+sites on the internal pore surfaces.This microporous compound can catalyze both the cyanosilylation of aromatic aldehydes and the Mukaiyama aldol reaction in a size-sieving pattern to yield homochiral products.
More recently,Tan et al.[192]employed a strategy of postsynthetic exchange to obtain a series of single-M(salen)and mixed-M(salen)crystals adopting the UiO-68 type structure.These compounds displayed high catalytic activity for various asymmetric reactions,including the oxidative kinetic resolution of secondary alcohols,cyanosilylation of aldehydes,aminolysis of stilbene oxide,and ring-opening of epoxides,as well as sequential asymmetric alkene epoxidation/epoxide ring-opening reactions.These MOFs are effective chiral catalysts with good stereoselectivity and easy recyclability,and are therefore appealing in the environmentally friendly synthesis of fine chemicals.
8.5.Polymerization
Polyethylene has entered daily life as one of the most common plastics since its unexpected discovery in 1933.Linear low-density polyethylene(LLDPE),a member of the polyethylene family,is produced from the key co-monomer 1-butene,which can be obtained by cracking higher alkanes.In contrast,ethylene dimerization(ED)yields 1-butene in higher purity,as required for LLDPE production.Therefore,the ED reaction accounts for half of the 1-butene demand for LLDPE production in industry—a staggering annual amount of 7×105t[193].
Metzger et al.[194]designed a MOF catalyst—namely,Ni-MFU-4l—via post-synthetic metal exchange in the known MFU-4l.A new set of heterogeneous catalysts was thus developed for producing 1-butene from ethylene with a TOF of 4.15×104h-1and a selectivity of 96.2%.These catalysts have been shown to outperform the homogeneous species(TpMes)NiCl(TpMes=HB(3-mesitylpyrazolyl)3)in almost all aspects,and present outstanding regenerability;therefore,they may be a valid solution for the industrial implementation of ED(Fig.8).
Fig.8.(a)Mechanism,(b)activity,and(c)product distribution of ethylene dimerization with Ni-MFU-4l.Reproduced from Ref.[194]with permission of the American Chemical Society,©2016.
Aside from the important and prevalent reactions promoted by MOF-based catalysts mentioned above,MOFs can participate in an additional range of reactions,including C–C coupling,Knoevenagel condensation,the methanolysis of epoxides,transesterification,acetalization,and even tandem reactions or multicomponent coupling reactions[43,195,196].Although amazing processes have been demonstrated,pristine MOF-based catalysts are still in their infancy for practical implementation.Inherent issues still remain to be solved,such as the trade-off between catalytic activity and substrate selectivity.Meanwhile,more attention should be paid to green reactions associated with high value-added products for long-term economic and environmental benefits.Given the preeminent advantages of the adjustable MOF structure,we believe that pristine MOF-based materials have a bright future in industrial catalysis.
9.Commercialization
MOF chemistry has developed to the point where MOFs are now promising candidates for implementation in real systems and processes.In the academic community,MOFs are generally synthesized on milligram scales under relatively complex reaction conditions.However,the feasibility of fabricating MOFs on a desirable scale is central to the evolution of these emerging materials into earthshaking technologies,especially in terms of expenditure for implementation.BASF was the first company to show an interest in the large-scale production of MOFs.BASF researchers have optimized the solvothermal synthesis of AlFu into a hydrothermal preparation for industrial implementation in tonnes,which has been commercialized by BASF as Basolite A520.As of 2019,several classical MOFs are commercially available online in relatively small amounts(from 1 to 500 g),at a quoted price of about 129 CNY·g-1or more(Table 1).This situation is unfavorable for most industrial applications,indicating that researchers have a long way to go in their pursuit to defeat industrial zeolites and activated carbon materials with MOFs in terms of cost,despite the functional superiority of MOFs.
Table 1 Examples of commercially available MOFs in 2019.
A strong imperative has arisen to develop effective and universal methods to produce MOFs on a large scale in order to bridge the huge gap between academic research and commercial application.The cost of MOF production could be largely curtailed by optimizing the synthetic approaches,including rationally screening the starting materials and varying the synthesis conditions,with the well-defined hierarchy of theoretical knowledge as a guideline.Therefore,it is reasonable to believe that MOF commercialization will be realized in the near future and will benefit humanity in diverse aspects of both living and production.
The pioneer company BASF has initiated a MOF-based natural gas storage system,which has been tested since 2013 in a group of demonstration vehicles.Following BASF’s interest in MOFs,Decco registered a MOF-based post-harvest freshness management tool(TruPick)for fruit and vegetables,and NuMat developed a MOF-based system for the storage of hazardous gases,which is typically employed in the electronics industry.These groundbreaking projects have inspired researchers to focus on MOF-enabled technologies and devote increasing effort toward their rapid industrialization[200].
10.Outlook
By rationally selecting inorganic and organic components,the judicious construction of MOFs provides a wide synthetic scope and permits the fine-tuning of the textural properties of these crystalline frameworks.The advantages of MOFs over conventional materials strongly rely on their well-defined and highly porous structures assembled by linking units,which are amenable to precise chemical modification by elegant design.During the first two decades of intense research on MOFs,a great wealth of academic knowledge was gathered,and proficiency was acquired in both computational and experimental tools among investigators worldwide.
Fortunately,as clearly shown in this review,research on these exquisite products has gradually evolved from methodological studies confined to the laboratory scale to material,energy,and environmental research.As part of this shift,interested pioneers have already begun producing commercial products for real use.Stability and price-performance ratio are two main parameters for the application of MOFs in the green chemical engineering community.Future trends will involve the preparation of robust MOFs and MOF-based devices using a facile,green,and economical approach and in large amounts capable of meeting industrial requirements.As many novel functional MOFs have been advancing in recent years and will soon be ready for practical implementation,we are optimistic that MOFs will be able to compete in the near future with today’s well-known industrial materials,thus mitigating the global pressure on energy and the environment and establishing their indispensability to the modern world.Diverse applications,covering almost every aspect of production and daily life,illustrate the ascending interest in and grand opportunities for MOF development[46].
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China(51621003,21771012,and 22038001)and the Science&Technology Project of Beijing Municipal Education Committee(KZ201810005004).
Compliance with ethics guidelines
Xiang-Jing Kong and Jian-Rong Li declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Selective Laser Melting under Variable Ambient Pressure:A Mesoscopic Model and Transport Phenomena
- Programmable Adaptive Security Scanning for Networked Microgrids
- Flexibility Prediction of Aggregated Electric Vehicles and Domestic Hot Water Systems in Smart Grids
- Atomic Force Microscopy Measurement in the Lignosulfonate/Inorganic Silica System:From Dispersion Mechanism Study to Product Design
- Engineered Biomimetic Platelet Membrane-Coated Nanoparticles Block Staphylococcus aureus Cytotoxicity and Protect Against Lethal Systemic Infection
- Advances and Strategies for Controlling the Quality and Safety of Postharvest Fruit

