钛酸钡压电臭氧化降解水中的硝基苯
庄 玮,杨 婧,龚冰柔,郑 莹,赵 纯
钛酸钡压电臭氧化降解水中的硝基苯
庄 玮1,2,杨 婧,龚冰柔,郑 莹,赵 纯*
(1.重庆大学环境与生态学院,重庆 400030;2.四川省城乡建设研究院,四川 成都 610043)
将臭氧(O3)体系与压电(PE)体系相结合提出了压电臭氧化(PE-O3)体系,探究了该体系对难降解有机污染物硝基苯(NB)的降解效果,考察了转速、O3浓度、钛酸钡(BT)投加量和初始pH值对NB去除的影响.此外,探讨了PE-O3体系降解NB过程中存在的活性物质,并分析了反应机理.结果表明:PE-O3体系对NB的降解体现出明显的协同效应(协同系数高达5.04),在15min内对NB的去除率高达85.37%,反应符合一级反应动力学规律,为0.1256min-1.此外,PE-O3体系在120min内对NB实现了74.06%的矿化.随着磁力转子转速的增加,体系反应速率提升,当转速提高到1500r/min时,反应速率常数可达到0.1446min-1.反应速率随体系中BT浓度和O3浓度的增加而增加,但一定程度后,增长趋势变缓.NB降解速率随pH值的增加而增大,当pH值为9.0时,在15min后体系中的NB降解率达85.69%.反应过程中产生的是降解NB的主要活性物质.
钛酸钡;压电臭氧化;硝基苯;压电;臭氧
硝基苯(NB)在石油化学工业、燃料、化学原料和焦化工业中的广泛应用导致了大量含硝基苯的废水排放到环境中[1].NB具有潜在的致癌、致畸和致突变性[2],被列为中国的58种优先污染物之一[3].但是传统的水处理工艺难以有效降解NB.
臭氧作为一种清洁高效的氧化剂在水处理高级氧化过程中被广泛研究[4-5],但单独臭氧体系对难降解污染物的去除率低,难以彻底矿化难降解污染物[6-7].为了实现对污染物的高效降解,研发了多种基于臭氧的高级氧化技术,其中电催化臭氧化体系(E-O3)因对污染物去除能力强、矿化率高等优点受到广泛关注[8-10].但该体系对电极性质要求较高,且电极、臭氧、反应液之间的传质条件限制了该体系去除污染物的效果[11-12].
目前已有通过电化学反应法,利用臭氧高效降解硝基苯的实验研究.Wang等[13]研究比较了以颗粒活性炭(GAC)为三维电极电解、臭氧氧化以及电解、GAC与臭氧(E-GAC-O3)联用对硝基苯(NB)的去除矿化效果,证实了电解法、活性炭法和臭氧法相结合可以有效降低电能的投入,降低水处理费用.
压电材料可以将外部机械能转化为电能和化学能,这一过程被称为压电效应.近年来,基于压电效应的压电催化过程由于对水中有机污染物的有效降解而引起了广泛关注.但压电催化过程产生•OH的能力较弱,因此只能对部分易降解的污染物进行高效降解.
将臭氧体系与压电催化相结合,利用压电过程为臭氧反应提供能量,可能是一种更具吸引力的类似电催化臭氧化的新技术.通过水力驱动的压电处理技术也能有效利用水处理流程中富余的水力能量.同时,粉末状催化剂相较于电极板而言具有更好的传质能力.因此,本研究将臭氧体系(O3)与压电体系(PE)相结合提出了压电臭氧化体系(PE-O3).本文将系统的研究压
电臭氧化体系(PE-O3)对NB的降解,以及转速、BT投加量、O3的浓度、初始pH值对PE-O3体系降解NB的影响,并揭示其反应机理.
1 材料与方法
1.1 试验材料
硝基苯(AR)、硝酸银(AR)、超氧化物歧化酶(200u/mg)、过氧化氢酶(200m/mg)、全氟磺酸(~5%)购于阿拉丁试剂有限公司;钛酸钡(BaTiO3,99.99%),购于成都市麦卡希化工有限公司;甲醇、叔丁醇、草酸铵、无水乙醇、无水硫酸钠均为分析纯,购于成都市科龙化工试剂厂;硫酸和氢氧化钠购于重庆川东化工(集团)有限公司;氧气(99%)购于重庆瑞信气体有限公司.
1.2 试验方法
试验过程:所有的试验都在圆柱形反应器内进行(PTFE材质,底部直径7cm,高12cm)反应液有效体积为300mL,反应装置被展示在图1中.将反应器置于磁力搅拌器上后投入转子,将转速调至200r/min,保持五分钟,该步骤是为了让BT对NB达到吸附平衡.然后利用臭氧发生器(3S-X,北京同林)将纯氧制成臭氧,并将臭氧气体臭氧气体通入反应液中,同时将磁力搅拌器转速调整至需要的转速.在一个典型的实验过程中:气体臭氧浓度为14.0mg/L,气体臭氧流量为300mL/min,BT投加量为3.0g/L,污染物NB浓度为10mg/L,反应液初始pH为9.6,磁力搅拌转速为1000r/min.开始计时,取样时间点一般为0、1、3、5、7、10、15min.在预先设定的时间间隔用注射器取样2.0mL,而后采用0.22μm的PTFE滤膜将其过滤至小瓶内,然后用移液枪量取1.5mL过滤后的样品至预先加入了50μL甲醇的液相进样瓶内.所有采集的样品都被储藏在4℃条件下,以待测试.
电化学测试:将150mg BT投加至0.2mL全氟磺酸溶液和1.8mL乙醇的混合液中,然后将其置于超声中分散20min,待其完全分散即得BT悬浊液.然后,将BT悬浊液均匀地涂抹在清洗并晾干的ITO玻璃基质上.最后,将被涂覆的ITO玻璃置于室温下干燥12h,即得BT电极.ITO电极也以同样的方法被制得,唯一不同的地方在于未添加BT.
制得BT和ITO电极后即可进行电化学测试,以测量压电过程中产生的瞬态压电电流.使用三电极体系的电化学工作站测定各个体系中的压电电流,三个电极分别为:Ag/AgCl参比电极、Pt对电极和BT(或ITO)工作电极.

图1 压电催化、臭氧氧化和压电催化臭氧氧化处理NB的反应器示意
1.3 分析方法
NB浓度检测:采用高效液相色谱法(HPLC)测定本研究中硝基苯(NB)的浓度,采用美国Waters公司的高效液相色谱,配备了COSMOSIL3C18-MS-II (5μmparticlesize, 4.6×150mm, Nacalai Tesque, Inc., Japan)型号的色谱柱,流动相为水、乙腈和甲醇(55:35:15,//),流动速度为1.0mL/min,检测波长为263nm,进样量为10 μL.
TOC测定方法:利用TOC-VCPH分析仪(Shimadzu Co., Japan)测定了反应过程中溶液的TOC.
采用X射线衍射(XRD, Cu Kα source, 40kV- 40mA, Spectris Pte. Ltd)以7°min-1的扫描速率在20°到80°的2范围内分析了相组成和晶体结构,并使用室温下配备的波长为532nm的100mW激光器进行了拉曼光谱分析,以进一步分析BT的相态(HORIBA Jobin Yvon S.A.S company, France).用X射线光电子能谱(XPS,ESCALAB250Xi,Thermo, USA)分析了BT中元素的变化及其化学状态.
2 结果与讨论
2.1 NB在不同体系中的降解
由图2(a)所示,在15min内,PE体系对NB几乎没有去除效果,O3体系对NB的去除率为29.88%, PE-O3体系对NB的去除率为85.37%.此外,根据图2(b)看出,PE-O3体系、单独PE体系和单独O3体系的一级反应动力学常数分别为12.56×10-2min-1、0.08 ×10-2min-1和2.41×10-2min-1.其中,PE-O3体系的拟一级反应动力学常数明显大于单独PE体系和单独臭氧体系的一级反应动力学常数.臭氧体系和压电体系之间存在明显的协同作用,协同系数达到5.04.与NB降解相似,在PE-O3体系在120min内对NB的矿化率(74.06%)远高于PE体系(1.20%)和O3体系(24.93%)的矿化率(图2(c)).
根据以上实验结果可知,PE-O3体系可以实现对NB更有效的降解和矿化,这可能与更多活性物质(尤其是•OH)的生成有关[14].

2.2 影响因素
2.2.1 转速的影响 外部机械力的改变会影响压电材料压电势的大小,进而影响体系对污染物的降解效果[15].因此,水力条件是PE-O3体系降解NB的一个重要影响因素.实验结果如图3(a)所示,NB单位时间降解量随转子转速变化而改变.如,PE-O3体系对NB的降解率随转速增加而提高.反应进行至15min后,随着转速从0r/min增长到1500r/min,PE- O3体系对NB的降解率从54.33%增长到90.29%.
转速对NB降解速率的影响被展示在图3(b)中,当转速为0r/min时,值仅为5.17×10-2min-1.随着转速的不断增大,PE-O3体系对NB的降解率随之提高,值也不断增大.当转速为1500r/min时,值可达14.46×10-2min-1.易知,PE-O3体系降解NB的值与转速呈正相关.该结论与Feng等[16]的研究结论一致,即提高转速有利于压电体系对污染物的降解.这可能一方面是转速的增加提高了传质效果[17],另一方面转速的提高使压电材料产生的压电势提高,产生了更多的自由载流子[18],进而产生了更多的自由基(如•OH)[11,19].

2.2.2 O3浓度的影响 O3浓度对基于臭氧的高级氧化工艺有重要的影响.如图3所示, O3浓度从3.0mg/L增加至18.0mg/L时,反应15min后,PE-O3体系对NB的降解率从32.14%提高至89.02%,NB去除率与臭氧浓度呈正相关.此外,值随着O3浓度的增加而增加.当O3浓度从3.0mg/L增至14.0mg/L时,值从2.63 × 10-2min-1提高至12.56×10-2min-1.这是因为O3浓度的提高使更多的O3参与反应,进而生成了更多的活性自由基,提高了反应速率.而当O3浓度进一步提高到18.0mg/L时,NB降解率仅提高了3.33%,值也仅增至14.30 × 10-2min-1.这可能是因为,在此条件下,O3浓度不再是反应的限制因素,压电效应产生的自由载流子成为了限制体系反应速率的因素.过量的O3不能完全与有限的自由载流子反应,造成体系降解NB的增长速率减缓.综上,选择14.0mg/L的O3为最佳O3浓度进行下一步实验.
2.2.3 BT投加量的影响 压电材料BT的浓度也会影响PE-O3体系对NB的降解效果.如图4所示,提高BT的投加量有利于PE-O3体系对NB的降解.当BT投加量从0.7g/L增加至3.0g/L时,反应15min后,NB的去除率从48.71%增加至85.69%,值从4.60×10-2min-1增加至12.56×10-2min-1.当BT投加量继续提高至6.0g/L时,PE-O3体系对NB的降解率略有提高,为89.90%,值为14.87×10-2min-1.这是由于BT投加量的增加能够增加压电效应的活性位点,进而产生更多活性氧化物质,提高体系对污染物的降解效果[20].然而,进一步提高BT的投加量可能导致过量的自由载流子的自我淬灭,使PE-O3体系降解污染物的增长速率减缓.Wu等[21]研究也发现,压电催化体系中,存在压电材料的最佳投加量,投加过量的压电材料无法进一步提高压电催化体系对污染物的降解效果,甚至产生抑制作用.因此,本文选取的BT最佳投加量为3.0g/L.


2.2.4 pH值的影响 在各类臭氧高级氧化工艺中,pH值是一个重要的影响因素[22-23].使用0.1mol/L的NaOH和H2SO4溶液调节反应液的初始pH至3.0、5.0、7.0、9.0、11.0,以探究不同pH值下PE-O3体系对NB的降解效果.如图5(a)所示,反应15min后,当 pH值从3.0提高到9.0,PE-O3体系对NB的降解速率随着pH的增大而增加.其中,NB的去除率从35.67%增加至85.69%,值从2.96×10-2min-1增加至12.52×10-2min-1.当pH值进一步提高至11.0后, PE-O3体系对NB的降解率相对pH值为9.0时略有提高(1.49%),为87.18%,值为13.20×10-2min-1.酸性条件下,O3的氧化还原电位比碱性条件下[24],导致臭氧在酸性条件下更难活化.此外,研究表明[25],碱性条件下,体系中的OH-能够与O3反应产生•OH(式2-1和2-2),促进污染物的降解.因此,提高PE-O3体系的pH值有利于NB的降解.

2.3 反应机理
根据目前的研究可知,压电臭氧化体系中可能存在羟基自由基(•OH)、电子(e-)、空穴(+)、超氧自由基(•O2-)和过氧化氢(H2O2)等活性物质[26],为了确定压电臭氧化体系中的活性物质,选取叔丁醇(TBA)[27]、硝酸银(AgNO3)[28]、草酸铵(AO)[29]、超氧化物歧化酶(SOD)[30]、过氧化氢酶(CAT)[30]分别对这六种物质进行淬灭.由图6试验结果可知,当TBA、AgNO3、AO、SOD和CAT被加入后,PE-O3体系去除NB的反应速率常数分别为2.56,5.27, 10.81,11.28,12.37(×10-2min-1).而没有淬灭剂时,PE- O3体系去除NB的反应速率常数为12.56× 10-2min-1.TBA对PE-O3体系去除NB的抑制能力最强,说明•OH是最重要的活性物质.同时,超氧自由基(•O2-)、电子(e-)和空穴(+)也是压电臭氧化体系中存在的活性物质,而过氧化氢(H2O2)几乎不存在或不占主导作用.由此前的研究可知,•OH和+被广泛证明可氧化降解NB[31].而e-可能是作为产生•OH的前驱物对试验造成了影响.此外,•O2-在以往的研究中也被证实可以进一步产生•OH以降解有机污染物[32].

图6 不同淬灭剂对NB降解速率的影响
压电臭氧化过程起始于压电效应的激活,当压电材料受到外界机械压力时会发生形变和极化,电子和空穴向材料两端分离而形成内建电场(式(3))[33].被检测到的e-和+证明了这一反应的发生.此外,电化学测试被广泛应用于压电效应的检测和证明,测试结果被展示在图7中.当磁力搅拌出于关闭状态时,ITO电极系统和BT电机系统内均无感应电流生成.而当磁力搅拌打开后,BT电极系统内立即检测到感性电流的存在,而ITO系统却没有.这些结果表明水力驱动的方式可以有效激活BT的压电效应.此外,PE-O3体系产生的压电电流明显高于PE体系,这表明臭氧促进了BT的电子和空穴分离过程,出现了更强的电子转移反应,从而显示出更强的感应电流.这些结果表明了O2(式(4))和O3(式(5))的得电子反应的发生.根据以往的文献,推测了这些活性物质分别可以发生一系列链式反应(式(6)至(8)),从而生成•OH降解NB[34-35](式(12)).压电效应产生的+因其独特的氧化性也可以在降解NB过程中发挥作用,+一方面可以直接氧化降解NB(式(9))[31],另一方面可以与反应液中的水电离出的OH-发生反应生成•OH(式(10)).

图7 不同体系中的瞬态压电电流测试
此外,在反应体系中,O3可直接氧化NB(式2-11).并且,在弱碱性条件下(pH 9.8),O3和OH-反应产生•OH(式(1)和(2))[25].
因此,PE-O3体系对NB的降解主要分为自由基过程和非自由基过程.其中,自由基过程的主要活性物质为•OH,并可以通过以下四条路径生成:1)O3的单电子还原(式(5)、式(7)和式(8))2)O2的单电子还原(式(4)和式(6)至式(8))3)O3和OH-的反应(式(1)和式(2))和4)+和OH-的反应(式(10)).而非自由基过程主要包括O3(式(11))和+(式(9))由对NB的直接氧化降解.
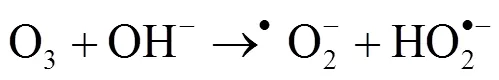
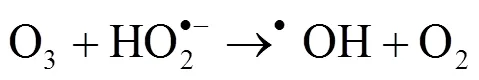








O3+R®byproducts (11)

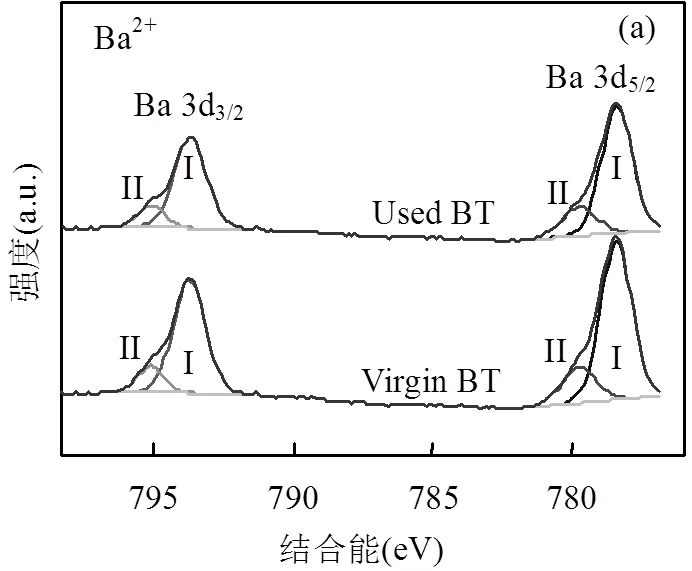
在大多数情况下,多相催化臭氧氧化涉及催化剂与臭氧之间的电子转移,这可能改变催化剂中过渡元素的价态,从而降低催化剂的稳定性[36-37].X射线光电子能谱(XPS)可对晶体中各种元素化学状态进行分析测量[38-39].对BT晶体在10次循环试验前后的XPS进行测量.如图8(b)所示,钛(BT中唯一的过渡金属)的化学状态保持在Ti4+,没有任何变化[40-41].此外,如图8(a)和8(c)所示,钡(Ba2+)和氧(O2-)的化学状态都没有变化[42-43].由此可见,参与氧化还原反应的电子可能来自压电反应产生的内置电场,能量由机械转化为电能[44-45],因此活性元素的化学状态不变.
3 结论
3.1 水力驱动的压电体系与臭氧体系对硝基苯的降解表现出较好的协同效果,协同系数高达5.04,并在120min内实现了对NB高达74.06%的矿化.
3.2 在PE-O3体系中,体系反应速率与转速呈正相关.这可能是因为,一方面搅拌使得压电效应增强,更多的电子参与反应,另一方面,体系中反应物充分混合,接触面积增大,反应更为迅速.
3.3 在PE-O3体系中,反应速率常数随着O3浓度和BT投加量的增大增加,但增大到一定程度以后,将不再是关键影响因素,增速将会变得缓慢.
3.4 在碱性条件下,O3和OH-反应产生更多的活性自由基,但在较高pH下,O3含量不足,导致O3的压电催化臭氧化与臭氧与OH-反应两个反应互相制约,减缓了反应速率的增加.
3.5 在PE-O3体系中,NB主要被•OH降解,同时也可被O3和+降解.
[1] Abdedayem A, Guiza M, Toledo F J R, et al. Nitrobenzene degradation in aqueous solution using ozone/cobalt supported activated carbon coupling process: A kinetic approach [J]. Separation and Purification Technology, 2017,184:308-318.
[2] Liu Q, Zhao H, Li L, et al. Effect of surface modification on carbon nanotubes (cnts) catalyzed nitrobenzene reduction by sulfide [J]. Journal of Hazardous Materials, 2018,357:235-243.
[3] Zhao L, Ma J, Sun Z. Oxidation products and pathway of ceramic honeycomb-catalyzed ozonation for the degradation of nitrobenzene in aqueous solution [J]. Applied Catalysis B: Environmental, 2008, 79(3):244-253.
[4] Ding W, Jin W, Cao S, et al. Ozone disinfection of chlorine-resistant bacteria in drinking water [J]. Water Research, 2019,160:339-349.
[5] Sgroi M, Anumol T, Vagliasindi F G, et al. Comparison of the new Cl2/O3/UV Process with different ozone- and UV-based aops for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter [J]. Science of the Total Environment, 2021,765:142720.
[6] Yan P, Shen J, Yuan L, et al. Catalytic ozonation by Si-doped α-Fe2O3for the removal of nitrobenzene in aqueous solution [J]. Separation and Purification Technology, 2019,228:115766.
[7] Guo Y, Zhao E, Wang J, et al. Comparison of emerging contaminant abatement by conventional ozonation, Catalytic ozonation, O3/H2O2and electro-peroxone processes [J]. Journal of Hazardous Materials, 2020,389:121829.
[8] Kishimoto N, Morita Y, Tsuno H, et al. Advanced oxidation effect of ozonation combined with electrolysis [J]. Water Research, 2005, 39(19):4661-4672.
[9] Li X, Wang Y, Wang B, et al. Combination of ozonation and electrolysis process to enhance elimination of thirty structurally diverse pharmaceuticals in aqueous solution [J]. Journal of Hazardous Materials, 2019,368:281-291.
[10] 李新洋,李燕楠,祁丹阳,等.电-多相臭氧催化工艺深度处理焦化废水[J]. 中国环境科学, 2020,40(10):4354-4361.
Li X Y, Li Y N, Qi D Y, et al. Study on electrochemical heterogeneous catalytic ozonation process for treatment of coking wastewater [J]. China Environmental Science, 2020,40(10):4354-4361.
[11] Xia G, Wang Y, Wang B, et al. The competition between cathodic oxygen and ozone reduction and its role in dictating the reaction mechanisms of an electro-peroxone process [J]. Water Research, 2017,118:26-38.
[12] Xiong Z, Lai B, Yang P. Insight into a highly efficient electrolysis- ozone process for N, n-dimethylacetamide degradation: Quantitative analysis of the role of catalytic ozonation, Fenton-like and peroxone reactions [J]. Water Research, 2018,140:12-23.
[13] Tuo W, Yunqian S, Haojie D, et al. Insight into synergies between ozone and in-situ regenerated granular activated carbon particle electrodes in a three-dimensional electrochemical reactor for highly efficient nitrobenzene degradation [J]. Chemical Engineering Journal, 2020,394.
[14] Lyu L, Zhang L, Wang Q, et al. Enhanced Fenton catalytic efficiency of γ-Cu–Al2O3By σ-Cu2+–ligand complexes from aromatic pollutant degradation [J]. Environ. Sci. Technol., 2015,49(14):8639-8647.
[15] Xue X, Zang W, Deng P, et al. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires [J]. Nano Energy, 2015,13:414-422.
[16] Feng Y, Ling L, Wang Y, et al. Engineering spherical lead zirconate titanate to explore the essence of Piezo-catalysis [J]. Nano Energy, 2017,40:481-486.
[17] 陈美辰.搅拌釜内液固非催化反应体系的动力学测定及颗粒悬浮的CFD模拟[D]. 上海:华东理工大学, 2020.
Cheng M C. Kinetics Determination of noncatalytic liquid-solid reaction system in stirred tank and CFD simulation of particle suspension [D]. Shanghai: East China University of Science and Technology in Shanghai, 2020.
[18] Wang,Z. L. Piezoelectric nanogenerators based on zinc oxide nanowire arrays [J]. Science, 2006, 312(5771):242-246.
[19] Zhang J, Xin B, Shan C, et al. Roles of oxygen-containing functional groups of O-doped G-C3N4in catalytic ozonation: Quantitative relationship and first-principles investigation [J]. Applied Catalysis B: Environmental, 2021:120155.
[20] Lin H, Wu Z, Jia Y, et al. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb (Zr0.52Ti0.48)O3fibers [J]. Applied Physics Letters, 2014,104:162907.
[21] Wu J, Xu Q, Lin E, et al. Insights into the role of ferroelectric polarization in piezocatalysis of nanocrystalline BaTiO3[J]. Acs Appl. Mater. Interfaces, 2018,10(21):17842-17849.
[22] Ratpukdi T, Siripattanakul S, Khan E. Mineralization and biodegradability enhancement of natural organic matter by ozone–vuv in comparison with ozone, Vuv, ozone–UV, and UV: Effects of pH and ozone dose [J]. Water Research, 2010,44(11):3531-3543.
[23] Zhao L, Ma J, Sun Z, et al. Mechanism of influence of initial pH on the degradation of nitrobenzene in aqueous solution by ceramic honeycomb catalytic ozonation [J]. Environ. Sci. Technol., 2008, 42(11):4002-4007.
[24] Bard A, Parsons R, Jordan J [M]. 1985.
[25] Song Y, Zhao C, Wang T, et al. Simultaneously promoted reactive manganese species and hydroxyl radical generation by electro- permanganate with Low Additive Ozone [J]. Water Research, 2021, 189:116623.
[26] Peng F, Yin R, Liao Y, et al. Kinetics and mechanisms of enhanced degradation of ibuprofen by piezo-catalytic activation of persulfate [J]. Chemical Engineering Journal, 2020,392:123818.
[27] Ding H, Zhu Y, Wu Y, et al. In situ regeneration of phenol-saturated activated carbon fiber by an electro-peroxymonosulfate process [J]. Environ. Sci. Technol., 2020,54(17):10944-10953.
[28] Mushtaq F, Chen X, Hoop M, et al. Piezoelectrically enhanced photocatalysis with Bifeo 3 nanostructures for efficient water remediation [J]. Iscience, 2018,4:236-246.
[29] Yang T, Peng J, Zheng Y, et al. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2nanocomposites [J]. Applied Catalysis B: Environmental, 2018,221: 223-234.
[30] Wang T, Song Y, Ding H, et al. Insight into synergies between ozone and in-situ regenerated granular activated carbon particle electrodes in a three-dimensional electrochemical reactor for highly efficient nitrobenzene degradation [J]. Chemical Engineering Journal, 2020,394: 124852.
[31] Zhang A, Liu Z, Xie B, et al. Vibration catalysis of eco-friendly Na0.5K0.5NbO3-based piezoelectric: an efficient phase boundary catalyst [J]. Applied Catalysis B: Environmental, 2020,279:119353.
[32] 苗志全,黄文璇,王 拓,等.碳气凝胶阴极用于电化学–臭氧体系去除布洛芬的机理试验研究[J]. 土木与环境工程学报(中英文): 1-9.
Miao Z Q, Huang W X, Wang T, et al. Removal of ibuprofen by electrolysis-ozone system with carbon fiber aerogel cathode [J]. Journal of Civil and Environmental Engineering: 1-9.
[33] Wu W, Wang L, Li Y, et al. Piezoelectricity of Single-atomic-layer MoS2for energy conversion and piezotronics [J]. Nature, 2014,514 (7523):470-474.
[34] Zhao L, Ma W, Ma J, et al. Characteristic mechanism of ceramic honeycomb catalytic ozonation enhanced by ultrasound with triple frequencies for the degradation of nitrobenzene in aqueous solution [J]. Ultrasonics Sonochemistry, 2014,21(1):104-112.
[35] Lin A Y, Panchangam S C, Chang C, et al. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition [J]. Journal of Hazardous Materials, 2012, 243:272-277.
[36] Wang J, Bai Z. Fe-based Catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater [J]. Chemical Engineering Journal, 2017,312:79-98.
[37] Wang J, Chen H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective [J]. Science of the Total Environment, 2020,704:135249.
[38] Gao C, Su Y, Quan X, et al. Electronic modulation of iron-bearing heterogeneous catalysts to accelerate Fe(iii)/Fe(ii) redox cycle for highly efficient fenton-like catalysis [J]. Applied Catalysis B: Environmental, 2020,276:119016.
[39] Li M, Sun M, Dong H, et al. Enhancement of micropollutant degradation in UV/H2O2process via iron-containing coagulants [J]. Water Research, 2020,172:115497.
[40] Srisombat L, Ananta S, Singhana B, et al. Chemical investigation of Fe3+/Nb5+-doped barium titanate ceramics [J]. Ceramics International, 2013,39:0-0.
[41] Zhang Q, Du L, Weng Y, et al. Particle-size-dependent distribution of carboxylate adsorption sites on TiO2nanoparticle surfaces: Insights into the surface modification of nanostructured TiO2electrodes [J]. Journal of Physical Chemistry B - J Phys Chem B, 2004,108:15077- 15083.
[42] Liao J, Wei X, Xu Z, et al. Effect of potassium-doped concentration on structures and dielectric Performance of barium-strontium-titanate films [J]. Vacuum, 2014,107:291-296.
[43] Mccafferty E,Wightman J. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative Xps method [J]. Surface and Interface Analysis, 1998,26:549-564.
[44] Gong S, Xie Z, Li W, et al. Highly active and humidity resistive perovskite LaFeO3based catalysts for efficient ozone decomposition [J]. Applied Catalysis B: Environmental, 2019,241: 578-587.
[45] Yan X, Li G, Wang Z, et al. Recent progress on piezoelectric materials for renewable energy conversion [J]. Nano Energy, 2020,77:105180.
Degradation of nitrobenzene from water by piezoelectric ozonation of barium titanate.
ZHUANG Wei, YANG Jing, GONG Bing-rou, ZHENG Ying, ZHAO Chun*
(College of Environment and Ecology, Chongqing University, Chongqing 400045, China)., 2021,41(10):4654~4661
The single ozone process is difficult to degrade refractory pollutants. Although the electrolysis ozonation process has been proven to effectively degrade and mineralize refractory organic pollutants, it is limited by electrode materials and mass transfer. The piezoelectric ozonation (PE-O3) process was proposed by combined the ozone (O3) process with the piezoelectric (PE) process. The PE-O3process showed a significant synergistic effect (the synergy index = 5.04) on the degradation of nitrobenzene (NB). Besides, the degradation ratio of NB was 85.37% in PE-O3process within 15min, and the reaction conformed to pseudo first order (= 0.1256min-1). The TOC removal ratio was 74.06% in PE-O3process within 120min. As the rotation speed increased, the reaction rate increased. However, the reaction rate constant could reach 0.1446min-1when the rotation speed increased to 1500r/min. The reaction rate increased with the increase of BT and O3concentration in PE-O3process, but the increasing trend slowed down after a certain degree. Moreover, the degradation rate of NB increased with the increase of pH value. When the pH value is 9.0, the degradation rate of NB in the system reached 80% after 15min. Notably, the•OH produced during the reaction is the main active species to degrade NB.
barium titanate;piezoelectric ozonation;nitrobenzene;piezoelectricity;ozone
X52
A
1000-6923(2021)10-4654-08
庄 玮(1995-),男,四川阆中人,重庆大学硕士研究生,主要从事基于臭氧的高级氧化研究.
2021-03-25
国家自然科学基金资助项目(22076015);重庆市自然科学基金(cstc2019jcyj-msxmX0463)
* 责任作者, 副教授, pureson@163.com

