Efficacy and adverse reactions of apatinib as secondline or later-line treatment in advanced lung cancer
Tao Ren ,Yan Wu
1 Department of Oncology,The First People’s Hospital of Nanning,Nanning 530022,China
2 Department of Pharmacy,The First Affiliated Hospital of Xi’an Medical University,Xi’an 710077,China
Abstract Objective The objective was to investigate the prognostic factors of advanced lung cancer treated with apatinib by log-rank regression analysis.Methods Sixty patients with advanced stage lung cancer confirmed at The First People’s Hospital of Nanning between January 2018 and December 2018 who had received a second-line treatment or a treatment above this level were included.All patients were treated with 425 mg/d apatinib orally for a 28-day course of treatment.Log-rank regression analysis of remission rates,disease control rates,adverse events,and prognostic factors in all patients was performed.Results After treatment,the total remission rate was 6.7%,and the disease control rate was 61.7%(37/60).Progression-free survival was 3.2 ± 0.1 months,and overall survival was 5.3 ± 0.5 months.The overall incidence of grade 3–4 adverse reactions was 15.0% (9/60),and these adverse reactions were significantly relieved by reducing the drug dose or suspending drug use.Differentiation degree,Eastern Cooperative Oncology Group (ECOG) score,and adverse reactions were all important and independent risk factors affecting the prognosis of patients (P <0.05).Conclusion Apatinib treatment could effectively inhibit the progress of the disease for patients with advanced lung cancer and prolong their survival with relatively mild toxicity and side effects,which is beneficial to patient tolerance.Moreover,the degree of differentiation,ECOG score,and adverse reactions could affect the prognosis of patients.
Key words: Log-rank regression analysis;apatinib;advanced lung cancer;prognostic factors
Lung cancer is a malignant tumor with relatively high morbidity and mortality worldwide,and most patients are at the advanced stage of lung cancer at the time of diagnosis,resulting in a low 5-year survival rate[1].When lung cancer develops to an advanced stage,lymph nodes and cancer cells infiltrate into adjacent tissues and metastasize to distant tissues and organs;thus,the best opportunity for surgery is often missed upon diagnosis[2].Currently,patient conditions are effectively alleviated through radiotherapy,chemotherapy,and targeted therapy.Platinum-combined chemotherapy can effectively kill cancer cells,prolong the disease-free life of patients,and significantly improve their quality of life;however,recurrence is frequent after one year of treatment[3].Radiotherapy can effectively control tumors;however,there are still limitations in the treatment of patients with advanced lung cancer whose tumors have metastasized[4].As the first generation of oral anti-angiogenic drugs in China,apatinib,whose main target is vascular endothelial growth factor receptor (VEGFR)-2,has shown significant efficacy in the treatment of advanced stomach cancer,significantly prolonging the total survival of patients with advanced gastric cancer for whom second-line treatment has failed[5–6].Researchers have also found that apatinib combined with conventional chemotherapy has a good effect on patients with advanced non-small cell lung cancer[7].However,the clinical efficacy of apatinib alone for the treatment of patients with advanced lung cancer for whom second-line treatment has failed has not been reported.In this study,patients with advanced lung cancer for whom second-line treatment or a treatment above this level failed were treated with 425 mg/d of apatinib administered orally in a 28-day course of treatment,to explore the efficacy and safety of this treatment.
Materials and methods
Patient population
In this study,a total of 60 patients with advanced lung cancer were included.The enrolled patients received therapy at The First People’s Hospital of Nanning between January 2018 and December 2018.The patients included 38 males and 22 females;their age was 39–75 years (average 64.1 ± 4.6),12 patients had small cell lung cancer,48 patients had non-small cell lung cancer;28 cases showed poor differentiation,22 showed moderate differentiation,and 10 showed high differentiation.The inclusion criteria were as follows:(1) patients for whom second-line treatment or a treatment above this level failed,(2) all patients who presented with pathologically confirmed advanced lung cancer,and (3) patients with a life expectancy of at least 3 months.The exclusion criteria were as follows:(1) patients with severe cardiac dysfunction,(2) mental disorders that lead to an inability to cooperate with the researchers,(3) pregnant and lactating women,and (4) patients who had received surgical treatment.This study was approved by the Ethics Committee of The First People’s Hospital of Nanning.Written informed consent was obtained from all patients for the use of their clinical information and for obtaining their blood and DNA samples.
Methods
All patients were treated with apatinib alone as follows:oral administration of 425 mg apatinib (Jiangsu Hengrui Pharmaceutical Co.,Ltd.,China) once a day,30 min after a meal for 28 days (28-day course).During the treatment period,if the patients showed adverse reactions,the dosage could be reduced to 250 mg/d.
Observational index
Clinical efficacy evaluation
Patients were evaluated for clinical efficacy,mainly using the solid tumor efficacy evaluation criteria,which were mainly divided into progressive disease (PD),stable disease (SD),partial remission (PR),and complete remission (CR).The total response rate=(PR+CR)/total number of cases,and the disease control rate=(PR+CR+SD)/total number of cases.Progression-free survival refers to the time from the patient’s further disease development or from being enrolled to drug intolerance.The overall survival is the time from enrollment to death.
Adverse reaction monitoring
The occurrence of adverse reactions in patients was observed and classified into grade 1,2,3,or 4,depending on the severity of the adverse reactions,according to the World Health Organization criteria for the evaluation of the toxic side effects of antineoplastic drugs,with higher grades indicating that the adverse reactions are relatively serious.
Factor analysis
Single and multifactorial analyses of factors affecting patient prognosis,including patient age,gender,pathology type,degree of tissue differentiation,tumor diameter,tumor stage,Eastern Cooperative Oncology Group (ECOG) score,and grade of adverse events,were performed.
Statistical analyses
The data were analyzed by SPSS 21.0,and theχ2(%) test was used for counting.The log rank method was used for univariate analysis,and prognostic factors were screened.The Cox regression model was used for multivariate analysis to confirm the independent influencing factors.P<0.05 showed significant differences.
Results
Clinical efficacy
After treatment,CR accounted for 0.0% (0/60),PR accounted for 6.7% (4/60),SD accounted for 55.0%(33/60),PD accounted for 38.3% (23/60),the total remission rate was 6.7%,and the disease control rate was 61.7% (37/60).The progression free survival was 3.2 ± 0.1 months,and the overall survival was 5.3 ± 0.5 months.
Occurrence of adverse effects
The overall incidence of grade 3–4 adverse reactions was 15.0% (9/60),which was significantly improved by either dose reduction or suspension of drug administration,as shown in Table 1.
Single-factor analysis of the prognosis of patients with advanced lung cancer
The degree of differentiation,ECOG score,and adverse effects were all important factors (P<0.05) affecting patient prognosis,as shown in Table 2.
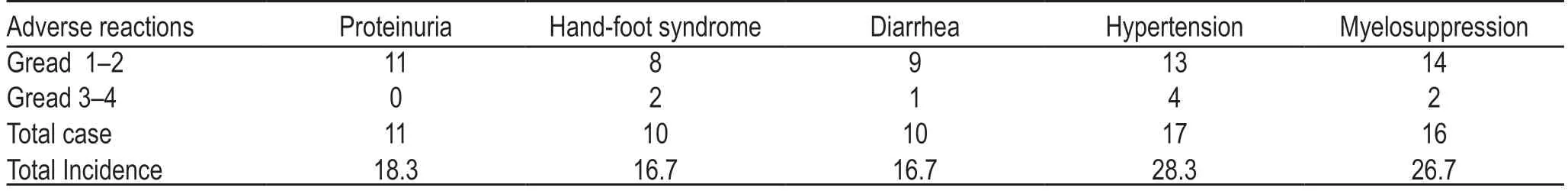
Table 1 Occurrence of adverse effects
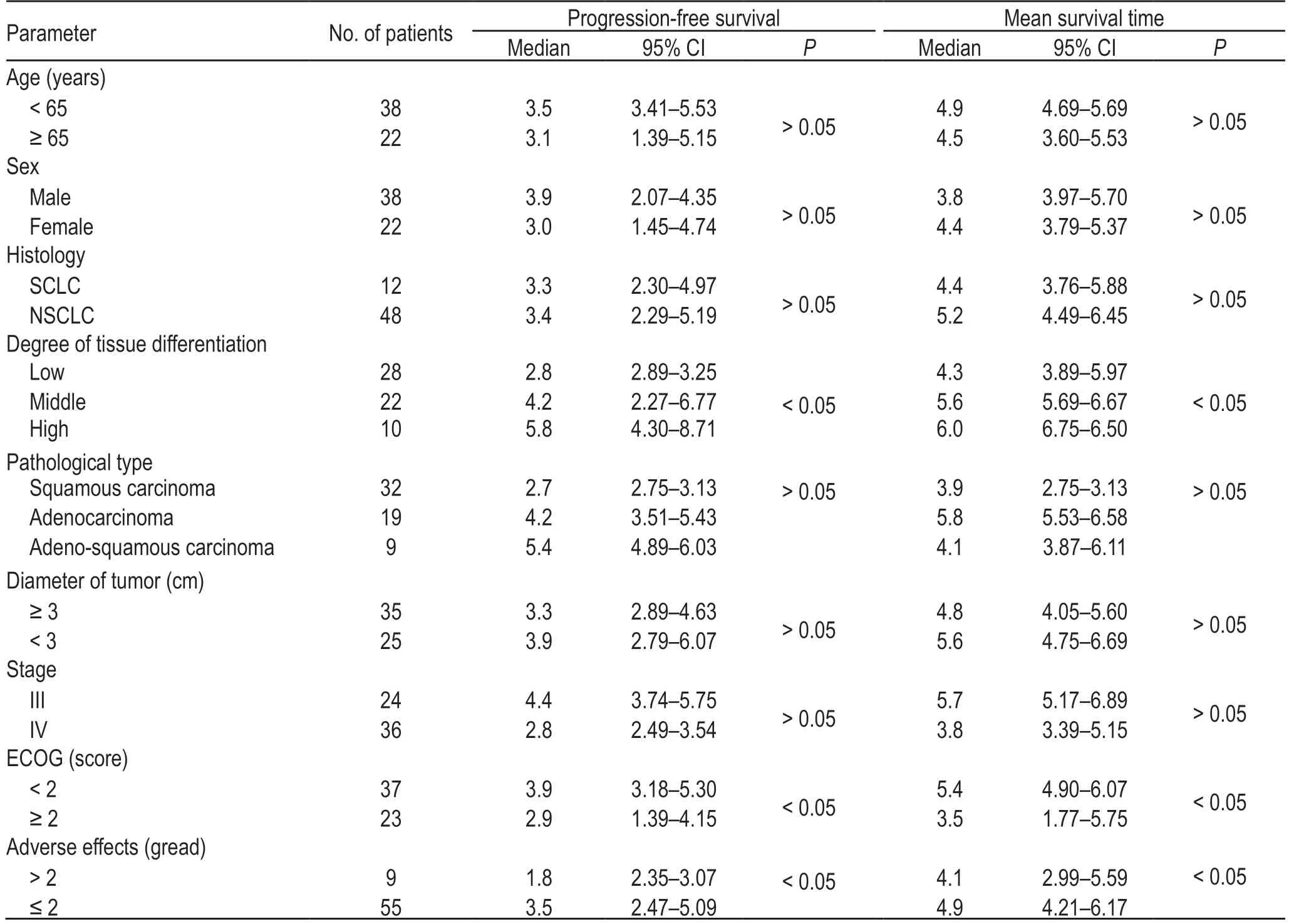
Table 2 Single-factor analysis of the prognosis of patients with advanced lung cancer (eg)
Multifactorial analysis of the prognosis of patients with advanced lung cancer
The degree of tissue differentiation,ECOG score,and adverse events were all independent risk factors for patient prognosis (P<0.05;Table 3).
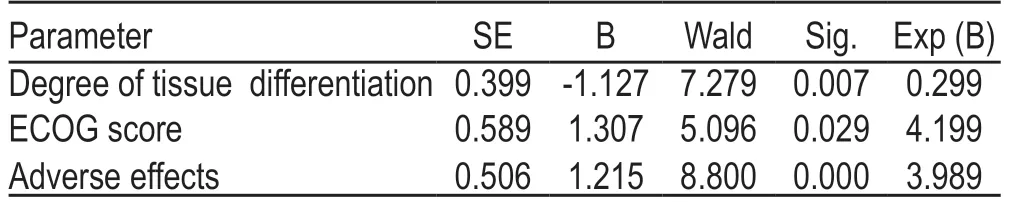
Table 3 Multifactorial analysis of the prognosis of patients with advanced lung cancer (eg,%)
Discussion
Lung cancer is a serious threat to human life and health,and the incidence rate in male patients is higher than that in women[1].Traditional chemotherapy can kill tumor cells as well as normal tissue cells,resulting in serious adverse reactions in the body[3].The index of chemotherapy is relatively narrow,the specificity is poor,and drug resistance leads to disease recurrence,which is why targeted therapy is gradually replacing treatment using chemical drugs[2].In recent years,targeted epidermal growth factor receptor tyrosine kinase inhibitors (EGFRTKIs),which are relatively well tolerated by patients with few side effects,have been widely used.Relevant studies have shown that the use of EGFR-TKIs can be applied to the treatment of patients with a positive EGFR gene mutation,and a good therapeutic effect can be obtained[8].Despite the significant initial efficacy of targeted drug therapy,drug resistance occurs in this treatment as well,similar to chemotherapy.With the progressing drug administration time,the likelihood of developing resistance increases,reducing the effectiveness of later treatment and eventually leading to further development of the disease[9].Apatinib is a first-generation oral antiangiogenic drug invented in China that targets VEGFR-2 and receptor tyrosine kinases (RTKs),such as c-kit,RET,and c-src[10].VEGF-2 has now been found to promote endothelial cell proliferation during angiogenesis by activating the mitogen-activated protein kinase signaling pathway[11].By blocking VEGFR-2,apatinib can inhibit endothelial cell proliferation,which ultimately leads to anti-angiogenesis,and this has been shown to have antitumor effects in a variety of cancers[12].This also provides the theoretical basis for the treatment of lung cancer with apatinib.The present study,which assessed the effect of apatinib treatment by log-rank analysis,found that patients were treated with an overall remission rate of 6.7% and a disease control rate of 61.7%.Progressionfree survival (3.2 ± 0.1 months) and overall survival (5.3 ±0.5 months) were observed.Therefore,the use of apatinib for patients with advanced lung cancer could effectively control the disease.Because apatinib is an angiogenesis inhibitor with high affinity,it can effectively block the transmission of downstream signaling pathways with a significant inhibitory effect on the neovascularization of endothelial cells[13].Sun Luet al.showed that apatinib effectively regulates and contributes to the process of kinase phosphorylation by inhibiting extracellular signals downstream of the signaling pathway[14].In recent years,several clinical trials have confirmed that apatinib effectively exerts its unique antitumor effect in the treatment of breast[15],gastric[16],and colorectal cancer[17],as well as other malignant cancers.Chemotherapeutic drugs have a more general cytotoxic effect,and they can cause damage to normal tissue cells of the body while killing cancer cells,thus leading to patient intolerance and relatively poor treatment compliance.Apatinib,in contrast,makes it easier for patients to tolerate and accept the treatment[18–19].The present study showed that the overall incidence of grade 3-4 adverse reactions was 15.0%,and the adverse reactions were significantly reduced after dose reduction or suspension of drug administration.Thus,apatinib has a relatively mild adverse reaction profile in the treatment of patients with advanced lung cancer,which is more conducive to treatment acceptance by patients.In this study,data were analyzed separately for regression using the log-rank method.The results showed that the degree of differentiation,ECOG score,and adverse reactions could influence patient prognosis,and all the three were independent risk factors.This shows that the factors that affect the prognosis of patients with advanced lung cancer are mainly the degree of tissue differentiation,ECOG score,and severity of adverse reactions.However,the influence of other factors on the prognosis of patients with advanced lung cancer needs further confirmation via a larger sample size in order to make the results of the study more accurate and reliable.
In summary,the treatment of patients with advanced lung cancer using apatinib could effectively inhibit the progression of the disease and prolong the survival of patients,with relatively mild toxic side effects that could be well tolerated by patients.In addition,the degree of differentiation,ECOG score,and adverse effects could have an impact on patient prognosis.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年4期
Oncology and Translational Medicine2021年4期
- Oncology and Translational Medicine的其它文章
- Mechanism of tumor synthetic lethal-related targets
- Relationship between miR-7-5p expression and 125 I seed implantation efficacy in pancreatic cancer and functional analysis of target genes*
- The clinical efficacy of percutaneous ethanol-lipiodol injection (PEI) combined with high-intensity focused ultrasound (HIFU) for small hepatocellularcarcinoma in special or high-risk locations*
- Enhancing the treatment effects of tumor cell purified autogenous heat shock protein 70-peptide complexes on HER-3-overexpressing breast cancer *
- Relationship between molecular changes in epidermal growth factor receptor (EGFR)and anaplastic lymphoma kinase (ALK)mutations in lung adenocarcinoma *
- The expression of vascular endothelial growth factor (VEGF)/ endostatin (ES) and VEGF receptor 2(VEGFR2)/ES is associated with NSCLC prognosis *
