The protective effect of a standardized hydroalcoholic extract of Prosopis farcta(Banks&Sol.)J.F.Macbr.fruit in a rat model for experimental ulcerative colitis
Ali Akbar Safari, Mohammad Sharifzadeh, Gholamreza Hassanzadeh, Mohammad-Reza Delnavazi, Mohammad Mahdi Ahmadian-Attari , Amir Hossein Abdolghaffari7,8,9,0,Maryam Baeeri,Mehran Mirabzadeh Ardakani*
1Department of Traditional Pharmacy, School of Persian Medicine, Tehran University of Medical Sciences, Tehran, Iran.2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.3Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.4Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.5Evidence-based Phytotherapy and Complementary Medicine Research Center, Alborz University of Medical Sciences, Karaj, Iran.6Department of Pharmacognosy, Faculty of Pharmacy, Alborz University of Medical Sciences, Karaj, Iran.7Department of Toxicology & Pharmacology, Faculty of Pharmacy, Tehran Medical Sciences,Islamic Azad University, Tehran, Iran.8Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Tehran, Iran.9Toxicology and Diseases Group(TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran,Iran.10GI Pharmacology Interest Group (GPIG), Universal Scientific Education and Research Network (USERN), Tehran,Iran.11Pharmaceutical Sciences Research Center,Tehran University of Medical Sciences,Tehran,Iran.
Abstract Background: Investigating new therapies for inflammatory bowel disease among natural products has recently been gaining attention.Although Prosopis farcta has repeatedly been mentioned in traditional Persian medicine as a therapeutic option for inflammatory bowel disease, no evidence-based investigations have been conducted on this topic.The aim of this study was to assess the impact of P.farcta on acetic acid-induced colitis in rats.Methods: A hydroalcoholic extract of P.farcta fruits was prepared.Thirty-six adult Wistar rats were divided into six groups, and colitis was induced in five groups, except the sham group, using acetic acid solution.The animals received distinctive daily doses of P.farcta (50, 75, and 100 mg/kg/day,p.o.) and dexamethasone (1 mg/kg/day, i.p.) as standard treatment for two progressive days,starting from colitis induction.Microscopic and histopathological examinations were performed on inflamed colonic tissue.Tissue concentrations of interleukin-1β and tumor necrosis factor-α were assessed using enzyme-linked immunosorbent assay kits.To identify the role of oxidative stress in ulcerative colitis, the levels of malondialdehyde and myeloperoxidase were measured in colon tissues.Results:Treatment with all concentrations of P.farcta attenuated inflammation and ulcers compared with saline treatment in the control group (P < 0.01 for 50 mg/kg; P <0.001 for 75 and 100 mg/kg/day).The best suppression of tumor necrosis factor-α and interleukin-1β was observed at a dose of 100 mg/kg P.farcta (P<0.001).This dose showed the best effect in reducing myeloperoxidase and malondialdehyde in ulcerative colitis-induced rats(P < 0.001).Conclusion: P.farcta can be considered a promising candidate for treating ulcerative colitis; thus, it requires further confirmation by clinical trials.
Keywords: Prosopis farcta; colitis; interleukin-1β; tumor necrosis factor; malondialdehyde;traditional Persian medicine
Tradition
Literature regarding the medicinal properties ofProsopis farctahave been found in manuscripts of Iranian origin.Rhazes,in his famous bookAl-Hawi(10thcentury),quoted the hemostatic activities ofProsopis farctaas outlined by Yahya ibn Masa,a Persian physician from Marv Hospital in the 9thcentury.In the same book,Rhazes also suggested the use ofProsopis farctafruits for abdominal cramps and diarrhea,which was also mentioned in Avicenna’s famous book,Canon of Medicine(completed in 10thcentury).In traditional Persian medicine,the fruits of this herb have been extensively used as an astringent,anti-diarrheal,stomach tonic,and blood clotting agents.Moreover,later scholars like Mo’men-Tonekaboni(11thcentury)and Aghili-Khorasani(12thcentury)specifically suggested this herb for the treatment of proctitis.
Background
Inflammatory bowel disease (IBD) is a chronic and recurrent inflammatory disease of the gastrointestinal tract.The etiology of IBD is unclear; however, environmental factors and genetic defects are the key parameters involved in IBD.Generally, IBD is divided into two main conditions: ulcerative colitis and Crohn’s disease, which share common symptoms such as abdominal pain, severe diarrhea, fatigue,and weight loss.ulcerative colitis is a long-term (chronic) condition with certain manifestations, such as irritation, inflammation, and ulcers in the colon.Of factors that precipitate ulcerative colitis, proand inflammatory mediators such as cytokines and chemokines, some microorganisms, oxidative stress, and leukocyte migration are known to have the most effective roles [1–6].Accumulating evidence has confirmed that reactive oxygen and/or nitrogen species, such as hydrogen peroxide, superoxide anion or hydroxyl radicals, and peroxynitrite are implicated in the pathogenesis of IBD.Reactive species can act as pro-inflammatory triggers, leading to the accumulation of neutrophils at the site of inflammation and the release of pro-inflammatory or inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).In contrast, inflammation induces oxidative stress by stimulating reactive oxygen and/or nitrogen species production, such as myeloperoxidase(MPO).Inhibition of lipid peroxidation, scavenging of oxygen free radicals, and suppression of inflammatory mediators establishes an important protective and/or therapeutic approach for the treatment of ulcerative colitis [7].Amino salicylates and corticosteroids are the first-line standard drug treatment options for IBD.Despite considerable progress, IBD pharmacotherapy is ineffective (for salicylate derivatives) and causes severe side effects (for corticosteroids and immunosuppressants) in both short- and long-term regimens [8–9].Thus, the discovery of new therapeutic options is still of interest.
The use of traditional medical information is a common approach to drug discovery.Iran has a long history of traditional and folk medicine.In traditional Persian medicine,IBD is well explained under the title of Sahaj ul-Am’a [10].As a type of Sahaj ul-Am’a, Zahir is characterized by tenesmus of the rectum during defecation after bloody diarrhea and secretion of mucosa and can be considered as proctitis.[11].Prosopis farcta(Banks&Sol.) J.F.Macbr.(Kharnoob-e Nabati in Persian) is a plant used as one of the treatments traditionally recommended for the management of Zahir[12, 13].
Literature regarding the medicinal properties ofP.farctahave been found in manuscripts of Iranian origin.Rhazes, in his famous bookAl-Hawi(10thcentury), quoted the hemostatic activities ofP.farctaas outlined by Yahya ibn Masa, a Persian physician from Marv Hospital in the 9thcentury [14].In the same book, Rhazes also suggested the use ofP.farctafruits for abdominal cramps and diarrhea, which was also mentioned in Avicenna’s famous book,Canon of Medicine(completed in 10thcentury) [14, 15].In traditional Persian medicine,P.farctafruits have been widely used as an astringent, anti-diarrheal,stomach tonic, and blood clotting agents [13–16].In addition, in later periods, scholars such as Mo’men-Tonekaboni (11thcentury) and Aghili-Khorasani (12thcentury) specifically suggested the use of this herb for the treatment of Zahir(proctitis) [12, 13].
P.farctabelongs to the family Fabaceae and is an acanaceous shrub native to North Africa, Syria, Caucasus, Pakistan, Afghanistan, and Iran [17].Recent phytochemical analysis showed that this herb contains tannins, flavonoids,fatty acids,and pectin,as well as calcium,magnesium, and selenium [18–20].Pharmacological studies have indicated thatP.farctapossesses therapeutic activities, including wound healing, fertility enhancement, and blood glucose reduction properties [21–23].Moreover,P.farctaexhibited hepatoprotective activity against acetaminophen-induced hepatotoxicity [24].
Given the significance ofP.farctain cellular protection, antioxidant activity, and wound healing, we decided to assess the efficiency of a hydroalcoholic extract ofP.farctafruit in attenuating the oxidative and inflammatory biomarkers of colitis in rats with acetic acid(AA)-induced ulcerative colitis.The aim of this study was to investigate the impact ofP.farctaon treating AA-induced colitis in rats.
Materials and methods
Plant material
P.farctafruits were collected from Zabol city, Systan and Baluchestan provinces, Iran, in July 2018.The plant was identified by an expert,and a voucher specimen (TEH 7050) was kept for this collection at the Herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, for future reference.
Extraction
Fruits were dried in a shed, powdered, and extracted using the maceration method.Ethanol (70%) was used as the extraction solvent and the extract was concentrated using a vacuum rotary evaporator(Hei-VAP value/digital G3, Heidolf, Germany) prior to being dried in a desiccator, and stored in a dark, cold, and dry environment for future examinations.The yield of extraction was calculated as follows:(dry extract weight/dry starting material weight) × 100%.
Preliminary phytochemical screening
The chemical content of theP.farctafruit extract was assessed for the presence of alkaloids, tannins, saponins, and flavonoids.For alkaloids,15 mg of sample was dissolved in 6 mL HCl (1%) in a bath of hot water for 5 min and then filtered.The filtered solution was divided into three parts and examined using Dragendorff, Mayer, and Wagner’s tests.Dragendorff’s reagent (1 mL) was added to 1 mL of the filtered solution.The presence of alkaloids was confirmed by the formation of an orange-red precipitate.For the Mayer’s test, the formation of a cream-colored precipitate after mixing 1 mL of Mayer’s reagent with 1 mL of the filtered solution confirmed the presence of alkaloids.Wagner’s test was performed by dissolving 2 g of potassium iodide and 1.27 g of iodine in 5 mL of distilled water and then diluting 5 mL of the solution with 95 mL of distilled water.The addition of a few drops of this solution to the filtrate and formation of a brown-colored precipitate indicated the presence of alkaloids [25].
For tannins, dried extract powder (0.5 g) was dissolved in 10 mL of distilled water.Following filtration, a few drops of ferric chloride (5%)were added.A color change to black or blue-green was considered a positive result.For saponins, the sample (0.5 g) was dissolved in 10 mL of distilled water.The formation of foam that remained during heating was considered a sign of the presence of saponins [25].For flavonoids, 2 mL of 5% ferric chloride solution was added to the diluted extract in distilled water.The formation of blue, green, or violet colors indicated the presence of phenolic compounds [26].Qualitative results were considered (+) for the existence and (−) for the absence of phytochemicals.
Total phenolic content measurement
Total phenolic content was measured using the Folin-Ciocalteu reagent [27].For this analysis, 1 mL of theP.farctaextract (100 mg dry extract in 1 mL methanol) was mixed with Folin-Ciocalteu reagent(0.2 N).After 5 min, 1.5 mL of sodium bicarbonate (60 g/1000 mL)was added.After 2 h of incubation in the dark, the absorbances of the sample and blank (methanol) were measured by spectrophotometry at 760 nm.
Diphenyl-2-picrylhydrazyl(DPPH) antioxidant assay
Various concentrations of theP.farctaextract were prepared in methanol (0.007–2 mg/mL) and mixed with 2 mL of DPPH solution[28].The solutions were incubated at 25 °C for 70 min.Next, the absorbance of each solution was measured against the blank at 517 nm.Inhibition of free radicals by DPPH in percent (I%) was calculated as follows: I% = (A blank −A sample/A blank)× 100%.
Butylated hydroxytoluene was utilized as a positive control.
High performance liquid chromatography (HPLC)standardization of the P.farcta extract based on gallic acid
Gallic acid 97% (Sigma Aldrich Chemie, Steinheim, Germany) was used as a marker for standardization of the extract.To determine the gallic acid content in theP.farctaextract through HPLC, 1 g of the dried extract was placed in an ultrasonic bath for 1 h to dissolve in 50 mL methanol.Next, the mixture was cooled and centrifuged for 15 min at 500 × g.The supernatant was filtered through a 0.45 µm filter and collected in specific vials.Then, this sample was diluted in the HPLC mobile phase (1:5), and 10 µL was injected for analysis using HPLC(Knauer Co., Ltd.,Berlin, Germany).The mean of three runs was considered the final result.Pure gallic acid solutions (4, 20, 40, and 100 ppm) were used for the construction of a calibration curve and standardization [29].The area under the curve was used to measure the content of gallic acid in the sample.Column: Eclipse-XBD®-C18 column (Agilent Technologies, Santa Clara, CA, USA), 5 µm × 4.6 cm× 25 cm; UV detector: K-2501 (Knauer Co., Ltd., Berlin, Germany),280 nm; HPLC mobile phase: phosphoric acid 1%: acetonitrile(80:20);flow rate: 1.0 mL/min; temperature: 20 ±1 °C.
Animals
Thirty-six adult male Wistar rats (200–230 g) were provided by the Animal Laboratory of Medicinal Plants Research Center, Institute of Medicinal Plants, Alborz, Iran.Animals were housed in groups of six per cage with free access to standard food and water (12 h light/dark cycle; at a temperature of 23 ± 1°C).All animal tests were performed in accordance with the Guidelines for the Care and Utilization of Laboratory Animals published by the US National Institute of Health(NIH Publication No.85-23, reviewed 1996) and under the license of the ethical committee(IR.TUMS.VCR.REC.1397.135).
Acute toxicity
For acute toxicity assessment, three doses (500, 1000, and 2000 mg/kg) of theP.farctaextract were administered by gavage to 15 rats.The animals were monitored for 72 h, and related symptoms were recorded [30].
Experimental design for colitis induction
Following induction of colitis, the drugs were dissolved in normal saline and administered orally for 2 days (once a day).The rats were randomized into six groups, each containing six animals (n = 6).
Group I, sham group: healthy rats without colitis were treated with 1 mL saline (gavage) for 2 days,once a day.
Group II, control group: colitis induced by AA (4%); rats were treated with 1 mL normal saline orally for 2 days.
Group III, dexamethasone/Dexa group: colitis induced by AA (4%);rats were treated with 1 mL dexamethasone 1 mg/kg intraperitoneally for 2 days[13].
Group IV (AA + 50): colitis induced by AA (4%);P.farctawas administered at 50 mg/kg (1 mL) via gavage for 2 days.
Group V (AA + 75): colitis induced by AA (4%);P.farctawas administered at 75 mg/kg (1 mL) via gavage for 2 days.
Group VI (AA + 100): colitis induced by AA (4%);P.farctawas administered at 100 mg/kg (1 mL) via gavage for 2 days.
It should be noted that due to the traditional background ofP.farctaas a herbal medicine for the treatment of IBD, the doses of the extract in this investigation were calculated by converting the traditional human dose (34.3 mg/kg/day) to an animal dose [31].This was calculated as an animal dose of 214.4 mg/kg/day (particularly the dose in rats).As a preliminary assessment, colitis was induced in 9 rates via acetic acid and then, doses of 100, 200, and 300 mg/kg (3 rats for each dose) were orally administered for 2 consecutive days.Macroscopic evaluation of colon tissues showed that the 100 mg/kg dose was superior to the other doses.Therefore, we selected 100, 75,and 50 mg/kg/day as the doses for our final study.
AA-induced colitis and sample preparation
The experiment was performed for 4 days.The animals were fasted for 24 h before any colonic intervention while having access to water.Rats were anesthetized with an intramuscular injection containing a mixture of ketamine (4 mg/100 g) and xylazine (1 mg/100 g).A medical-grade polyurethane cannula for enteral feeding (external diameter, 2 mm) was embedded into the anus of each animal.Furthermore, the tip progressed by 7 cm proximal to the anus verge[32].AA (1 mL, 4% v/v in 0.9% saline) or saline alone (sham) was instilled into the colon using the cannula for 30 s, after which the liquid was pulled back.Following induction of colitis, drugs were dissolved in normal saline and administered orally on days 1 and 2 after colitis induction.On the 4thday, all animals were euthanized with ether overdose(inhalation),and their colons were collected.A 10 cm distal section of the colon of each animal was excised and longitudinally separated.Colon sections were washed with saline to remove fecal residues, and the colons were cut into two parts.For minuscule and histopathological investigations, portions of the colonic samples were preserved in 10% formalin.The remaining half were kept at −80 °C until required for biochemical measurement studies[33].
Evaluation of ulcer index
Macroscopic scoring was performed under a magnifying glass by an independent observer using the following 0–5 scale criteria described by Morris et al.in 1989: 0, no macroscopic modifications; 1, localized hyperemia but no ulcer; 2, linear ulcer with no critical inflammation;3, linear ulcer with inflammation at one location; 4, two or more sites of ulcer and inflammation; 5, two or more locations of ulcer and inflammation amplifying over 1 cm[34].
Microscopic evaluation
For microscopic characterization, the colon tissue was placed in phosphate buffered formaldehyde, embedded in paraffin, and then 5µm sections were obtained.The tissue was stained with hematoxylin and eosin, assessed by light microscopy, and scored in a blinded manner by a master pathologist (Professor Gholamreza Hassanzadeh,Department of Anatomy, Tehran Medical University of Medicine).A histological grading scale of crypt damage, inflammation seriousness,and irritation degree described for the first time by Murthy et al.in 1993 was utilized, and microscopic parameters were graded from 0 to 3 (0, no change; 1, mild; 2, direct; 3, extreme) [35].Histological assessment and scoring were performed using a Zeiss®magnifying lens fitted with an Olympus®color video camera for digital imaging.Samples were prepared in a blinded manner by assigning a number to each sample.
Determination of colonic TNF-α and IL-1β levels
On an ice-cold bath, the colon samples were immediately weighed,minced, and suspended in a tube containing potassium phosphate buffer (10 mmol/L, pH 6) and 0.5% hexamethylammonium bromide.The tubes were placed in a shaking water bath (40 °C) for 20 min and then centrifuged at 4,000 ×gfor 30 s at 4 °C.The supernatant was frozen and stored at −80°C until assay.
TNF-α was quantified using enzyme-linked immunosorbent assay kits (ab100785, Abcam, Cambridge, UK).Standards and samples were pipetted into the wells.The wells were washed, and biotinylated anti-rat TNF-α antibody was added.After washing away the unbound biotinylated antibody, horseradish peroxidase-conjugated streptavidin was pipetted into the wells.The wells were washed again, and a TMB substrate solution was added to each well.All samples and standards were measured in triplicate, and the optical density of the changing color (from blue to yellow) was determined at 450 nm.The intensity of the color was directly proportional to the concentration of TNF-α in the colon samples.The results are presented as pg/mg protein [36].
For IL-1β, the samples and standards were added to plate wells coated with IL-1β antibodies labeled with horseradish peroxidase(ab100768, Cambridge, UK).Next, the TMB substrate solution was pipetted into the wells.After that, stop buffer was added and the optical density of the changing color (from blue to yellow) was measured at 450 nm.The intensity of the color was directly proportional to the concentration of IL-1β in the colon samples.The results are presented as pg/mg protein [37].
Evaluation of colonic MPO activity
Briefly, colonic tissues were homogenized in a solution containing 0.5% (w/v) hexadecyltrimethyl ammonium bromide dissolved in 10 mM sodium phosphate buffer (pH 7.4) in an ice bath using a polytron homogenizer (50 mg tissue/mL).The homogenates were centrifuged at 20,000 ×gat 4 °C for 30 min and then added to a solution of tetramethyl benzidine (1.6 mM) and hydrogen peroxide (0.1 mM).The absorbance was measured spectrophotometrically at 650 nm.MPO activity was defined as the quantity of enzyme that can degrade 1 μmol of peroxide/min at 37 °C and is expressed in units/mg wet tissue[38].
Lipid peroxidation analysis via thiobarbituric acid (TBA)-reactive substance(TBARS) measurement
Malondialdehyde is a product of polyunsaturated fatty acid oxidation and an indicator of lipid peroxidation.In this experiment, TBA was reacted with lipid peroxide to create a TBARS complex that was then measured spectrophotometrically.Segments of colons were homogenized in buffered saline (1:5).Thereafter, 800 mL of TBA(28% w/v) was added to 400 mL of this mixture and centrifuged at 3,000×gfor 30 min.Then,600 mL of the supernatant and 150 mL of TBA (1% w/v) were mixed.After 15 min of incubation in a 100 °C water bath, n-butanol (4 mL)was added.The solution was centrifuged,and the absorbance was determined at 532 nm using a spectrophotometer.The calibration curve of a 1,1,3,3-tetraethoxypropan standard was used to calculate the TBARS concentration asµmol/g of colon [39].
Statistical analysis
Statistical analysis was performed using one-way analysis of variance.For the post-hoc test, Tukey’s test was applied to analyze the data(SPSS version 19, Chicago, IL, USA).All values are expressed as the standard error of the mean.Significance was set atP< 0.05.
Results
Phytochemical analysis
The extraction yield fromP.farctawas 26.5%.The entire total phenolic content of the extract was 13.78 ± 0.39 mg/g equivalent to gallic acid.As shown in Figure 1, HPLC analysis showed that the amount of gallic acid in theP.farctaextract was 7.36 ± 0.26 mg/g(LOD: 6.87 ppm, LOQ: 20.61 ppm, and R² = 0.97).In the case of DPPH analysis, the IC50of theP.farctaextract was 29.00 ± 2.45µg/mL, while butylated hydroxytoluene, used as a positive control,exhibited an IC50of 18.70 ± 2.10 μg/mL.In the preliminary phytochemical analysis, the presence of tannins, flavonoids, saponins,and alkaloids was observed.
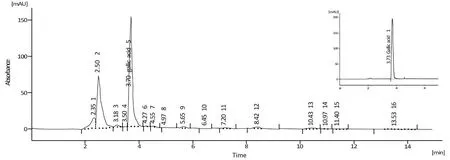
Figure 1 Assay of gallic acid by HPLC.The peaks of the extract and standard gallic acid(a single peak in up and right of the picture)are presented.Retention time, 3.700 min and 3.733 min for the extract and standard gallic acid, respectively; area under the curve, 1188.099 mAU.s and 1417.476 mAU.s for the extract and standard gallic acid, respectively.The results showed that the sample contains 7.36 ± 0.26 mg/g gallic acid.HPLC, high performance liquid chromatography.
Animal studies
Macroscopic assessment of colonic damage.Macroscopic assessment showed no signs of inflammation in the sham group.The administration of AA caused ulcers, adhesions, intestinal wall necrosis,and severe inflammation in the control group compared to the sham group (P< 0.001).The administration of dexamethasone to animals with colitis improved macroscopic symptoms of the disease.Administration ofP.farctaat all concentrations attenuated inflammation and ulcers compared with saline-treated animals(control group) (P< 0.01, 50 mg/kg;P< 0.001 for 75 and 100 mg/kg/day).There was no significant difference (P> 0.05) between the improved macroscopic scores from theP.farcta(100 and 75 mg/kg/day) and Dexa groups.P.farctaat a dose of 100 mg/kg exhibited a greater healing effect than all the other treatments (Figure 2).
Histopathological assessment of colonic damage.The normal mucosal layers and epithelial surfaces of sham group animals were observed.The control group showed severe hemorrhage in the mucosa and submucosal area, polymorphonuclear infiltration, and macrophage accumulation within normal muscle externa.In animals treated withP.farctaextract (AA + 50), the intestinal glands, goblet cells, and muscle externa were found to be normal, with slight hemorrhage and very low polymorphonuclear infiltration in the submucosa.There was slight mucosal and submucosal polymorph nuclear infiltration and normal intestinal glands in AA + 75-treated rats.The appearance of intestinal glands, goblet cells, neutrophil density, and macrophages was normal in AA + 100-treated rats.Animals in the Dexa group showed normal colons and mild cellular infiltration (Figure 3).
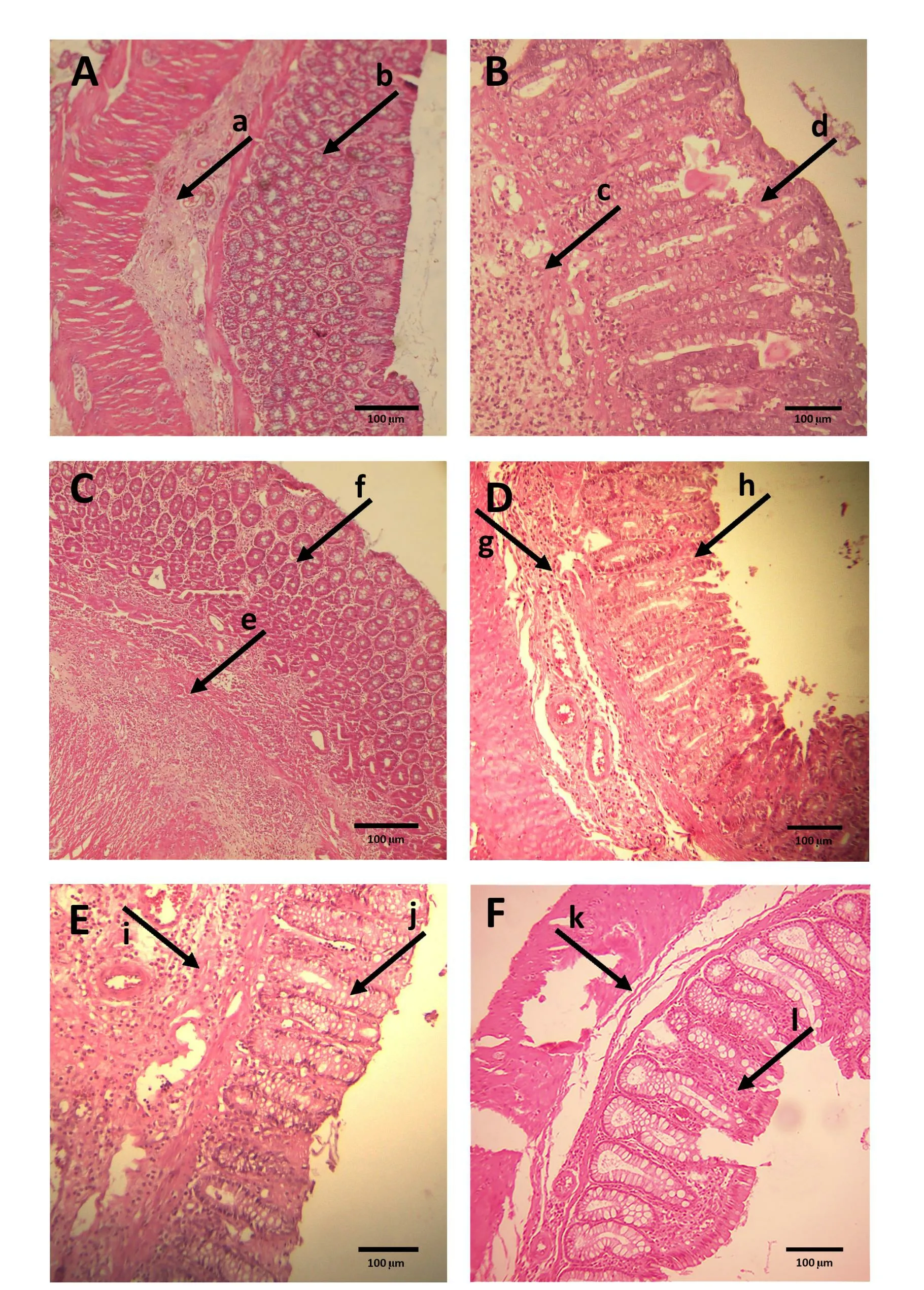
Figure 3 Photomicrograph of heamatoxylin and eosin stained portions of rat colonic tissues.A,received intrarectal NS(sham)and no histological modification with normal crypts (a) and submucosal tissue (b); B, received intrarectal AA and no treatment (Ctr),destruction of crypts (c) and hemorrhages in the mucosa and submucosa and inflammatory cell infiltration (d); C, received intrarectal AA and treated with dexamethasone, minimal cellular infiltration (e) in otherwise normal large bowel (f); D, received intrarectal AA and treated with 50 mg/kg of the extract (AA + 50),with normal view of bowel glands, low inflammatory cell infiltration(g) and normal gablet cell (h); E, received intrarectal AA and treated with 75 mg/kg of the extract (AA + 75), the bowel glands with normal view (i) and very low inflammatory cell infiltration (j); F,received intrarectal AA and treated with 100 mg/kg of the extract (AA+ 100), the bowel glands with normal view (k) without inflammatory cell infiltration (l) (scale bar: 100 µm).AA, acetic acid.
Impact on colonic TNF-α levels.In the control group, the levels of TNF-α were significantly higher than those in rats of the sham group(P<0.001).P.farctaextract at all concentrations(AA+50,AA+75,AA + 100) reduced TNF-α levels in the animals in comparison with the control rats (P< 0.001).Similarly, this reduction was significant when rats were treated with dexamethasone (P< 0.001) (Figure 4).
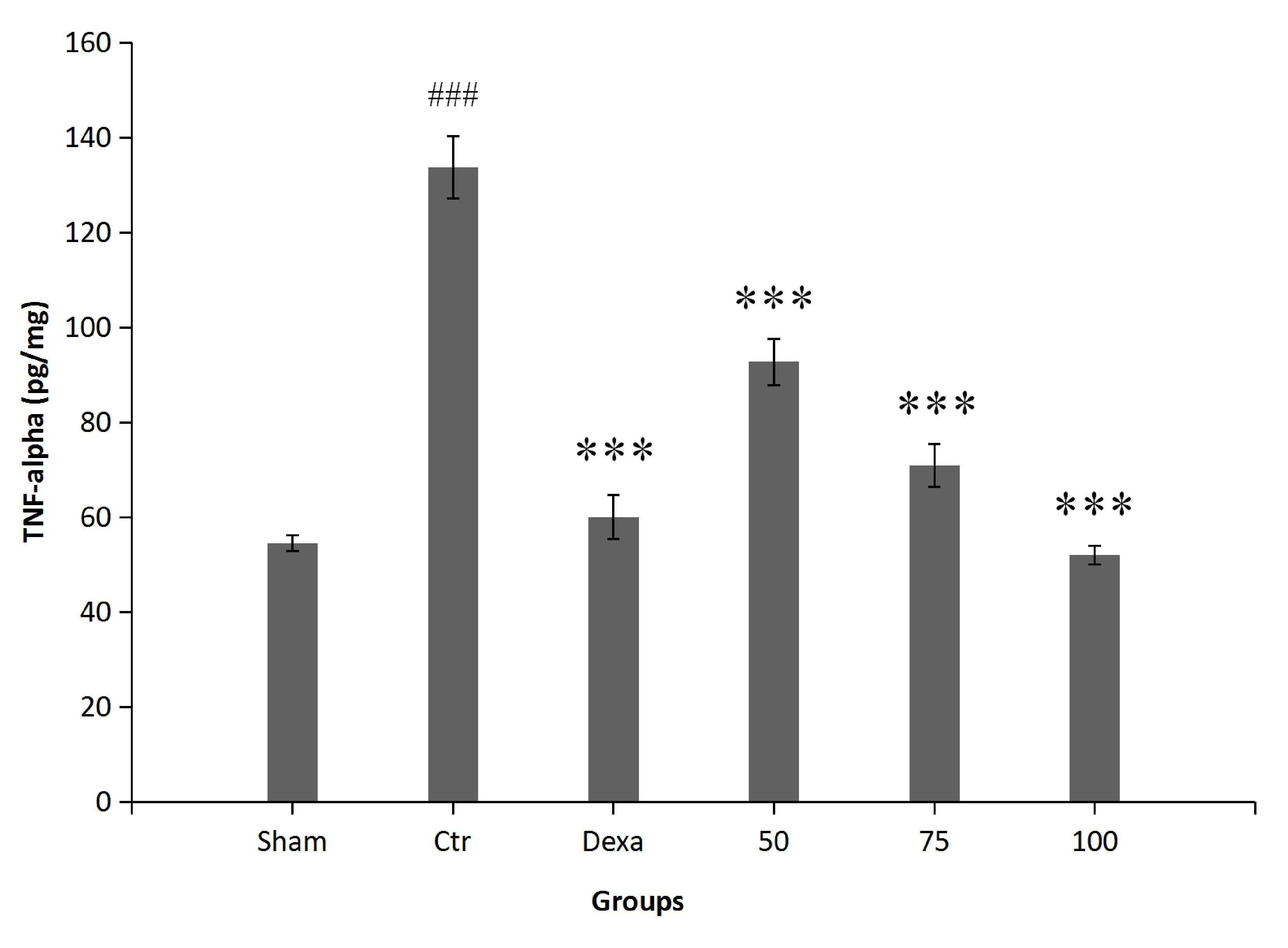
Figure 4 The effect of P.farcta (50, 75, and 100 mg/kg/day) on colonic activity of TNF-α.###, significant difference with sham (P <0.001);***, significant difference with control (P < 0.001).Values are expressed as mean ±SEM.Ctr,control; Dexa, dexamethasone; 50, 75,and 100, groups receiving 50, 75, and 100 mg/kg/day of the extract.SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
Colonic IL-1β levels.As expected, the levels of IL-1β were higher in control group than in sham group animals(P< 0.001).Rats in the AA+ 100 and Dex groups showed significantly alleviated IL-1β levels in comparison with the control animals (P< 0.001).The extract at 75 mg/kg was also able to reduce IL-1β activity (P< 0.01); however,extract treatment at a dose of 100 mg/kg showed the highest inhibitory activity of IL-1β in comparison with all the other treatments(Figure 5).
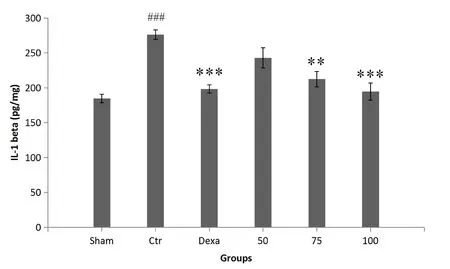
Figure 5 The effect of P.farcta (50, 75, and 100 mg/kg/day) on IL-1β level in colon tissue.###, significant difference with sham (P< 0.001); **, significant difference with control (P < 0.01); ***,significant difference with control (P < 0.001).Values are expressed as mean ± SEM.Ctr, control; Dexa, dexamethasone; 50, 75, and 100,groups receiving 50, 75, and 100 mg/kg/day of the extract.SEM,standard error of the mean; IL-1β, interleukin-1β.
Colonic lipid peroxidation levels as TBARS.The inflammatory condition caused a considerable increase in TBARS levels compared with those observed in the sham group (P< 0.001).Treatment with the plant extract at all concentrations(AA+50,AA+75,AA+100)and dexamethasone significantly reduced lipid peroxidation activity compared with saline treatment (control group) (P< 0.001) (Figure 6).
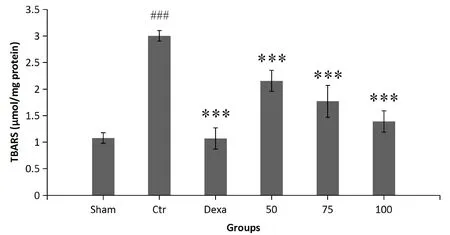
Figure 6 The effect of P.farcta (50, 75, and 100 mg/kg/day) on lipid peroxidation as TBARS in colon.###, significant difference with sham (P < 0.001); ***, significant difference with control (P <0.001).Values are expressed as mean ± SEM.Ctr, control; Dexa,dexamethasone; 50, 75, and 100, groups receiving 50, 75, and 100 mg/kg/day of the extract.SEM, standard error of the mean; TBARS,thiobarbituric acid-reactive substance.
Colonic MPO activity.Colitis induction caused a significant increase in MPO activity in the control group compared to that in the sham group (P< 0.001).MPO levels were considerably lower in rats treated with 100 mg/kg of the extract and dexamethasone than in control group animals (P< 0.001) (Figure 7).
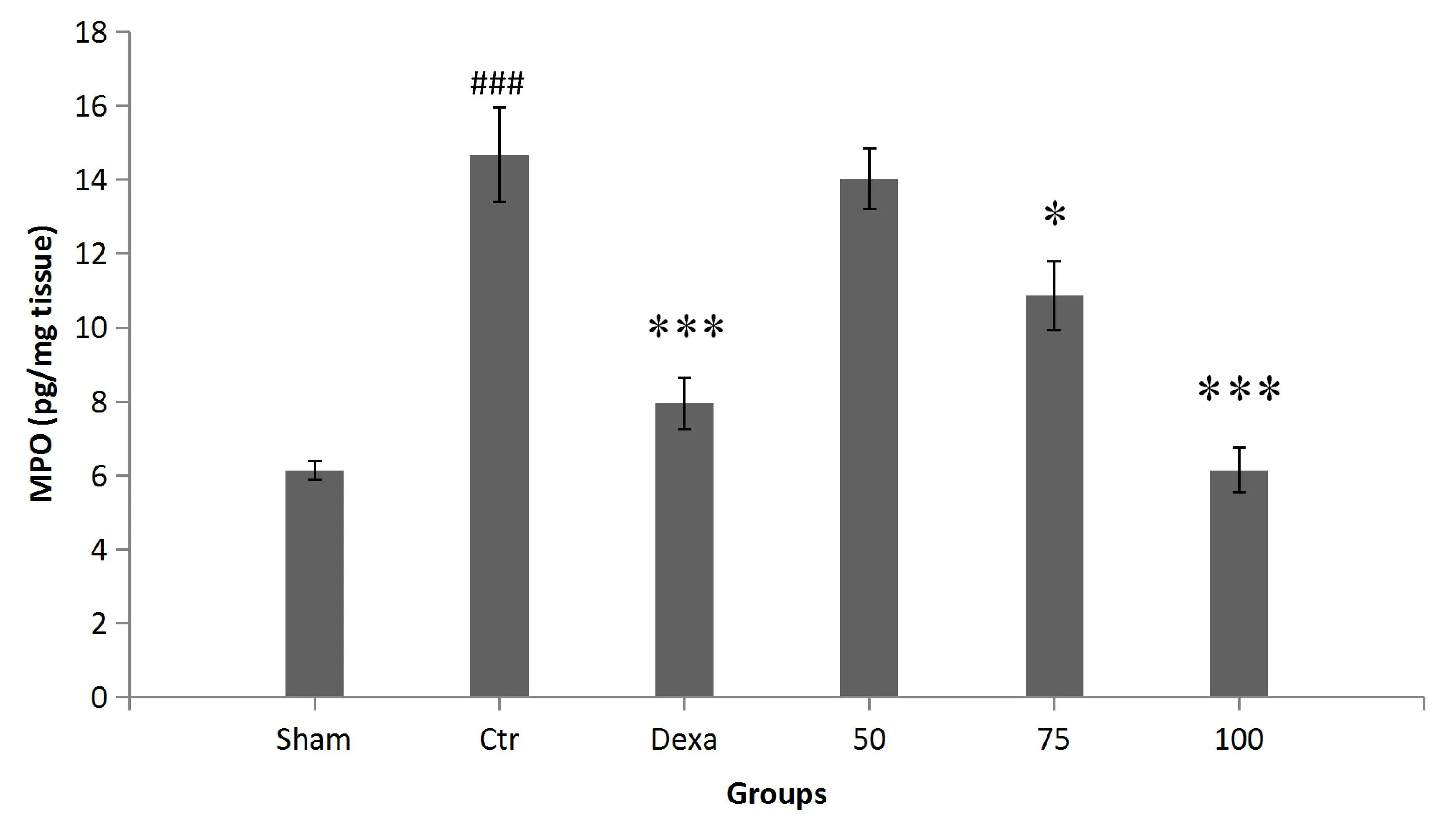
Figure 7 The effect of P.farcta (50, 75, and 100 mg/kg/day) on MPO activity in colon tissue.###,significant difference with sham(P< 0.001);*, significant difference with control (P < 0.05),***,significant difference with control (P < 0.001).Values are expressed as mean ± SEM.Ctr, control; Dexa, dexamethasone; 50, 75, and 100,groups receiving 50, 75, and 100 mg/kg/day of the extract.SEM,standard error of the mean; MPO, myeloperoxidase.
Discussion
IBD is one of the most common diseases of the gastrointestinal tract,which affects the quality of life of those affected and incurs many costs to patients.Due to the lack of certain therapies for IBD management,the exploration of new medications is still ongoing.A hydroalcoholic extract ofP.farctafruits was investigated in an AA-induced colitis rat model through macro- and micro-histological analyses as well as determining oxidative and inflammatory markers.Our findings showed thatP.farctacould ameliorate macro- and microscopic manifestations of colitis, by inhibiting inflammatory mediators (IL-1β,TNF-α), reducing oxidation activity by suppressing MPO and lipid peroxidation, and by providing potent antioxidant activity.
The AA-induced colitis animal model is well-known in experimental investigation of IBD.During the establishment of this animal model,intrarectal administration of AA leads to non-transmural inflammation.This condition is similar to the pathogenesis of human colitis,characterized by increased neutrophil infiltration into the intestinal tissue, massive necrosis of mucosal and submucosal layers, vascular dilation, edema, and submucosal ulceration [40].It has been proposed that IBD might be a result of an imbalance between oxidants and antioxidants.For instance, the infiltration of neutrophils leads to superoxide anion production and initiates a cascade of events,resulting in excessive production of various reactive species that contribute to tissue necrosis and mucosal dysfunction [41].
The current study showed thatP.farctacould improve AA-induced colitis in rats, mainly through its antioxidant and anti-inflammatory activities.We found thatP.farctaat all investigated concentrations(AA + 50, AA + 75, and AA + 100) significantly reduced tissue damage by reducing lipid peroxidation in rat colon tissue.It was previously shown thatP.farctadecreased malondialdehyde in the testes of rats [22], and other studies confirmed the reduction of malondialdehyde in rat hepatocytes byP.farcta[42–44].In the present study,P.farctareduced MPO expression in the colonic mucosa,particularly at a dose of 100 mg/kg.An increase in MPO is a quantitative marker of disease severity, indicating that the activity ofP.farctaagainst MPO might be correlated with its anti-inflammatory action[38].
We also demonstrated thatP.farctadownregulated the tissue levels of IL-1β, especially when administered at a dose of 100 mg/kg.IL-1β is a potent inflammatory cytokine involved in the modulation of autoimmune inflammation [45].A recent study reported that IL-1β is a mediator of enhanced microvascular thrombosis, which mainly occurs in extra-intestinal tissues during colonic inflammation [46].In addition to its anti-inflammatory activity,P.farctacan prevent thromboembolic events in patients with IBD by inhibiting IL-1β activity.Currently, IL-1β is considered one of the main clinical biological markers of ulcerative colitis.TNF-α is also considered a biological marker for evaluating the severity of ulcerative colitis in patients [47].It has been shown that the amount of TNF-α is elevated in individuals with ulcerative colitis and is likely an important component in the pathophysiology of IBD [48].Our results indicated that all investigated concentrations ofP.farctacould reduce the levels of TNF-α, representing the therapeutic importance ofP.farctain controlling ulcerative colitis, since anti-TNF-α medicaments are recommended for IBD [49].
From a chemical point of view,P.farctacontains significant amounts of gallic acid and other bioactive phytochemicals.In addition,gallic acid can improve ulcerative colitis in rats [50]; thus, the presence of these bioactive substances might be another reason for the significant efficacy ofP.farctain ulcerative colitis.
To the best of our knowledge,this is the first study on the effect ofP.farctafruit extract on experimental colitis.In addition to the positive effects of the extract on inflammatory and oxidative biomarkers,macroscopic and microscopic histopathological evidence showed thatP.farctaimproves colitis using an animal model.Moreover, its low toxicity and market availability make this herb a good candidate for further experimental and clinical studies.
Conclusion
The reported study supports the therapeutic effect ofP.farctain ulcerative colitis.Inhibition of oxidative markers and suppression of inflammatory mediators are the main mechanisms underlying the therapeutic effect ofP.farctain ulcerative colitis.The chemical content, antioxidant activity, and anti-inflammatory properties ofP.farcta,as well as its healing effect in ulcerative colitis, make it worth considering for investigation in clinical trials.
 Traditional Medicine Research2021年5期
Traditional Medicine Research2021年5期
- Traditional Medicine Research的其它文章
- Neuroprotective effect and mechanism of daidzein in oxygen-glucose deprivation/reperfusion injury based on experimental approaches and network pharmacology
- Understanding the prevention and cure of plagues in Daoist medicine
- Efficacy of Xianglian pill for antibiotic-associated diarrhea:a protocol for systematic review and meta-analysis
- Effects of Dendrobium candidum polysaccharides on microRNA-125b and mitogen-activated protein kinase signaling pathways in diabetic cataract rats
- Research advances concerning the mechanism of glucocorticoid resistance in relation to traditional Chinese medicine for patients with chronic obstructive pulmonary disease
- Light and color therapy: the role of light and color in architecture from the perspective of traditional Persian medicine
