污泥超高温好氧发酵去除氟喹诺酮类抗生素及其降解产物
宋相通,杨明超,张 俊,郭亚丽,董 滨,*
污泥超高温好氧发酵去除氟喹诺酮类抗生素及其降解产物
宋相通1,杨明超1,张 俊2.3,郭亚丽2,董 滨1,2*
(1.同济大学环境科学与工程学院,上海 200092;2.中国长江三峡集团有限公司长江生态环境工程研究中心,北京 100038;3.长江生态环保集团有限公司,湖北 武汉 430070)
对比分析了污泥传统高温好氧发酵(TC)和超高温好氧发酵(HTC)对诺氟沙星(NOR)、氧氟沙星(OFL)及其降解产物的去除性能.结果表明,超高温好氧与高温好氧发酵25d时NOR去除率分别为91.8%,92.1%,产物诺氟沙星脱乙基(NORP)残留含量分别为628,668μg/kg;OFL去除率分别为92.1%、88.1%,产物氧氟沙星脱乙基(OFLP)残留含量分别为191,675μg/kg,相较于传统高温好氧发酵,超高温好氧发酵使得NOR、OFL的生态风险分别降低3.1%、30.5%,这表明超高温好氧发酵可以更有效地去除氧氟沙星及其降解产物,降低发酵产物中OFL的环境暴露风险.同时,超高温好氧发酵产物DOC/DON更低,种子发芽指数高,发酵产物植物毒性小,有利于污泥的安全土地利用.
污泥;超高温;好氧发酵;氟喹诺酮;抗生素;降解产物
氟喹诺酮类(FQs)是最常用的抗生素之一,目前普遍使用的是第三代药物,包括诺氟沙星(NOR)、环丙沙星(CIP)、氧氟沙星(OFL)等.研究表明长江中下游的65个湖泊的水和沉积物中检测到5种氟喹诺酮类抗生素,对湖泊中的藻类、细菌构成中等风险[1].Wang等[2]研究表明,吸附是活性污泥污水处理过程中FQs的主要去除途径,占不同FQs总去除量的50%~91%.大量抗生素的聚集与残留使得污泥已成为环境中抗生素最重要的归宿之一[3],随着污泥土地利用过程,对土壤生态造成威胁.
好氧发酵是一个传统且经济的技术,将污泥等有机固废转化为土壤改良剂.前期研究表明好氧发酵能够去除基质中FQs,去除效果与发酵堆体温度密切相关[4].Yang等[5]研究指出,鸡粪好氧发酵过程中OFL等5种FQs去除率可达45.3%~75.4%, Jałowiecki等[6]研究表明,NOR与OFL在好氧发酵过程中去除率分别达到80%、60%,Selvam等[7]研究猪粪好氧发酵过程中CIP去除率最高达到82.9%, Zhang等[8]研究城市污水污泥好氧发酵过程中NOR与OFL去除率均达85%以上.近年来研究也发现,多地牛、猪粪便的发酵产物残留高浓度FQs[9],这表明传统高温好氧发酵无法充分去除氟喹诺酮类抗生素.
超高温好氧发酵(HTC)是利用极端嗜热菌群使发酵过程中最高温度达到80℃以上,并维持3d以上的新型发酵工艺[10].与传统高温好氧发酵(TC)相比,最高温度提高20℃以上,Liao等[11]研究表明,HTC过程中抗生素耐药基因的去除率显著提高.前期研究中,Paul等[12]观察到CIP中哌嗪环转化使得抗菌活性降低, Zhu等[13]认为FQs降解产物对DNA拓扑异构酶的结合亲和力相比母体较低,因此抗菌活性降低.但是目前关于FQs好氧发酵过程中降解产物的定量研究较少.参考Prieto等[14]提出的FQs生物降解路径,本研究选取NOR、OFL及其哌嗪环脱乙基产物,使用LC/MS-MS定量检测NOR、OFL在HTC、TC两种发酵过程中的残留含量及其哌嗪环脱乙基产物含量,以期从降解产物层面解释FQs在不同好氧发酵过程中的降解行为,为污泥的安全土地利用提供理论参考.
1 材料与方法
1.1 实验材料
生污泥取自上海某养殖业污水处理厂,使用前生污泥储存在4℃冰箱中.稻谷壳取自某农产品加工厂,平均直径约0.9mm,作为发酵辅料以调节含水率.超高温好氧发酵腐熟料来自实验室运行的超高温发酵堆体,原材料的理化性质如表1所示.

表1 生污泥、腐熟料、稻谷壳的理化性质(%)
注:*以干基重量计,数据以平均值±3个重复的标准差表示.
1.2 实验方法
发酵反应器如图1所示,容积为15L(高31cm,直径25cm),外层为厚2cm的恒温水浴层,在距反应器底部7cm处设置气流均布板,发酵堆体由安装在距反应器底部12cm的不锈钢格栅支撑.采样时在分别距反应器顶部10,20和30cm的堆体中心取样.
本研究预实验中确定了各发酵原料的最佳配比,本研究中HTC组添加生污泥、稻谷壳与超高温好氧发酵腐熟料干重比为2.0:1.0:0.8,TC组添加生污泥与稻谷壳干重比为2.0:1.0,2组均添加10mg/kg的NOR与OFL.HTC堆体总质量湿重10.4kg,干重5.7kg,TC堆体总质量湿重8.2kg、干重4.5kg,在启动发酵之前确保堆体的均质性.
2组发酵堆体初始水分含量调节至55%左右,发酵过程持续40d,空气泵持续运行,通风量为0.50L/(min·kg).每天10:00记录3个采样位置的温度,并从3处采样位置各取10g子样本并混匀,收集重量为30g的代表性样本.样品分为3部分,其中一部分立即分析,一部分经风干研磨,过50目筛后保存,另一部分储存在-80℃冰箱中,冷冻干燥后用于FQs及其产物分析.

图1 发酵反应器示意
(1)反应器;(2)气体出口;(3)不锈钢格栅;(4)气流均布板;(5)保温层;(6)恒温水循环泵;(7)气体流量计;(8)曝气风机
1.3 检测分析方法
取样前在分别距反应器顶部10,20和30cm的堆体中心取样位置,采用Pt100型电子温度计监测堆体温度.预先在105℃下干燥至恒重的样品在马弗炉中600℃燃烧4h,确定挥发分含量(VS/TS).准备新鲜样品的水提取液(1:10/样品:水),使用TOC分析仪(日本岛津SSM-5000A)测定溶解性有机碳(DOC)与溶解性有机氮(DON),种子发芽指数(GI)试验用于评估发酵产物的植物毒性,参考Gao等[15]提出的方法.
根据Yuan等[16]的方法,分析了发酵产物中NOR、OFL残留含量及其降解产物含量:称取均质试样1.0g,置于50mL聚丙烯离心管中,分别用20, 20,10mL,0.1mol/L EDTA-Mcllvaine缓冲液冰水浴超声提取3次,每次涡旋混合10min,冰水浴超声10min,10000r/min离心5min,合并上清液.取5mL上清液以2.5mL/min的速度过预先用6mL甲醇,6mL水活化的SAX-HLB串联固相萃取柱(200mg,6mL),将小柱抽干,用6mL甲醇+乙酸乙酯洗脱,收集洗脱液用氮气吹干,用2mL初始流动相溶解,过0.45μm尼龙滤膜,转移至2mL琥珀色玻璃小瓶中,待液相色谱/串联质谱(LC/MS-MS, ThermoFisher)测定.色谱柱:Eclipse Plus C18柱(4.6mm×150mm,3.5μm),流动相为体积比70:30的质量分数为0.2%甲酸和乙腈,流速为0.5mL/min,柱温40℃,进样体积20μL.质谱条件为多反应监测模式(MRM),正离子模式,其余质谱参数采用仪器微量进样优化结果,各化合物质谱参数如表2所示.

表2 诺氟沙星、氧氟沙星及其降解产物测定质谱参数
1.4 诺氟沙星与氧氟沙星生态风险评估
本研究采用风险熵权法(RQ)评估发酵产物中氟喹诺酮类抗生素的生态风险.RQ值通常表示为特定污染物的测量浓度(MEC),与预测无影响浓度(PNEC)之比,具体计算公式为:


式中:PNECSoil为氟喹诺酮类抗生素土壤预测无影响浓度,μg/kg;PNECWater为氟喹诺酮类抗生素水预测无影响浓度,μg/L;d为土壤-水分配系数;EC50为半最大效应浓度,mg/L.EC50为急性毒性时, AF取1000;EC50为慢性毒性时,AF取100.NOR及OFL相关参数如表3所示.

表3 NOR与OFL生态风险评估参数
2 结果与讨论
2.1 温度变化曲线
温度是好氧发酵过程中重要参数,与微生物活动密切相关,不仅影响微生物的代谢速率与群落结构,而且影响发酵产物的理化特性[19].根据温度变化,本研究将发酵过程分为4个阶段(图2):升温阶段0~4d;超高温/高温阶段5~10d;降温阶段11~23d;腐熟阶段24~40d. 1d时开启50℃水浴保温,11d时堆体温度出现明显下降趋势,将水浴保温下降至35℃, 24d时堆体温度开始接近水浴温度,认为堆体进入腐熟阶段,进一步将水浴保温下调至30℃,40d时,HTC与TC组堆体温度降低至水浴温度,发酵结束.HTC发酵开始时有机物快速分解,温度迅速升高,在4d达到80℃,在6d达到最高温度83.3℃, 80℃以上维持了6d,17d时温度快速下降,进行翻堆维持发酵过程.TC组6d达到最高温度60.3℃,在22d时翻堆维持发酵过程.
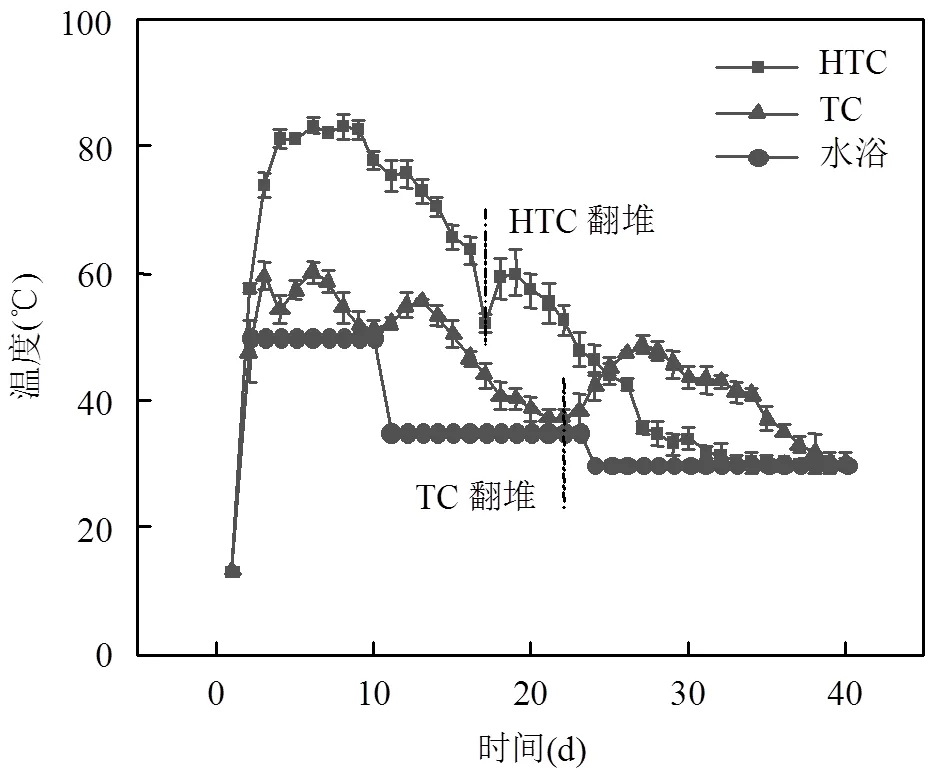
图2 HTC和TC过程温度变化曲线
2.2 VS总量与VS/TS变化曲线

图3 HTC和TC过程VS总量与VS/TS变化曲线
有机质含量是评估好氧发酵腐熟度的重要参数通常用挥发分含量(VS/TS)来表示.有机质含量通常在发酵末期达到相对稳定的状态,表示好氧发酵进入腐熟阶段[20],发酵堆体中有机质总量采用VS总量表示,VS总量=堆体总质量´(1-含水率)´(VS/TS).
如图3所示, HTC与TC过程有机质含量均呈下降趋势, HTC在超高温阶段有机质的分解速率高于降温和腐熟阶段,而TC过程中各阶段有机质分解速率差异较小.HTC和TC发酵过程中VS/TS分别降低了24.9%和20.3%,考虑取样对堆体总质量的影响后,发酵完成时HTC与TC堆体干重分别为3.5kg与3.1kg,有机质总量分别降低了53.8%与44.6%.值得注意的是HTC过程中, 24d时VS/TS值已趋于稳定,而TC组此时VS/TS值仍略有下降,表明HTC过程堆体达到稳定状态较快,发酵周期更短.
2.3 DOC、DON及DOC/DON变化曲线
溶解性有机碳是有机固废中活性最强的组分之一,研究指出DOC可作为反映好氧发酵稳定性的一个重要参数[21-22].如图4所示,HTC过程中DOC呈持续下降趋势,尤其是前11天DOC快速下降,TC组有机物分解速率低于HTC组,HTC组发酵过程中,第24天DOC趋于稳定,与此同时,TC过程DOC仍有小幅降低,表明HTC过程堆体较快趋于稳定,发酵周期更短.

图4 HTC和TC过程DOC与DON变化曲线
溶解性有机氮主要由低分子量的化合物,如氨基酸、氨基糖、尿素和嘌呤,以及高分子量的化合物,如蛋白质、叶绿素、DNA 和多酚等组成[23].2种发酵过程初期,堆体中DON含量均呈上升趋势.这表明溶解性有机物快速分解,使得堆体的干基质量下降,同时堆体中有机氮水解活性高,使得发酵初期DON含量增加.之后DON含量逐渐下降,一方面DON易于被微生物同化利用,另一方面,硝化和反硝化等过程使得DON含量下降[21].值得注意的是,由于HTC过程启动时添加超高温好氧发酵腐熟料,相当于进行微生物接种,而TC过程未接种,微生物活性较低,DON利用速率小于有机氮水解速率,使得TC过程前期DON含量上升.
DOC和DON容易被微生物利用,与常规C/N相比,DOC/DON更能充分说明发酵产物的稳定性.如图5所示,HTC与TC过程中DOC/DON均呈现出逐渐下降趋势,Said-Pullicino等[21]研究指出,当DOC/DON比值约为5~6时,发酵达到了腐熟稳定.发酵完成时,HTC组与TC组均达到腐熟稳定的标准,不过HTC组发酵周期显著短于TC组.

图5 HTC和TC过程DOC/DON变化曲线
2.4 发芽指数变化曲线

图6 HTC和TC过程GI变化曲线
通常,未完全腐熟的发酵产物会产生有植物毒性的物质抑制植物生长,此外有研究指出,抗生素的存在会显著抑制植物根系生长[24-25].GI不仅是一个腐熟化程度指标,也是一个评估发酵产物植物毒性变化的指标,具体计算公式为:

式中对照组为新鲜样品水提取液,空白组为无菌水.2种发酵过程初期,GI值均低于20%(图6),表明生污泥具有较强的植物毒性,HTC组与TC组稳定产物GI分别为136.7%和119.5%,发酵过程不仅消除了植物毒性,而且对植物根系生长有促进作用.值得注意的是,HTC过程中在16d已经满足GI不低于80%的阈值范围,而TC过程28d时GI才超过80%.这个事实符合2.2与2.3节中HTC发酵周期较短的结论.
2.5 NOR与OFL残留含量变化与生态风险评估

如图7a所示,HTC与TC过程中NOR与OFL含量均显著下降.HTC与TC组初始NOR含量分别为9210,9750μg/kg,25d时NOR残留含量为752, 775μg/kg,去除率分别达到91.8%,92.1%,两者去除率无显著差别.如图7b所示,HTC与TC组初始OFL含量分别为8181,7815μg/kg,25d时OFL残留含量为645,929μg/kg,去除率分别达到92.1%、88.1%, HTC过程对OFL去除率更高.有研究表明是因为高温破坏了OFL分子结构,或是高温促进了OFL的生物降解活性[26].TC过程中,OFL比NOR更加难以去除,这与Jałowiecki等[6]研究结果相符.本研究生态风险评估中MEC值采用25d时NOR与OFL残留含量,PNECSoil采用表3中数据,如图8所示,HTC相较于TC,NOR生态风险下降3.1%,OFL生态风险下降30.5%.将含有FQs的发酵产物施用进土壤需格外关注,因为FQs可能被蔬菜吸收和积累[27],对人类健康构成潜在的风险,且FQs残留可促使污染的土壤中FQs耐药基因的产生.这项研究中的风险评估是根据细菌的毒性数据进行的,未考虑FQs降解产物的毒性作用,风险水平可能被高估或低估.

图8 发酵产物中NOR与OFL风险熵值
2.6 诺氟沙星与氧氟沙星降解产物含量变化
研究指出在FQs氧化过程中形成的各种氧化中间体与产物,极有可能保留其母体化合物的生态毒性,甚至发展出新的可遗传毒性[28].Zhu等[13]研究表明,与FQs母体化合物相比,氧化后的FQs对绿藻表现出相同甚至更高的毒性.因此,好氧发酵过程应该尽可能降低FQs降解产物的含量.如图9a所示,HTC与TC过程中由于前期NOR快速分解,NORP产生积累,导致含量上升,NORP含量最高点分别出现在4d与5d,最终浓度分别为628,668μg/kg.如图9b所示,由于HTC与TC组在OFL去除速率方面的差异, HTC过程OFLP含量最高点在4d,最终含量191μg/ kg,TC过程OFLP含量最高点出现在10d,最终含量675μg/kg.这表明HTC能有效降低污泥中氧氟沙星脱乙基降解产物的含量,相比较TC而言,HTC稳定产物的土地利用更安全.
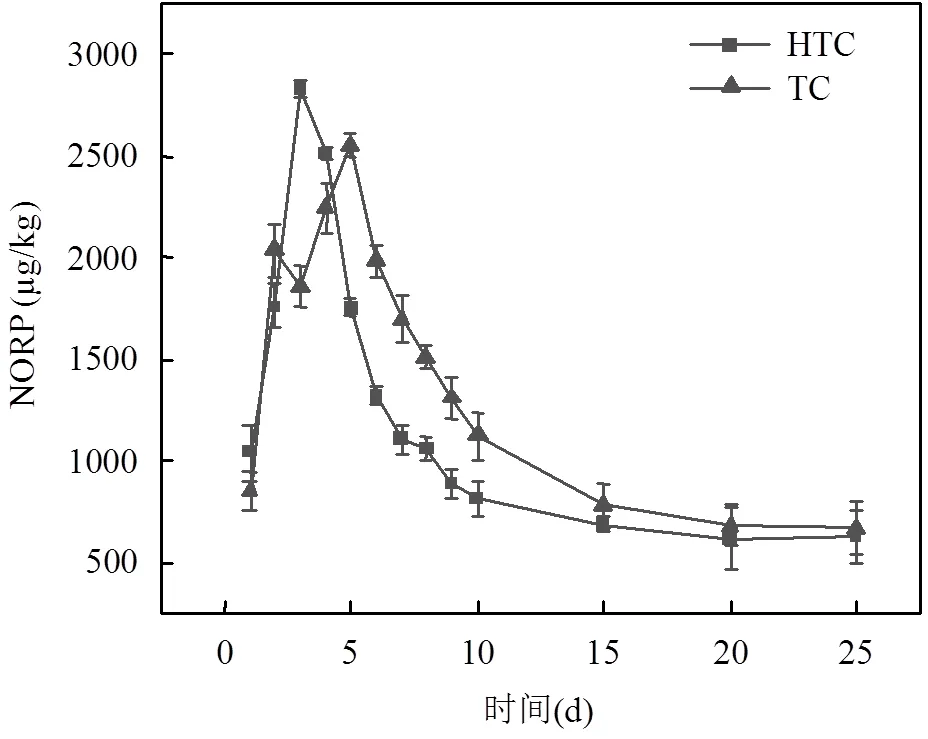
3 结论
3.1 超高温好氧与高温好氧发酵25d时NOR去除率分别为91.8%,92.1%,降解产物NORP残留含量分别为628,668μg/kg;OFL去除率分别为92.1%、88.1%,降解产物OFLP残留含量分别为191,675μg/ kg.超高温好氧发酵可以显著提高OFL的去除率,对中间降解产物OFLP的降解效果显著优于传统高温好氧发酵.
3.2 相较于传统高温发酵,超高温好氧发酵稳定产物中NOR、OFL的生态风险分别降低3.1%、30.5%,同时DOC/DON更低、腐熟度更高、种子发芽指数较高,有利于安全土地利用.
[1] Zhou L J, Li J, Zhang Y D, et al. Trends in the occurrence and risk assessment of antibiotics in shallow lakes in the lower-middle reaches of the Yangtze River basin, China [J]. Ecotoxicology and Environmental Safety, 2019,183:109511.
[2] Wang L, Qiang Z M, Ben W. et al. An insight into the removal of fluoroquinolones in activated sludge process: Sorption and biodegradation characteristics [J]. Journal of Environmental Sciences, 2017,56(6):263-271.
[3] Oberoi A S, Jia Y, Zhang H, et al. Insights into fate and removal of antibiotics in engineered biological treatment systems: A critical review [J]. Environmental Science and Technology, 2019,53(13):7234-7264.
[4] 姚全威,张 军,严沁颖,等.中温期和高温期污泥堆肥物料中典型氟喹诺酮类抗生素去除的影响因素[J]. 环境工程, 2020,38(9):200-207.
Yao Q W, Zhang J, Yan Q Y, et al. Main factors on dissipation of typical fluoroquinolones in sewage sludge compost during mesophilic and thermophilic phases [J]. Environmental Engineering, 2020,38(9):200-207.
[5] Yang B, Meng L, Xue N. Removal of five fluoroquinolone antibiotics during broiler manure composting [J]. Environmental Technology, 2017,39(3):1-9.
[6] Jalowiecki L, Plaza G, Ejhed H, et al. Aerobic biodegradation of norfloxacin and ofloxacin by a microbial consortium [J]. Archives of Environmental Protection, 2019,45(4):40-47.
[7] Selvam A, Zhao Z, Wong J. Composting of swine manure spiked with sulfadiazine, chlortetracycline and ciprofloxacin [J]. Bioresource Technology, 2012,126:412-417.
[8] Zhang J, Bao Y, Jiang Y, et al. Removal and dissipation pathway of typical fluoroquinolones in sewage sludge during aerobic composting [J]. Waste Management, 2019,95:450-457.
[9] Xie W Y, Yang X P, Li Q, et al. Changes in antibiotic concentrations and antibiotic resistome during commercial composting of animal manures [J]. Environmental Pollution, 2016,219:182-190.
[10] 薛兆骏,周国亚,俞肖峰,等.超高温自发热好氧堆肥工艺处理剩余污泥[J]. 中国环境科学, 2017,37(9):3399-3406.
Xue Z J, Zhou G Y, Yu X F, et al. Ultra high temperature aerobic composting processing treating municipal sludge [J]. China Environmental Science, 2017,37(9):3399-3406.
[11] Liao H, Lu X, Rensing C, et al. Hyperthermophilic composting accelerates the removal of antibiotic resistance genes and mobile genetic elements in sewage sludge [J]. Environmental Science & Technology, 2018,52(1):266-276.
[12] Paul T, Dodd M C, Strathmann T J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity [J]. Water Research, 2010,44(10):3121-3132.
[13] Zhu L, Santiago-Schubel B, Xiao H, et al. Electrochemical oxidation of fluoroquinolone antibiotics: Mechanism, residual antibacterial activity and toxicity change [J]. Water Research, 2016,102:52-62.
[14] Prieto A, Moder M, Rodil R, et al. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products [J]. Bioresource Technology, 2011,102(23): 10987-10995.
[15] Gao M, Li B, Yu A, et al. The effect of aeration rate on forced-aeration composting of chicken manure and sawdust [J]. Bioresource Technology, 2010,101(6):1899-1903.
[16] Yuan T, Zhang Z J, Jing L, et al. Multiresidue determination of fluoroquinolones in eggs by solid-phase extraction-LC-MS/MS [J]. Journal of China Pharmaceutical University, 2010,41(1):60-65.
[17] Wu X L, Xiang L, Yan Q Y, et al. Distribution and risk assessment of quinolone antibiotics in the soils from organic vegetable farms of a subtropical city, Southern China [J]. Science of the Total Environment, 2014,487:399-406.
[18] Liu X, Lu S, Guo W, et al. Antibiotics in the aquatic environments: A review of lakes, China [J]. Science of the Total Environment, 2018, 627:1195-1208.
[19] Hu Z, Lane R, Wen Z. Composting clam processing wastes in a laboratory- and pilot-scale in-vessel system [J]. Waste Management, 2009,29(1):180-185.
[20] Chen Z, Zhang S, Wen Q, et al. Effect of aeration rate on composting of penicillin mycelial dreg [J]. Journal of Environmental Sciences, 2015,37(11):172-178.
[21] Said-Pullicino D, Erriquens F G, Gigliotti G. Changes in the chemical characteristics of water-extractable organic matter during composting and their influence on compost stability and maturity [J]. Bioresource Technology, 2007,98(9):1822-1831.
[22] Lv B, Xing M, Zhao C, et al. Towards understanding the stabilization process in vermicomposting using PARAFAC analysis of fluorescence spectra [J]. Chemosphere, 2014,117:216-222.
[23] He X S, Xi B D, Zhang Z Y, et al. Composition, removal, redox, and metal complexation properties of dissolved organic nitrogen in composting leachates [J]. Journal of Hazardous Materials, 2015,283: 227-233.
[24] Pan M, Chu L M. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops [J]. Ecotoxicology & Environmental Safety, 2016,126:228-237.
[25] Liu F, Ying G G, Tao R, et al. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities [J]. Environmental Pollution, 2009,157(5):1636-42.
[26] Dorival-Garcia N, Zafra-Gomez A, Navalon A, et al. Removal of quinolone antibiotics from wastewaters by sorption and biological degradation in laboratory-scale membrane bioreactors [J]. Science of the Total Environment, 2013,443:317-328.
[27] Li X W, Xie Y F, Li C L, et al. Investigation of residual fluoroquinolones in a soil–vegetable system in an intensive vegetable cultivation area in Northern China [J]. Science of the Total Environment, 2014,468-469:258-264.
[28] Vasquez M I, Garcia-Kaufer M, Hapeshi E, et al. Chronic ecotoxic effects to Pseudomonas putida and Vibrio fischeri, and cytostatic and genotoxic effects to the hepatoma cell line (HepG2) of ofloxacin photo(cata)lytically treated solutions [J]. Science of the Total Environment, 2013,450-451:356-365.
Removal of fluoroquinolone antibiotics generated from the sludge using hyper-thermophilic composting and its degraded products.
SONG Xiang-tong1, YANG Ming-chao1, ZHANG Jun2,3, GUO Ya-li2, DONG Bin1,2*
(1.College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China;2.China Three Gorges Corporation Yangtze Ecology and Environment Engineering Research Center, Beijing 100038, China;3.Yangtze Ecology and Environment Corporation Limited, Wuhan 430070, China)., 2022,42(1):220~226
The removal performance of NOR, OFL and their degradation products from traditional thermophilic composting (TC) and from hyper-thermophilic composting (HTC) were compared. The results showed that after 25 days’ hyper-thermophilic composting, the removal rate of NOR with TC and HTC was 91.8% and 92.1% with the residual NORP of 628 and 668μg/kg, respectively, while the removal rate of OFL was 92.1% and 88.1%, respectively with the residual OFLP and 191 and 675μg/kg, respectively. Compared with TC, HTC reduced the ecological risk of NOR and OFL by 3.1% and 30.5%, respectively, indicating that HTC could more effectively remove the ofloxacin and its degradation products and thus reduced the risk of environmental exposure to OFL in composting products. At the same time, the DOC/DON of hyper-thermophilic composting products was lower with the higher seed germination index and the plant toxicity of composting products was also lower, which is must be beneficial to the safe land use of sludge.
sludge;hyper-thermophilic;composting;fluoroquinolone;antibiotic;degradation product
X705
A
1000-6923(2022)01-0220-07
宋相通(1996-),男,同济大学硕士研究生,研究方向为固体废弃物资源化利用.
2021-06-03
中国长江三峡集团有限公司资助项目(202003080);国家重点研发计划(2020YFC1908702);国家重点研发计划(2021YFC3200704)
* 责任作者, 教授, dongbin@tongji.edu.cn

