大黄?虫丸中17种质量标志物的含量测定
付传奎 许珂嘉 张子蒙 黄艳 陈志鹏 李伟东 吴丽

中图分类号 R286.0;R917 文献标志码 A 文章编号 1001-0408(2021)19-2353-05
DOI 10.6039/j.issn.1001-0408.2021.19.08
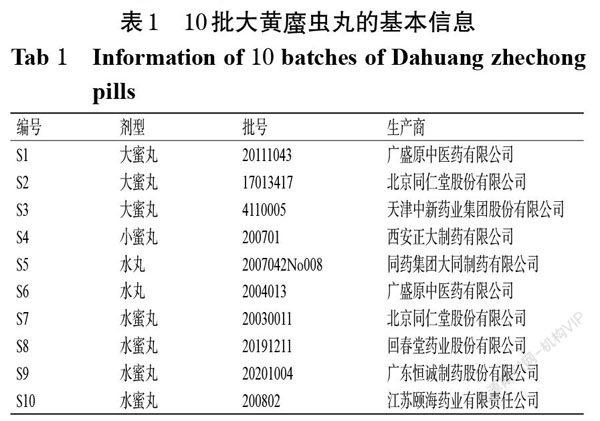
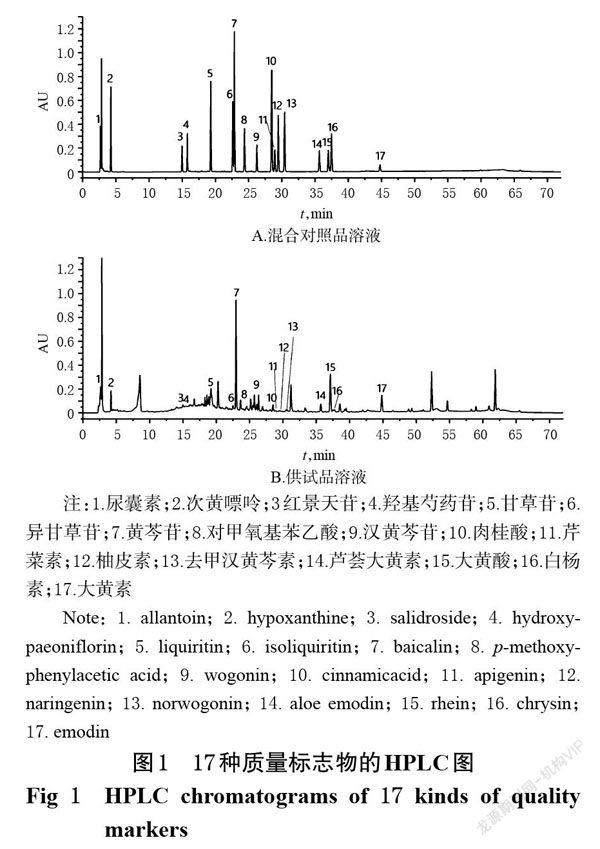
摘 要 目的:建立同时测定大黄?虫丸中17种质量标志物含量的方法。方法:采用高效液相色谱(HPLC)法测定10批市售大黄?虫丸中尿囊素、次黄嘌呤、红景天苷、羟基芍药苷、甘草苷、异甘草苷、黄芩苷、对甲氧基苯乙酸、汉黄芩苷、肉桂酸、芹菜素、柚皮素、去甲汉黄芩素、芦荟大黄素、大黄酸、白楊素、大黄素等17种质量标志物的含量。色谱柱为Kromasil 100-5-C18(250 mm×4.6 mm,5 μm),流动相为0.1%磷酸溶液-乙腈(梯度洗脱),流速为1.0 mL/min,柱温为30 ℃,检测波长为210 nm,进样量为20 μL。结果:上述17种质量标志物的质量浓度依次在5.74~183.53、6.51~208.24、4.30~137.65、4.60~147.06、4.12~131.76、4.25~135.88、6.31~201.76、4.60~147.06、1.94~62.06、4.47~142.94、0.69~22.06、2.29~73.24、2.33~74.41、1.42~45.29、6.65~212.94、1.11~35.44、1.47~47.06 μg/mL范围内与各自峰面积呈良好的线性关系(R2均不小于0.999 0)。该方法精密度、重复性、稳定性、耐用性的RSD均小于2%(n=6);17种质量标志物的平均加样回收率范围为96.31%~101.73%,RSD均小于3%(n=6)。结论:本方法简便、快速,专属性、精密度、重复性、稳定性、准确度和耐用性均良好,可用于完善大黄?虫丸的质量标准。
关键词 大黄?虫丸;质量标志物;高效液相色谱法;质量标准;含量测定
Content Determination of 17 Quality Markers in Dahuang Zhechong Pills
FU Chuankui1,XU Kejia1,ZHANG Zimeng1,HUANG Yan2,CHEN Zhipeng1,LI Weidong1,WU Li1(1. School of Pharmacy, Nanjing University of TCM, Nanjing 210023, China; 2. Anhui Province Laboratory of Inflammation and Immune Mediated Diseases, Anhui Medical Universtiy, Hefei 230032, China)
ABSTRACT OBJECTIVE: To establish the method for the content determination of 17 quality markers in Dahuang zhechong pills (DHZCP). METHODS: HPLC method was adopted to determine the contents of 17 quality markers in 10 batches of DHZCP, such as allantoin, hypoxanthine, salidroside, hydroxypaeoniflorin, glycyrrhizin, isoglycyrrhizin, baicalin, p-methoxyphenylacetic acid, wogonin, cinnamic acid, apigenin, naringin, norwogonin, aloe emodin, rhein, chrysin, emodin. The determination was performed on Kromasil 100-5-C18 (250 mm×4.6 mm, 5 μm) column with mobile phase consisted of 0.1% phosphoric acid solution-acetonitrile (gradient elution) at the flow rate of 1.0 mL/min. The column temperature was 30 ℃, the detection wavelength was 210 nm and the sample size was 20 μL. RESULTS: The linear range of above 17 quality markers were 5.74-183.53, 6.51-208.24, 4.30-137.65, 4.60-147.06, 4.12-131.76, 4.25-135.88, 6.31-201.76, 4.60-147.06, 1.94-62.06, 4.47- 142.94, 0.69-22.06, 2.29-73.24, 2.33-74.41, 1.42-45.29, 6.65-212.94, 1.11-35.44 and 1.47-47.06 μg/mL, respectively (all R2≥0.999 0). RSDs of precision, repeatability, stability and durability tests were all less than 2% (n=6); average recovery of 17 quality markers ranged from 96.31% to 101.73%, and the RSDs were less than 3% (n=6). CONCLUSIONS: The method is simple, rapid, speific, specise, reproducible, stable, accurate and durable, and can be used for improving the quality standard of DHZCP.

