右归丸中5种重金属元素的含量分析
李存金 谢婷 周云峰 邓杰华 吴喆 黄招光

中图分类号 R917 文献标志码 A 文章编号 1001-0408(2021)19-2377-06
DOI 10.6039/j.issn.1001-0408.2021.19.12
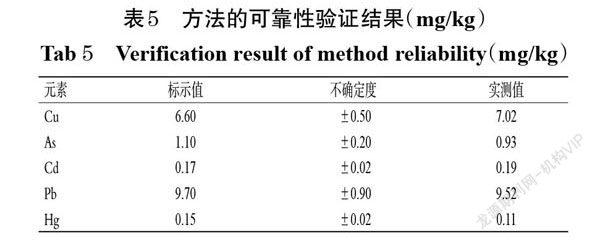
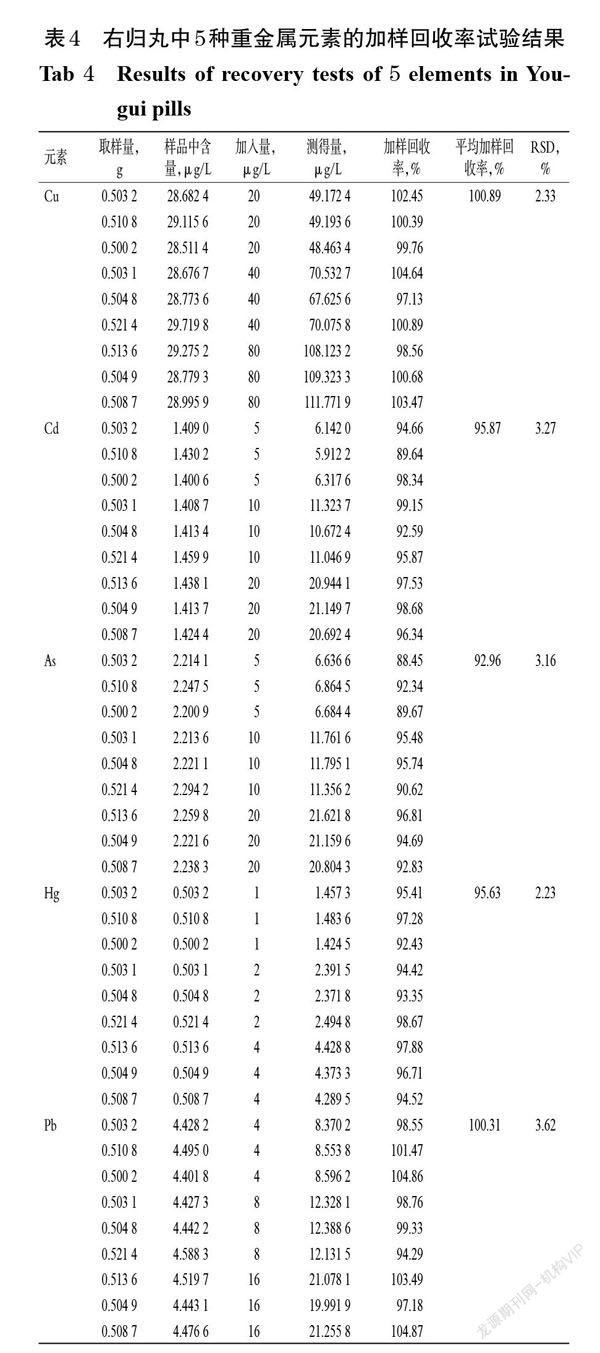
摘 要 目的:考察市售右归丸中铜(Cu)、砷(As)、镉(Cd)、汞(Hg)、铅(Pb)等5种重金属元素的含量,并评价其安全风险。方法:以钇(89Y)、铟(115In)、铋(209Bi)为内标,采用电感耦合等离子体质谱(ICP-MS)法测定各元素的含量。设置ICP-MS的条件为雾化气流量0.95 L/min,辅助气流量1.2 L/min,等离子体气(氩气)流量18 L/min,泵速30 r/min;设置电感耦合等离子体射频功率為1 200 W,模拟阶段电压为-1 750 V,脉冲阶段电压为1 300 V,偏转装置电压为-12 V,检测器为模拟和脉冲双模式。对各元素含量测定方法进行方法学考察,并对45批市售右归丸样品进行测定。运用危害指数(HI)对各元素非致癌性风险进行分析,并拟定各元素的最大残留阈值(MRL)。结果:Cu、As、Cd、Hg、Pb检测质量浓度线性范围分别为10~200、1~50、0.4~30、0.2~6、2~100 μg/L, r均大于0.999 0;定量限分别为0.67、0.23、0.20、0.07、0.27 μg/L,检测限分别为0.20、0.07、0.06、0.02、0.08 μg/L;精密度、稳定性、重复性试验的RSD均小于3.5%(n=6或n=5);平均加样回收率为92.96%~100.89%,RSD为2.23%~3.62%(n=3)。45批右归丸样品中Cu、As、Cd、Hg、Pb的平均含量分别为2.72、0.28、0.07、0.05、0.62 mg/kg,各元素的叠加HI小于1。右归丸中5种重金属元素的含量均低于拟定的MRL(Cu、As、Cd、Hg、Pb的MRL分别为20、2、1、0.2、5 mg/kg或者111.11、4.44、2.22、1.48、8.89 mg/kg)。结论:所建方法可用于测定右归丸中5种重金属的含量;市售右归丸的常见重金属污染率低,安全风险小。
关键词 右归丸;重金属;电感耦合等离子体质谱法;限度;最大残留阈值;危害指数
Content Analysis of 5 Kinds of Heavy Metal in Yougui Pills
LI Cunjin1,XIE Ting2,ZHOU Yunfeng3,DENG Jiehua3,WU Zhe3,HUANG Zhaoguang3(1. The Fourth Inspection Institute of Jiangxi Drug Inspector Center, Jiangxi Yichun 336000, China; 2. Science and Education Department, Yichun Municipal Peoples Hospital, Jiangxi Yichun 336000, China; 3. Drug Department, Yichun Institute for Food and Drug Inspection, Jiangxi Yichun 336000, China)
ABSTRACT OBJECTIVE: To investigate the contents of 5 kinds of heavy metal as copper (Cu), arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) in Yougui pills, and to evaluate its safety risk. METHODS: Using yttrium (89Y), indium (115In) and bismuth (209bi) as internal standard, the contents of each element were determined by ICP-MS. ICP-MS condition included that atomization gas flow rate was 0.95 L/min,auxiliary gas flow rate was 1.2 L/min,plasma gas (argon) flow rate was 18 L/min, pump speed was 30 r/min. RF power of inductively coupled plasma was 1 200 W, the voltage in simulation stage was 1 750 V, the voltage in pulse stage was 1 300 V, the voltage of deflection device was -12 V, and the detector was in analog and pulse dual-mode. The determination methods of various elements were investigated, and 45 batches of marketed Yougui pills were determined. Hazard index (HI) was used to analyze the non-carcinogenic risk of each element and calculate the maximum residual limit (MRL) of each element. RESULTS: The linear range of Cu, As, Cd, Hg and Pb ranged from 10-200, 1-50, 0.4-30, 0.2-6 and 2-100 μg/L (all r>0.999 0), respectively. The limits of quantitation were 0.67, 0.23, 0.20, 0.07, 0.27 μg/L. The limits of detection were 0.20, 0.07, 0.06, 0.02, 0.08 μg/L. RSDs of precision, stability and reproducibility tests were all lower than 3.5% (n=6 or n=5). Average recoveries were 92.96%-100.89% (RSD=2.23%-3.62%, n=3). Average contents of Cu, As, Cd, Hg and Pb in 45 batches of Yougui pills were 2.72, 0.28, 0.07, 0.05, 0.62 mg/kg, and superimposed HI of each element was less than 1. The contents of 5 kinds of heavy metals in Yougui pills were lower than the proposed MRL (MRL of Cu, As, Cd, Hg and Pb were 20, 2, 1, 0.2, 5 mg/kg or 111.11, 4.44, 2.22, 1.48, 8.89 mg/kg respectively). CONCLUSIONS: Established method can be used for the determination of content of 5 kinds of heavy metal in Yougui pills; the heavy metal pollution rate of marketed Yougui pills is low and the safety risk is small.

