Neuroprotective effect and mechanism of daidzein in oxygen-glucose deprivation/reperfusion injury based on experimental approaches and network pharmacology
Ming-Hua Xian,Si-Kai Zhan,Ke-Ning Zheng,Qu-liu,Ke-Ning Li,Jia-Yin Liang,Shu-Mei Wang*
1Key Laboratory of Digital Quality Evaluation of Chinese Materia Medica of State Administration of Traditional Chinese Medicine, Guangzhou 510006, China.2Engineering & Technology Research Center for Chinese Materia Medica Quality of the Universities of Guangdong Province, Guangzhou 510006,China.3School of Traditional Chinese Pharmacy,Guangdong Pharmaceutical University,Guangzhou 510006,China.4School of Pharmacy,Guangdong Pharmaceutical University,Guangzhou 510006,China.5School of Traditional Chinese Pharmacy,China Pharmaceutical University,Nanjing 211198,China.
Abstract Background:Daidzein, phytoestrogens derived from the Pueraria lobata (Willd.)Ohwi root used in traditional Chinese medicine, has a wide range of biological activities, including antioxidant,anti‑inflammatory, and neuroprotection.However, the neuroprotective role of daidzein in oxygen-glucose deprivation/reperfusion injury and its underlying mechanism are still unknown.Methods: In this study, we used pheochromocytoma cells induced by oxygen-glucose deprivation and reperfusion to study the potential effect in the protection of the nerve cells.Then, we used molecular docking simulation and network pharmacology to predict the possible targets and pharmacological pathways of daidzein.Western blot was used to verify the expression of target proteins with or without adding the inhibitors.Results: After daidzein treatment, cell vitality had an upward trend (P < 0.05) and the release of lactate dehydrogenase had a downward trend (P < 0.01) in dose-dependent compared with the model group by exposure to oxygen-glucose deprivation and reperfusion.Several core targets were analyzed through network pharmacology and molecular docking including catalase, peroxisome proliferator-activated receptor gamma, vascular endothelial growth factor A, interleukin-6,tumor necrosis factor, nitric oxide synthase 3, prostaglandin-endoperoxide synthase 2, and RAC-alpha serine/threonine kinase 1.These results suggest that catalase may be a first-ranked target for the neuroprotective role of daidzein.Gene Ontology enrichment analysis indicated the pathways mainly contained molecule metabolic process,while Kyoto Encyclopedia of Genes and Genomes enrichment analysis focus on pathways in terms of inflammation such as tumor necrosis factor signal pathway.Then, Western blot results showed that daidzein had a significant increase on the expression of protein catalase (P < 0.01).Daidzein reversed catalase level alterations after oxygen-glucose deprivation reperfusion injury in a dose-dependent manner which was consistent with the catalase antagonists-based experiments.Conclusion:These outcomes provide new insights into the neuroprotective effect and mechanism of daidzein in oxygen-glucose deprivation/reperfusion injury.
Keywords: daidzein; neuroprotection; catalase; oxygen-glucose deprivation and reperfusion;network pharmacology
Tradition
Pueraria lobata(Willd.)Ohwi root,widely known asRadix Puerariae(Gegen in Chinese)is one of the earliest medicinal herbs utilized in ancient China which first appeared in the Chinese bookShennong’sClassic of Materia Medica(traditionally attributed to Shennong and written between about 200 C.E.and 250 C.E.).With the research of its medicinal property,the therapeutic role ofRadix Puerariaehas been gradually discovered and various compounds extracted fromRadix Puerariaehave been found to be useful in the treatment of stroke.In 2004,Yan B et al.found that the ethanol extract ofRadix Puerariaehas an antidepressant effect in the treatment of poststroke depression.In 2011,Wong KH et al.have introduced the potential medicinal benefits ofPueraria lobata(Willd.)Ohwi root in diabetes and cardiovascular diseases including stroke.In 2016,a research among stroke patients suggested that receive traditional Chinese medicine includingRadix Puerariaecould improve a series of symptoms after suffering the stroke.Recent years,there were many researches have suggested that puerarin,a compound ofRadix Puerariae,could reduce infarction volume and ameliorated functional neurological outcome.
Background
Ischemic stroke, a type of cerebrovascular disease, is one of the deadliest diseases, endangering people’s daily lives [1].Most ischemic strokes are caused by thromboembolism with less oxygen and energy supply, which subsequently leads to severe damage of both brain tissue and neuron [2].Because ischemic stroke always occurs suddenly, preventive treatments are difficult to implement, even when patient risk factors have been identified.An ischemic stroke frequently causes long-term brain tissue damage, resulting in a reduction in motor, sensory, or cognitive functions [3, 4].Fibrinolytic therapy has been the most common treatment for cerebral stroke for decades [5].Neuroprotective therapies were promising in stroke.Therefore, it remains an urgent need to find compounds for neuroprotection or nerve recovery during or after stroke[6, 7].
To meet this demand, it was preferable in discovering candidates of active ingredients from traditional Chinese medicines.Pueraria lobata(Willd.) Ohwi (Kudzu in English) root, widely known asRadixPuerariae(Gegen in Chinese) is one of the earliest medicinal herbs utilized in ancient China which first appeared in the Chinese bookShennong’s Classic of Materia Medica(traditionally attributed to Shennong and written between about 200 C.E.and 250 C.E.)(Figure 1)[8].With the research of its medicinal property, the therapeutic role ofPueraria lobatahas been gradually discovered and various compounds extracted fromPueraria lobatahave been found to be useful in the treatment of stroke.In 2004, Yan B et al.found that the ethanol extract ofPueraria lobatahas an antidepressant effect in the treatment of poststroke depression.In 2011, Wong KH et al.have introduced the potential medicinal benefits ofPueraria lobata(Willd.)Ohwi root in diabetes and cardiovascular diseases including stroke [9].In 2016, a research among stroke patients suggested that receive traditional Chinese medicine includingPueraria lobatacould improve a series of symptoms after suffering the stroke [10].Recent years,there were many researches have suggested that puerarin, a compound ofPueraria lobata, could reduce infarction volume and ameliorated functional neurological outcome[11, 12].
Figure 1 The whole plant,medicinal site and active components of Pueraria lobata (Willd.) Ohwi root
Daidzein is a component of thePueraria lobata(Willd.) Ohwi root,has a history of usage in traditional Chinese medicine of more than 2,000 years to treat diabetes and cardiovascular diseases [13, 14].Daidzein has been shown to reduce neurological deficits by increasing neuronal count and enhancing hind limb activity [14].According to the result of the high-throughput screen and our previous research,daidzein was selected as a potential compound for the treatment of ischemic stroke.Due to its estrogenic effects, daidzein has been shown to have neuroprotective properties against brain injury and to influence various neurobiological regulating mechanisms such as behavior, cognition, and development [16].These effects are mediated by daidzein’s interaction with various signaling molecules and receptors, resulting in neuroprotection [15].Although daidzein’s neuroprotective effect has been reported, it remains unclear its mechanism of action in ischemic stroke.
To explore the mechanism of daidzein, combining the network pharmacology and molecular docking is a prospective approach for exploring the multi-targets regulation networks.Network pharmacology has been authenticated to be an essential way to investigate the pharmacological mechanism of effective compounds by incorporating bioinformatics, cheminformatics, and network biology [17, 18].Molecular docking is a well-established structure-based in silico process that is commonly used in drug discovery [19].In this study, we found the potential of daidzein protective effects on oxygen-glucose deprivation/reoxygenation(OGD/R) model in vitro by a high-throughput screen.Then, we combined molecular docking and network pharmacology to investigate the protective mechanism of daidzein in stroke.
Materials and methods
Chemical and reagents
Daidzein (98%; MUSF12042606) was purchased from Chengdu Chroma-Biotechnology Co., Ltd.(Chengdu, China).Nimodipine(66085-59-4)was purchased from Aladdin Co., Ltd.(Shanghai,China).Amitrol(61-82-5)was purchased from TCI Co.,Ltd.(Shanghai,China).Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Institute(Kumamoto, Japan).Lactate dehydrogenase (LDH) activity assay kit was purchased from Nanjing Jiancheng Bioengineering Institute(Nanjing, China).
Cell culture
The differentiated pheochromocytoma cells (PC12 cells) were derived from the Shanghai Institute of Cell Biology and its identification was done by Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China).The cells were grown in Dulbecco’s modified eagle medium in the presence of high glucose level, 10%heat-inactivated fetal bovine serum, streptomycin (100 g/mL), and penicillin (100 U/mL).Furthermore, the cells were transported to a chamber of 37°C containing 5% CO2.
OGD/R procedure and experiment grouping
In 96-well plates, PC12 cells (6 × 103cells/well) were plated and incubated for 24 hours before OGD/R.The experiment groups were divided into six different groups including the control group, model group, positive drug group and different concentrations of daidzein-treated groups.The model group and the control group were incubated in standard medium, while the positive drug control group was incubated with 5 μmol/L nimodipine, and the treatment group was incubated with 5, 20, and 50 μmol/L daidzein [20].After incubation for 24 hours, Earle’s balanced salt solution containing Na2S2O4(5 mmol/L) was added to all groups except the control group for 80 minutes.Lastly, all groups were placed in a normal culture medium (Dulbecco’s modified eagle medium with glucose) and incubator (with O2) for 24 hours of reperfusion.
Cell viability assay
To investigate the protective effect of daidzein on PC12 cells subjected to OGD/R, the viability of the cells was assessed using the CCK-8.PC12 cells were seeded in 96-well plates at a density of 6 × 103cells per well for 24 hours.Then, the cells were incubated with different concentrations (5, 20, and 50 μmol/L) of daidzein.After drug treatment and OGD/R, 10 μL of CCK-8 solution was added to each well and incubated at 37 °C for 2 hours.After incubation, we measure the absorbance at 450 nm using a multimode reader to obtain the viability of cell in each group.
Cell toxicity assay
PC12 cells were seeded in 96-well plates at a density of 6 × 103cells per well for 24 hours.Then, the cells were incubated with different concentrations (5, 20, and 50 μmol/L) of daidzein.After OGD/R procedure, the LDH assay was determined using an LDH assay kit(Jiancheng, Nanjing, China) according to the instructions of the manufacturer to assess cell toxicity.The LDH assay reagent was mixed with the collected cell medium supernatant.Absorbance was detected at 450 nm using a multimode reader (Bio-Rad Laboratories,CA,USA).
Target prediction
The information of ischemic stroke-related disease-targets was found from the GeneCards database (https://www.genecards.org/) and the target genes of ischemic stroke using only “Homo sapiens”.Then,targets from the GeneCards database which score over 10 were selected.
The information needed to predict molecular targets for daidzein was obtained from both TCMIP (http://www.tcmip.cn/TCMIP/index.php) and TCMSP (https://tcmspw.com/index.php) [21].Molecular targets whose similar score over 0.8 in TCMIP were selected and the default targets were totally chosen in TCMSP.
Prediction of “daidzein-ischemic stroke” common targets and construction for their protein-protein interaction (PPI) network
We used VENNY (version 2.0) to analyze the intersection targets between daidzein and ischemic stroke.The common targets were then added to the STRING database to create the PPI network interaction.The PPI network was built and visualized using Cytoscape 3.7.2 software.For topological analysis of PPI networks, CytoNCA, a network topology analysis plug-in in Cytoscape was used [22].Degree centrality, closeness centrality and betweenness centrality were used to screen the core targets of daidzein.Based on the literature, we chose the targets with degree centrality over 20, betweenness centrality and closeness centrality which over median value in all nodes, as the more accurate core target.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analysis
The KEGG and GO enrichment analysis of the common targets was performed using the David database (https://david.ncifcrf.gov/)which helped identify the molecular function and systemic involvement of target genes [23].According to the results of enrichment, the information about the processes and pathways involved in the core targets was visualized by R 4.3 software.Statistical significance threshold of enrichment analysis was set atP<0.05.
Construction and analysis of “daidzein -target-pathway-disease”network
Cytoscape 3.7.2 software was used to construct the connections among daidzein, targets, pathways, and diseases by making a network diagram, to systematically analyze the potential targets and pathways of daidzein in the treatment of stroke.
Component–target molecular docking
We used both ChemDraw and Chem3D to get a three-dimensional structure of daidzein.The RCSB PDB database(https://www.rcsb.org/)was used to obtain the three-dimensional structure of targeted proteins.Then, to improve protein structure, water molecules and the co-crystalline ligand was removed, but hydrogen atoms were added and the side chain was fixed.The ligand-based mode generated the binding site.Used the Surflex-Dock program in SYBYL-X 2.1.1 software, daidzein were docked into the ligands binding sites of these proteins in the way of semi-flexible docking and the total scores of Surflex-Dock represent binding affinities.
Western blot analysis
Based on molecular docking findings, we use 20 µg protein samples of cellular lysates were separated by protein electrophoresis (10%SDS-PAGE), followed by their transfer onto polyvinylidene fluoride membranes (Millipore, Burlington, MA, USA).After immersed in sealed liquid (5% nonfat milk) for 1 hour at room temperature, the membrane was incubated with catalase(CAT)-rabbit antibody(1:1000;ab209211; Abcam) at 4 °C overnight.After several time washing with TBST buffer solution, the membranes were then subjected for incubation with goat anti-rabbit IgG for secondary antibodies (1:5000;BA1054; BOSTER) for 2 hours at room temperature.With the help of an electrochemiluminescence detection reagent (Thermo Fisher Scientific, MA, USA), the resulting protein bands were visualized.Glyceraldehyde-3-phosphate dehydrogenase was used as a reference protein for normalization.Image J software was used to analyze the grey levels of the protein bands.
Verification experiment in vitro
To verify the objective goal of daidzein, the cells were divided into different classes including the control group, model group, positive drug nimodipine group (5 µmol/L), daidzein group (50 µmol/L),daidzein (50 µmol/L) + inhibitor group, and single inhibitor group.Based on the previous experiment, we used amitrol (20 µmol/L)protein based on the result of our previous study as the small molecule inhibitors of CAT protein and added it to the inhibitor group [24].The medicine treatment and OGD/R procedure were used according to the method mentioned in OGD/R procedure and cell treatment.After the OGD/R, cell viability was tested by CCK-8 assay.
Statistical analysis
The mean and standard deviation are used to express the data.A one-way study of variance was used to determine the statistical significance of the variations between groups.The statistical analyses were carried out using GraphPad Prism (version 8.0.1).P< 0.05 indicated that the difference was statistically important.
Result
Daidzein enhance the viability of PC12 cells injured by OGD/R
We first looked into the neuroprotective effects of daidzein on toxicity and cell viability under OGD/R conditions by pretreating PC12 cells for 24 hours with various concentrations of daidzein (5, 20, and 50 μmol/L).According to the result of the CCK-8 assay, we found significantly lower viability in the model group (54.70% ± 0.89%,P< 0.01) than in control cells, indicating the potential of OGD/R to cause significant cell injury under in vitro experiments.Also, such viability was higher in groups treated with various concentration of daidzein than in the model group which indicating that daidzein could improve the cells viabilities (high-dose group: 65.10% ± 2.06%,intermediate-dose group: 64.40% ± 1.85%, low-dose group: 62.50%±1.33%;P<0.05 vs.the model group, Figure 2A).Furthermore, the LDH assay showed significantly enhanced LDH leakage in the model group (193.00% ± 2.73%) in comparison to the control group(20.80% ± 3.87%;P< 0.01).while daidzein can reduce the LDH release compared with the model group (high-dose group: 132.00% ±6.10%, intermediate-dose group: 115.00% ±1.82%, low-dose group:109.00% ± 6.58%;P< 0.01, Figure 2B).These outcomes confirm that daidzein can improve the viability of PC12 cells and reduce OGD/R cytotoxicity which suggested its potential protection of stroke.
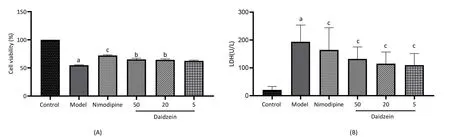
Figure 2 Effect of daidzein on cells exposed to OGD/R injury (n = 6).(A) Daidzein improved the cell viability of OGD/R-injured cells using CCK-8 assay.(B) Daidzein decreased the release of LDH from OGD/R-injured cells.a, P <0.01 vs.control group; b, P < 0.05 vs.model group; c, P< 0.01 vs.model group.LDH, lactate dehydrogenase; OGD/R, oxygen-glucose deprivation/reoxygenation.
Interaction of putative targets and construction for PPI network
UniProt database was used to correct irregular targets’ names.Then,we screened 141 daidzein compound targets predicted by TCMIP and TCMSP, respectively.After removing the duplicates, a total of 137 related targets were obtained (Figure 3A).From the GeneCards database, a total of 422 genes of ischemic stroke were retrieved using a correlation score > 10 as the screening criteria.There were 29 daidzein targets in the anti-ischemic stroke damage that were selected for further study after being processed by the Venn diagram (Figure 3B).In the ischemic stroke therapy,we chose 29 candidate targets that were the core targets of daidzein, then submitted them to STRING to construct the PPI network.Cytoscape software was used for further analysis.The PPI network exhibited strong correlations among complex interlaced networks and targets (Figure 3C and Figure 3D).Among them, hub genes included CAT, peroxisome proliferator-activated receptor gamma (PPARG), vascular endothelial growth factor A (VEGFA), interleukin-6 (IL6), tumor necrosis factor(TNF), nitric oxide synthase 3 (NOS3), prostaglandin-endoperoxide synthase 2 (PTGS2), and RAC-alpha serine/threonine kinase 1 (AKT1)(Supplementary Table S1).According to the results of Venn and PPI network, some hub targets associated with the therapeutic effect of daidzein were screened out and its mechanism needed to be further analyzed.Thus, we can choose probably the most effective target for verification.
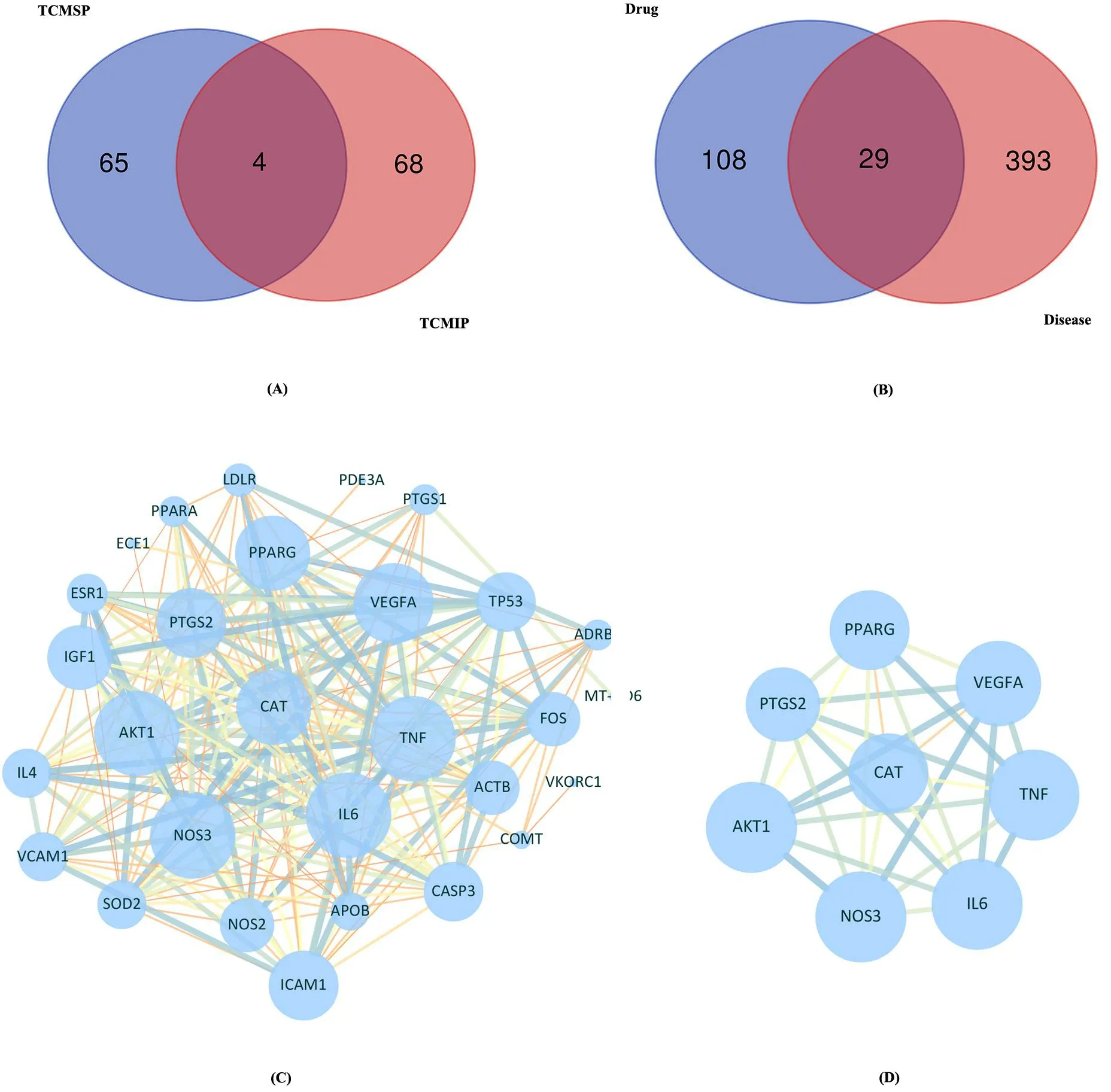
Figure 3 Venn diagram and construction of PPI network.(A) Venn diagram of daidzein.(B) Venn diagram of the prediction of“daidzein-ischemic stroke” common targets.(C) PPI network of 29 candidate targets.(D) PPI network of hub targets.The larger nodes in the ring represent more essential hub nodes.Other nodes are represented by the smaller nodes.The thicker lines represent stronger interaction between the proteins.The blue circle represents the hub targets.PPI, protein-protein interaction.
GO and KEGG pathway analysis
Using the David database, the GO process gene functions of 29 potential targets were analyzed, and biological process, molecular function, cellular component correlation scatter plots were generated.The findings of the biological process analysis focused primarily on the response to lipopolysaccharide, the response to bacteria-derived molecules, and the regulation of small-molecule metabolic processes,etc., suggesting the effect of daidzein on regulating metabolic rate.According to enrichment analysis of cellular component, the enrichment analysis mainly including membrane raft, membrane region, and membrane microdomain, etc.Some research showed that most membrane lipid classes have key roles in the hormonal control of plant development and regulate their metabolism [25].Also, the organization of lipid-driven membrane domains are essential in the integrity and function of brain which suggested that daidzein probably acts on the cell membrane [26].Molecular function terms mainly contained with heme binding and antioxidant binding, etc.,suggest that daidzein was closely related to oxidation-reduction reactions(Figure 4A).
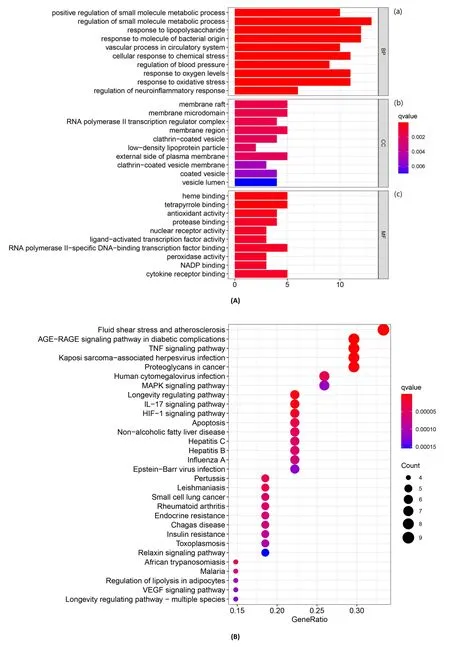
Figure 4 GO and KEGG pathway analysis.(A) GO enrichment analysis (biological process, molecular function, cellular component).(B) KEGG pathway enrichment analysis of core targets.The abscissa represents the proportion of the targets and the ordinate represents the ID of enriched terms.The length of the column refers to the number of genes involved in each pixel.Color from red to blue represents the q value from small to large which means the depth of color is proportional to the degree of enrichment.GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
KEGG pathway enrichment was conducted to cluster the major effects associated with daidzein.A total of 30 top-ranking pathways screened out according to their q value and mainly were associated with lipid metabolism and inflammation (Figure 4B).Based on the enrichment result of KEGG and literature researches, TNF-α was chosen to make further discussion since it is involved in every stage of stroke-related neuronal injury, including inflammatory and prothrombotic events.Therefore, the relationship of daidzein in the treatment of OGD/R and stroke between TNF-α signal pathway was worthy to be further study.By using pathway analysis, it is suggested that daidzein may play a therapeutic role through multiple pathways,especially some related to inflammation.At the same time, it also provides some basis for selecting the appropriate target in following experiment.
Construction and analysis of “daidzein-target-pathway-disease”network
To intuitively reflect the effect of daidzein on ischemic stroke, we constructed a network figure of “daidzein-target-pathway-disease” via Cytoscape software based on the above-mentioned “daidzein-disease”target, disease, and top 10 ranking KEGG pathway (Figure 5).The pattern above shows intuitively how daidzein works in the treatment of stroke and reflect its complex mechanism.
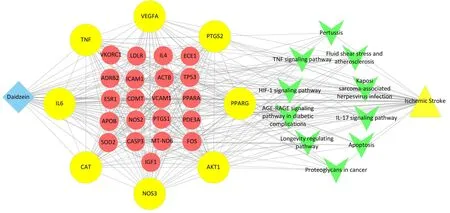
Figure 5 Construction and analysis of “daidzein-target-pathway-disease” network.The rhombus nodes represent daidzein, the red circle represents the candidate targets, the yellow circle represents the core targets, node size means a more important role.Green “V” nodes represent pathways, and the triangle nodes represent“ischemic stroke”.Edges represent the interactions of each part in the network.
Molecular docking and verification test
To see whether daidzein played a role in the regulation of the putative targets, researchers used molecular docking.A total of 8 hub targets obtained by Cytoscape 3.4.1 software through Network Analyze were docked with daidzein using the SYBYL 2.1.1 software (Supplementary Table S2).The greater the binding activity, the higher the docking score.
Thus, we used the PyMol 2.3.4 software to visualize daidzein and CAT docking results and performed an verification test (Figure 6A).According to the result of western blot, we found that the CAT protein levels were substantially downregulated in the model group compared to the control group (P< 0.01).Also, upon comparison with the model group, the expression of CAT protein was upregulated in the nimodipine and daidzein groups at different concentrations (P<0.01).More importantly, there was a dose-dependent relationship between the expression of CAT protein and the concentration of daidzein (Figure 6B and Figure 6C).Based on the results of molecular docking and western blot, we used amitrol (inhibitor of CAT protein)for further validation and the protective effect of daidzein on OGD/R injured cells was detected by the CCK-8 assay.The results showed that daidzein + amitrol group could reduce the viability of cells as compared to the amitrol group (P< 0.05) (Figure 6D).These indicated that the neuroprotective effects of daidzein might directly depend on CAT.CAT may be the most essential target for the therapeutic effect in the treatment of OGD/R model according to molecular docking.The descent of CAT expression after OGD/R injury could be restored by different concentrations of daidzein and its role in enhancing cell viability could be attenuated by using CAT inhibitor.
Discussion
We discovered that daidzein has a protective effect on PC12 cells that have been impaired by the OGD/R model and can boost cell vitality[27, 28].Also, it can relieve cytotoxicity by reducing the release of LDH [29].The results suggested that daidzein may be an active compound for the treatment of ischemic stroke.However, its specific mechanism needs further study.Thus, we used the network pharmacology method to obtain the active target of daidzein and 29 potential targets related to the intervention of cerebral ischemia were identified.Then, using topological parameters, we screened and obtained 8 primary targets, including CAT, PPARG, VEGFA, IL6, TNF,NOS3, PTGS2, and AKT1.
IL-6 is a kind of inflammatory cytokine that is significantly elevated in the acute ischemic stroke stage and can be used as a neurotrophic mediator to participate in the repair process of cerebral injury after ischemia [30].VEGFA, a member of the VEGF family, plays an important role in angiogenesis, neurogenesis, and neuroprotection just after cerebral ischemia [31].AKT1 is one of the serine/threonine protein kinases, which is involved in the regulation of both survival and cell proliferation and has an anti-apoptotic effect [32].According to some research, silencing the PTGS2 gene inhibits the nuclear factor kappa-B (NF-κB) signaling pathway, promoting endothelial progenitor cell proliferation, migration, and angiogenesis while inhibiting apoptosis [33].NOS3, an isoform of NOS, can confer protection during ischemia primarily by causing vasodilatation and improving cerebral circulation [34].PPARG is a transcription factor that exerts a role in atherosclerosis and other diseases, some research suggested the importance of activation of PPARG in nerve repair [35].TNF-α is involved in every stage of stroke-related neuronal injury, including inflammatory and prothrombotic events.TNF-α is involved in the central nervous system.TNF-α has been shown to have an essential role within the central nervous system [36].CAT has long been thought to be a key regulator of oxidative stress, with the basic purpose of breaking down toxic H2O2into water and oxygen[37].Also,CAT is one of the antioxidant factors involved in ROS scavenging.After cerebral ischemia-reperfusion, oxidative stress-induced neuronal apoptosis is a primary pathological process and often lead to the damage of cerebral vascular and blood-brain barrier [38].
Based on the above mentioned reasons, CAT could be an important target for preventing neuron apoptosis and improve the damage of brain vascular during the stroke.Daidzein may be able to control the recovery of ischemic stroke from multiple aspects by analyzing the position of critical targets.The findings of network pharmacology were confirmed by molecular docking, which revealed that daidzein had a high binding activity with certain receptor proteins, with CAT having the highest binding activity.As a result, we based on the CAT objective in the following experiments.
Next, to validate the findings of network pharmacology and molecular docking, cell culture experiments were carried out.Then,western blotting documented that daidzein downregulated the expression of CAT in OGD/R-injured cells.To further investigate whether the daidzein plays a neuroprotective role by directly targeting CAT, we introduced the antagonists of CAT.The results indicated that the involvement of the activated CAT in the neuroprotection by daidzein in PC12 cells injured by OGD/R (Figure 6C).TNF-α is a significant determinant of inflammatory responses in stroke patients [39].Some researchers hypothesized that CAT effectively reduced intracellular ROS produced by the cell culture system and mitigated the negative effects of TNF-α [40].Network pharmacology has sowed the relation of daidzein impact on ischemic stroke with TNF signaling pathway.TNF signaling pathway induces the activation of many genes, regulates cell apoptosis and necrosis,and inflammatory response.Furthermore, daidzein is an estrogen mimic that promotes cell proliferation and prevents neuronal loss,suggesting that it may protect neurons from damage via CAT targets during ischemic stroke.However, the potential relationship between the CAT and TNF signaling pathways in stroke needs to be investigated further.
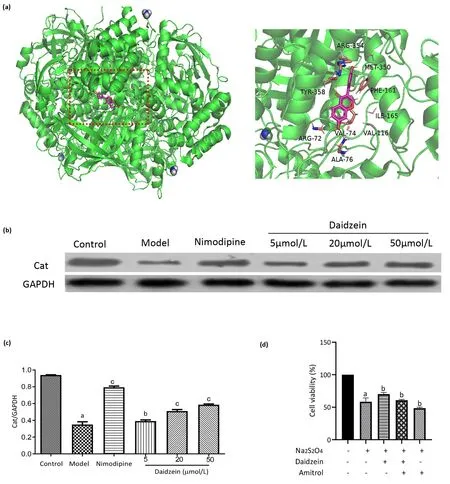
Figure 6 Molecular docking and verification test.(A) Docking results of daidzein with CAT.The red dotted box marks the site where CAT binds to daidzein and its large version is on the right.(B)CAT protein bands.(C)CAT protein expression relative densities(n=3).a,P<0.01 vs.control group; b, P < 0.05 vs.model group; c, P < 0.01 vs.model group.(D) Further verification of CAT proteins to the effects of OGD/R injured cells.a,P < 0.01 vs.control group; b, P < 0.05 vs.model group; c, P < 0.01 vs.model group.CAT, catalase; OGD/R, oxygen-glucose deprivation/reoxygenation.GAPHD, glyceraldehyde-3-phosphate dehydrogenase
It is difficult to properly explain the daidzein mechanism in middle cerebral artery occlusion/reperfusion, and our work has some limitations.First, we used differentiated PC12 cells to mimic neurons in the neuroprotection experiments.Despite the fact that PC12 cells can also represent neurons, there are several differences between PC12 cells and primary cultured neurons.
According to the bioinformatics analysis and experimental validation performed in the current study, it is suggested that daidzein ameliorates the damage of neurons associated with oxygen-glucose deprivation by regulating the CAT expression.Besides, through its antioxidant mechanism, CAT can scavenge the reactive oxygen with toxicity and inflammatory factors generated during the stroke phase,thus playing a neuroprotective role.
Conclusion
This study discovered daidzein’s neuroprotective activity and confirmed that CAT mediates its effects.Daidzein may play a role in the pathological processes of necroptosis and inflammation.This work helps to understand the neuroprotective effect of daidzein on neuronal damage induced by OGD/R.The conclusions reached, however, are based on bioinformatics and in vitro experiments and must be confirmed in vivo.
 Traditional Medicine Research2021年5期
Traditional Medicine Research2021年5期
- Traditional Medicine Research的其它文章
- The protective effect of a standardized hydroalcoholic extract of Prosopis farcta(Banks&Sol.)J.F.Macbr.fruit in a rat model for experimental ulcerative colitis
- Understanding the prevention and cure of plagues in Daoist medicine
- Efficacy of Xianglian pill for antibiotic-associated diarrhea:a protocol for systematic review and meta-analysis
- Effects of Dendrobium candidum polysaccharides on microRNA-125b and mitogen-activated protein kinase signaling pathways in diabetic cataract rats
- Research advances concerning the mechanism of glucocorticoid resistance in relation to traditional Chinese medicine for patients with chronic obstructive pulmonary disease
- Light and color therapy: the role of light and color in architecture from the perspective of traditional Persian medicine
