Enhancing the treatment effects of tumor cell purified autogenous heat shock protein 70-peptide complexes on HER-3-overexpressing breast cancer *
Xia Chen ,Xiaoming Zhang ,Xiangji Lu ,Meng Ren ,Rina Su ,Weishi Gao ,Yanwei Gao (✉)
1 Department of Apheresis,Inner Mongolia Red Cross Blood Center,Hohhot,Inner Mongolia 010010,China
2 Department of Ultrasound medicine,Inner Mongolia People’s Hospital,Hohhot,Inner Mongolia 010017,China
3 Department of General Surgery,Inner Mongolia Armed Police Hospital,Hohhot,Inner Mongolia 010010,China
4 Department of Surgical Oncology,Inner Mongolia People’s Hospital,Hohhot,Inner Mongolia 010017,China
Abstract Objective The aim of this study was to enhance the treatment effect of tumor purified autogenous heat shock protein 70-peptide complexes (HSP70-PCs) on HER-3-overexpressing breast cancer.Methods In this study,we first studied the expression of HER-3 in breast cancer tissues and its relationship with patient characteristics.We then purified HSP70-PCs from primary breast cancer cells with different HER-2 and HER-3 expression profiles and determined the cytotoxicity of autogenous dendritic cells (DCs) and CD8+T cells induced by these complexes.Third,recombinant human HSP70-HER-3 protein complexes were used to inhibit the autogenous HSP70-PCs purified from HER-3–overexpressing breast cancer cells,and the resulting immunological response was examined.Results The results show that HSP70-PCs can be combined with recombinant HSP70-HER-3 protein complexes to induce stronger immunological responses than autogenous HSP70-PCs alone and that these treatments induce autogenous CD8+T cell killing of HER-3-positive breast cancer cells.Conclusion These findings provide a new direction for HSP70-DC-based immunotherapy for patients with HER-3-overexpressing breast cancer.
Key words: heat shock protein 70 peptide complexes (HSP70-PCs);HER-3 protein;recombinant protein;dendritic cells (DCs);cellular immunotherapy
Breast cancer is the most common cancer in women,and the incidence of breast cancer increases every year.The Globocan 2012 data,released by the International Agency for Research on Cancer,reports that there are approximately 1,671,000 new cases of breast cancer reported worldwide each year,with 522,000 reported deaths from breast cancer[1].
HER-2 is the most important therapeutic target in breast cancer,with several novel therapeutics showing a high degree of efficacy for HER-2-positive breast cancers.These include Herceptin.However,no effective treatment has been identified for HER-2-negative breast cancer.Therefore,current research is focused on identifying novel therapeutic targets for HER-2-negative breast cancer and developing effective treatment strategies for these malignancies[2–3].
Cellular immunotherapy,a form of biological tumor therapy,is one of the most effective treatments for breast cancer.These therapies use biological agents to isolate,activate,and transfuse the patient’s own or allogeneic tumor-specific and nonspecific killer cells.Many approaches for cellular immunotherapy targeting tumors have been developed and dendritic cell (DC)-based active immunotherapy shows good potential as a novel strategy for breast cancer.DCs are the most powerful antigen presenting cells (APCs) and can activate cytotoxic T lymphocytes[4–5].
Heat shock protein 70 (HSP70) is an important molecular chaperone that binds to tumor antigen peptides in tumor cells to form HSP70-peptide complexes (PCs).Tumor-derived HSP70-PCs can specifically interact with DCs and drive their antigen presentation.Once the antigen is presented,the immune response will produce a series of antigen-specific cytotoxic CD8+T cells.Our previous study showed that we could purify HSP70 complexes from HER2-2-overexpressing breast cancers(HSP70-HER-2-PCs),and that these HSP70-HER-2-PCs produced more comprehensive tumor antigen peptides and induced stronger immune activity against the tumor cells from which the complex was derived[6–7].However,this method did not achieve decent results in HER-2-negative breast cancers.Recent studies have shown that HER-3 may be a potential target for many kinds of tumors;however,this result remains controversial[8–9].
In this study,we evaluated whether HER-3 can be used as a target for HSP70-DC-based cell immunotherapy.We also explored whether our established method could be used against breast cancers with high HER-3 expression.
Materials and methods
Patient selection and postoperative tumor tissue treatment
We selected 70 patients with breast cancer who were scheduled to undergo radical surgery at the Oncology Department of the Inner Mongolia People’s Hospital between January 2017 and December 2018.No patient had received preoperative chemotherapy or radiotherapy.The age of the patients ranged from 27 to 68 years,and all patients were females;all pathological types were diagnosed as invasive breast cancer by fine needle puncture and cytological examination.Postoperative tumor tissues were divided into two sections:one section was used for postoperative pathology and immunohistochemical examination (HER-2 and HER-3 staining),and the other section was used for primary cell culture.The study was approved by the Human Research Ethics Committee at the Inner Mongolia People’s Hospital and all of the patients provided informed consent for the collection of the tissue samples.In cases where primary tumor cell culture was successful we went on to isolate peripheral blood mononuclear cells (PBMCs) from the peripheral blood samples of the same patients.
Immunohistochemistry
Tissue samples from primary tumors were fixed in 10% neutral buffered formalin and embedded in paraffin.Serial sections (4 μm) were stored in a deep freezer(-20℃) until immunostaining could be completed.For antigen retrieval,the sections were immersed in citrate buffer (pH 6.0),and samples were heated in a pressure cooker.The primary antibodies were mouse anti-human HER-2 monoclonal antibody and rabbit anti-human HER-3 monoclonal antibody.Isotype-matched rabbit IgG or mouse IgG was used as a negative control.Following incubation with primary antibody,sections were incubated with horseradish peroxidase (HRP)-labeled goat anti mouse or rabbit antibody and immunoreactions were visualized using diaminobenzidine.
HER-2 expression was scored using the following parameters:(a) the intensity of the membrane staining(score 0:no staining,score 1:+,score 2:++,score 3:+++),and (b) the percentage of HER-2-positive cells (score 1:1–25%,score 2:26–50%,score 3:51–75%,and score 4:>75%).The total score was calculated as the sum of the two separate scores and ranged from 0 to 7.A score of 3 or higher was considered positive for HER-2 immunostaining and marked as HER-2 (+).
The HER-3 sections were scored as follows:(a) the intensity of the cytoplasmic staining (score 0:no staining,score 1:+,score 2:++,score 3:+++),(b) the percentage of HER-3-positive cells (score 1:0–25%,score 2:26–50%,score 3:51–75%,and score 4:>75%),and (c) the presence or absence of membrane staining (score 0:absent;score 1:present).The total score was calculated as the sum of these three scores and ranged from 0 to 8.A score of 4 or higher was considered positive for HER-3-immunostaining and marked as HER-3 (+).
Breast cancer primary cell culture
The breast cancer primary cell culture was completed as previously described,with some modifications[10].The resected breast cancer tissues were immediately placed in ice-cold RPMI-1640 medium containing 100 U/mL penicillin G and 100 μg/mL streptomycin,and transported to the laboratory within 10 min.After the removal of the necrotic tissues,the samples were rinsed twice in phosphate buffered saline (PBS) and cut into small fragments.These fragments were then incubated with 1% collagenase type II in a gently shaking water bath for 1 h at 37°C before being passed through a 38-μm mesh sieve.The resulting cell suspension was then washed twice and centrifuged at 300 ×gfor 10 min before the cells were diluted to 5 × 105cells/mL and incubated in RPMI-1640 supplemented with 10% heat inactivated fetal calf serum (FCS) at 37°C and 5% CO2.The fibroblasts were removed by reducing the FCS concentration to 5%during the second week of culture and then returned to 10% in the third week.Cells were passaged at 75%confluency.
Grouping
Patients and their corresponding breast cancer primary cells were divided into four groups based on their HER-2 and HER-3 immunohistochemistry results:Group A was HER-2 (+) and HER-3 (+),group B was HER-2 (+) and HER-3 (-),Group C was HER-2 (-) and HER-3 (+) and Group D was HER-2 (-) and HER-3 (-).Six cases were randomly selected from each group for analysis.
Purification of autogenous HSP70-PCs
Purifications of the autogenous HSP70-PCs were performed as previously described[7].The breast cancer primary cells were heated in a water bath at 42°C for 12 h,and then allowed to recover for 2 h at 37°C and 5% CO2.After heat shock,the tumor cells were digested using 0.02% trypsin,and then 5×106cells from each cell line were homogenized for 15 min on ice in a hypotonic buffer consisting of 50 mM Tris-HCl,150 mM NaCl,1 mM phenylmethylsulfonyl fluoride,1 mM sodium fluoride,and 5 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) at pH 7.2.After ultrasonication at 0°C for 30 min,the homogenate was centrifuged at 10,000 ×gfor 90 min at 4°C before the supernatant was dialyzed against buffer A (20 mM Tris-HCl,150 mM NaCl,1 mM CaCl2,1 mM MnCl2,0.5 mM phenylmethylsulfonyl fluoride,and 15 mM β-mercaptoethanol,pH 7.4) overnight at 4°C.The sample was then loaded onto a ConA-Sepharose column and unbound protein was collected at a flow rate of 12 mL/h.The fraction of interest was then dialyzed against buffer B (20 mM Tris-HCl,20 mM NaCl,3 mM MgCl2,1 mM MnCl2,0.5 mM phenylmethylsulfonyl fluoride and 15 mM β-mercaptoethanol,pH 7.4) overnight at 4°C before being applied to an ADP-agarose column equilibrated with buffer B at a flow rate of 12 mL/h.The column was washed with buffer B containing 0.5 M NaCl until no protein could be detected by the Bradford assay.The target protein was then eluted using buffer B supplemented with 3 mM ADP.The endotoxin level in the preparations was determined using the limulus amebocyte lysate assay.
The amounts of HER-2 and HER-3 protein in the purified products were quantified using HER-2 and HER-3 ELISA kits according to the manufacturer’s instructions.
Preparation of DCs and CD8+T cells
DCs were generated as previously described[11].Briefly,PBMCs were isolated from the hepa¬rinized venous blood of the patients using density gradient centrifugation and Ficoll-Hypaque and cultured in RPMI-1640 medium containing 10% FCS for 2 h.Non-adherent cells were collected and used to generate CD8+T cells,and the adherent cells were cultured for 7 days in RPMI-1640 medium containing 10% FCS,800 U/mL recombinant human granulocyte-macrophage colony stimulating factor,and 500 U/mL recombinant human interleukin(IL)-4 to generate DCs.Half the media volume was replaced every other day,and 50 U/mL of tumor necrosis factor-α was added to the culture medium on the sixth day.
CD8+T cells were harvested from the non-adherent fraction as previously described.Briefly,non-adherent cells were resuspended in RPMI-1640 medium containing 10% FCS,100 U/mL penicillin G,and 100 μg/mL streptomycin.Recombinant human interferon(IFN)-γ (1,000 IU/mL) was added on day 0.Then 50 ng/mL mouse anti-human monoclonal antibody against CD3,100 U/mL recombinant human IL-1β,and 300 U/mL recombinant IL-2 were added to the culture and the cells were incubated at 37°C in a humidified atmosphere with 5% CO2and subcultured every third day in fresh complete medium with 300 U/mL IL-2 at 2×106cells/mL.
Induction of CD8+T cells by DCs pulsed with autogenous HSP70-PCs and in vitro cytotoxicity testing
An LDH release assay was used to determine the specific cytolytic activities of the CD8+T cells as previously reported[7].DCs from each of the four groups(1×105each) were pulsed with their autogenous HSP70-PCs (10 μg) for 12 h and then washed in PBS before being co-cul¬tured with autogenous CD8+T cells at a ratio of 1:10.The co-cultures were treated with 300 U/mL IL-2 in a 96-well plate for 1 week and then the four groups of CD8+T cells were used as effector cells in the cytotoxicity assays evaluated using an LDH cytotoxicity detection kit.The corresponding primary breast cancer cells were used as the target.Target and effector cells were resuspended in assay medium (RPMI-1640 with 1% BSA),and then target cells (1×104cells/well) were co-cultured with effector cells at a 1:10 ratio in a 96-well round-bottomed culture plate at 37°C.After incubation for 4 h,the cells were centrifuged at 50 ×gfor 10 min,and the supernatant was collected and transferred to another 96-well plate for the LDH assay.The LDH detection mixture (100 μL/well)was added and the plates were then incubated in the dark for 30 min at room temperature (15–25°C).These reactions were stopped following the addition of 50 μL of stop solution and the absorbance of the samples was measured at 490 nm using a microplate reader.
Preparation of recombinant human HSP70-HER-3 protein complexes and their application in the immunoprecipitation binding analysis
The recombinant protein complexes were prepared as previously described[7].The recombinant HSP70-HER-3 protein complex was generated by incubating recombinant human HSP70 and recombinant human HER-3 protein at a 1:1 molar ratio at 43°C for 30 min and then at 37°C for 1 h.Binding was evaluated by coimmunopre¬cipitation and western blot analysis.Briefly,the HSP70-HER-3 protein mixture was incubated with rabbit anti-human mono¬clonal antibody against HER-3(1:100) at room temperature for 2 h.The complex was then precipitated by incuba¬tion with protein A-Sepharose CL-4B (20 μl/mL) and rotating for 8 h on ice.Samples were then washed eight times with washing buffer (1 M Tris-HCl,5 M NaCl,0.5 M EDTA and 0.1% Triton X-100,pH 7.4) at 4°C to remove any nonspecific binding of the recombinant proteins to the protein A-Sepharose and then the beads were mixed with 2X SDS sample buffer,boiled for 5 min,and subjected to SDS-PAGE.Samples were transferred to a nitrocellulose membrane and then probed with the mouse anti-human monoclonal antibody against HSP70 (1:100) at room temperature for 1 h.Membranes were then incubated with HRP-conjugated goat anti-mouse IgG (1:10,000) and then proteins were visualized by autoradiography.
Immunological activity of DCs pulsed with the autogenous HSP70-PCs combined with the recombinant HSP70-HER-3 protein complexes
Each group of DCs was divided into three parts(1×105cells in each part) and each part was pulsed using different antigens for 12 h.Antigen a was 10 μg of autogenous HSP70-PCs.Antigen b was 10 μg of thein vitrogenerated antigen complex:autogenous HSP70-PCs and recombinant human HSP70-HER-3 protein complexes mixed at a 1:1 ratio.Antigen c was 10 μg of the recombinant human HSP70-HER-3 protein complex.The pulsed DCs were then co-cultured with autogenous CD8+T cells at a ratio of 1:10 and the cytolytic activities of the CD8+T cells were determined using LDH release as described above.
Statistical analysis
Values are expressed as the mean ± standard deviation(SD) or as a percentage.The correlations between the study variables were investigated using the Spearman’s correlation coefficient and all analyses were conducted using SPSS18.0 software.T-test and chi square test are applied.The results were considered statistically significant atP<0.05.
Results
Correlation between HER-3 expression and the clinical parameters of patients
A total of 70 patients with breast cancer were included in this study.Patient characteristics are listed in Table 1.We used the scoring approach described in the Methods section to categorize HER-2 and HER-3 expression in the tumor samples from the patient cohort as follows:37 cases (53%) were HER-3-positive,33 cases (47%) were HER-3-negative,29 cases (41%) were HER-2-positive,and 41 cases (59%) were HER-2-negative.The expression of HER-3 was positively correlated with tumor size,histological type,lymph node metastasis,and the HER-2 expression status of the patients.There was no significant association between HER-3 expression and the age of onset in these patients (Table 2).
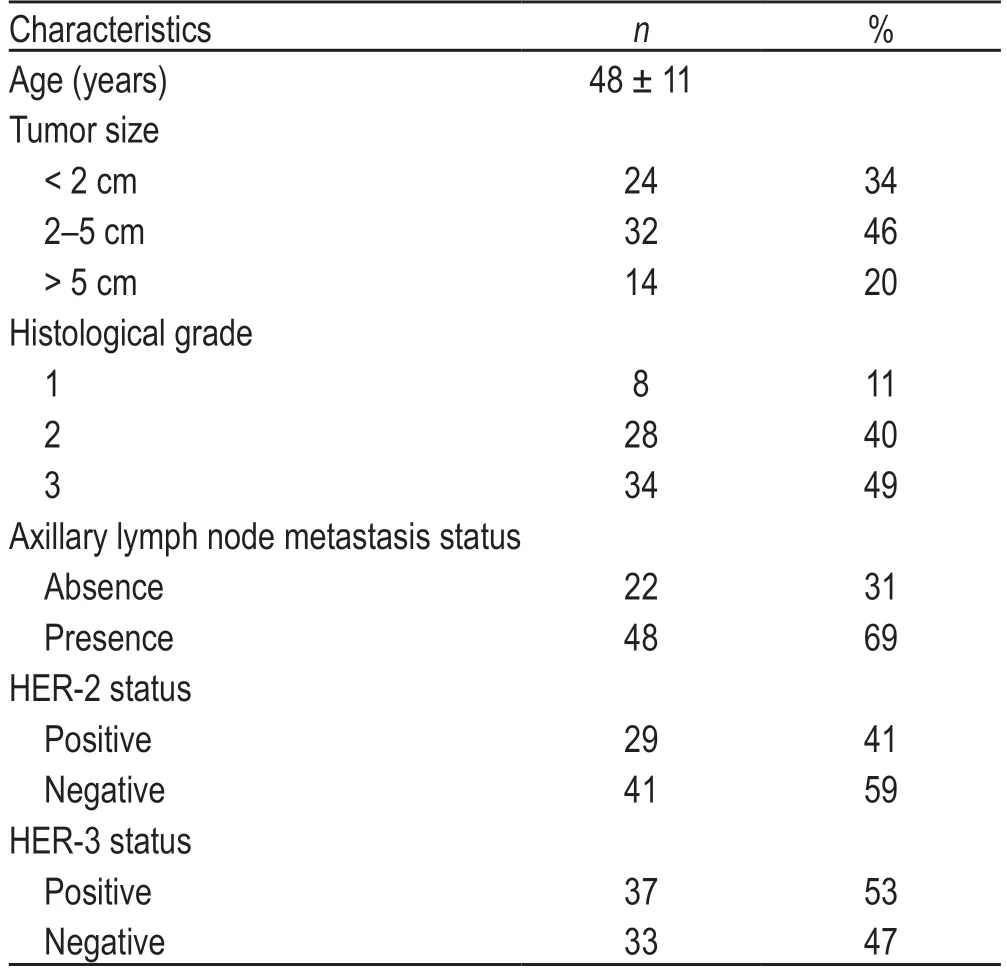
Table 1 Characteristics of breast cancer patients included in this study(70 cases)
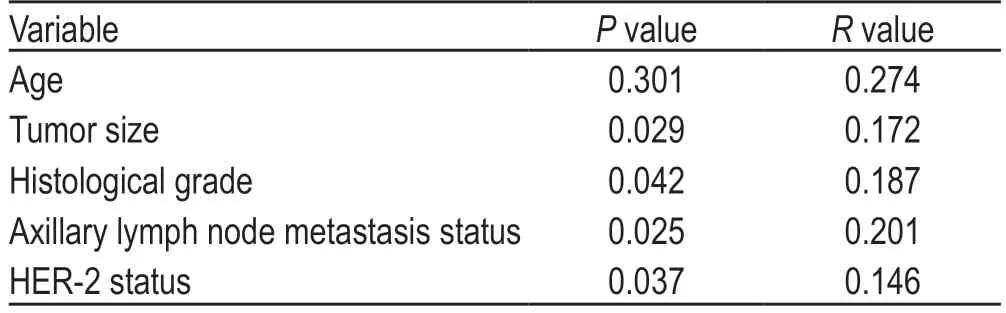
Table 2 Correlations between HER-3 immunohistochemical expression and clinicopathological characteristics of the breast cancer patients
Primary breast cancer cell culture and grouping
Of the 70 primary breast cancer cell cultures obtained from the surgical specimens,three became contaminated during culture and four developed senescence.The remaining cultures were passaged 4–5 times until we achieved sufficient cell density (1×107cells) to complete the preparation of the autogenous HSP70-PCs and populate the target cell quota in the further experiments.We categorized the 63 tumor cell cultures into four groups as described in Methods:Group A was HER-2 (+)and HER-3 (+) (n=17),group B was HER-2 (+) and HER-3 (-) (n=8),Group C was HER-2 (-) and HER-3 (+) (n=16),and Group D was HER-2 (-) and HER-3 (-) (n=22).
Quantitative detection of autogenous HSP70-PCs
We then randomly selected six cases from each of the four groups and purified autogenous HSP70-PCs from the primary breast cancer cells (5×106cells for each case).Protein was then quantified using the Bradford method and the standard curve showed that the total protein in the autogenous HSP70-PCs was 165.89 ± 20.77 μg (n=24).We performed quantitative analysis of HER-2 and HER-3 expression using ELISA kits and found that the amount of HER-2 protein in the HER-2 (+) groups (A and B) was 1.35 ± 0.24 μg (n=12) and that the HER-3 content in the HER-3 (+) groups (A and C)was very low,at only 5.21 ± 1.16 ng (n=12).The endotoxin levels in the preparations were lower than 0.03 EU/mg as determined by the LAL assays.
In vitro cytotoxicity tests
We next induced autogenous CD8+T cells by coculturing these cells with the four groups of autogenous DCs pulsed with their autogenous HSP70-PCs.The specific cytolytic activities of each of the four groups of CD8+T cells were then examined using an LDH release assay following 4 h of co-culture of the effector (CD8+T cells) and target cells (primary breast cancer cells) at a 10:1 ratio.There were no significant differences in the amount of LDH released in groups A and B (HER-2 (+)/HER-3 (+) and HER-2 (+)/HER-3 (-),respectively),but there was a significant increase in LDH in groups C and D (HER-2 (-)/HER-3 (+) and HER-2 (-)/HER-3 (-),respectively) (P<0.05;Fig.1).There was no significant difference between groups C and D.This result suggests that the HER-3 protein in the autogenous HSP70-PCs purified from HER-3-positive primary breast cancer cells did not exhibit any specific immunological activity when inducing autogenous CD8+T cells.
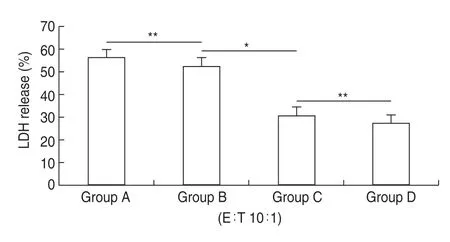
Fig.1 LDH release assays using CD8+T cells induced with autogenous HSP70-PCs.The HSP70-PC induced CD8+T cells were used as effector cells,and the primary breast cancer cells from which the antigen was derived were used as target cells.Group A is HER-2 (+) and HER-3 (+),group B is HER-2 (+) and HER-3 (-),Group C is HER-2 (-) and HER-3 (+),Group D is HER-2 (-) and HER-3 (-).Assays were performed in triplicate.Results are expressed as the mean ± SD (n =6).*P <0.05,**P >0.05.
Recombinant human HSP70-HER-3 protein complex prepared in vitro
Recombinant human HSP70 was incubated with recombinant human HER-3 protein at a 1:1 molar ratio at 43°C for 30 min,and then at 37°C for 1 h.The complex was then evaluated by co-immunoprecipitation and western blot.The protein A-Sepharose-immune complex was found to bind to a mouse anti-human monoclonal antibody against HSP70.We then used a chemiluminescence reagent to demonstrate the successfulin vitropreparation of the recombinant human HSP70-HER-3 protein complexes (Fig.2).
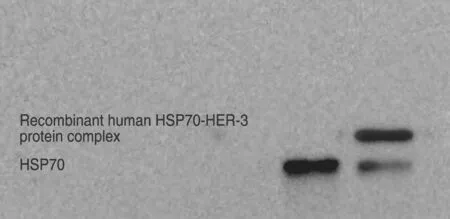
Fig.2 Co-immunoprecipitation-based analysis of the recombinant human HSP70-HER-3 protein complexes.Recombinant human HSP70 and HER-3 proteins were mixed and then immunoprecipitation was performed using HER-3 antibody,before being evaluated by western blot.
Immunological activity of DCs pulsed with autogenous HSP70-PCs combined with recombinant HSP70-HER-3 protein complexes
Finally,we examined the immunological activity of autogenous HSP70-PCs combined with recombinant HSP70-HER-3 protein complexes against breast cancer cells.Each of the four groups of DCs was pulsed using different antigens:the autogenous HSP70-PCs alone(antigen a),the autogenous HSP70-PCs and the recombinant HSP70-HER-3 protein complexes (antigen b),or the recombinant human HSP70-HER-3 protein complexes alone (antigen c).The pulsed DCs were then used to induce autogenous CD8+T cells,and the cytolytic activities of these T cells against primary breast cancer cells were evaluated by LDH release assays.
Groups A and C,which were positive for HER-3 expression (HER2+/HER3+and HER2-/HER3+,respectively),presented with significantly higher levels of LDH in response to antigen b when compared with antigens a and c (P<0.05).In group B and D,the LDH release level was not significantly different between antigens a and b,but significantly increased in response to antigen c (P<0.05;Fig.3).This result suggests that the combination of autogenous HSP70-PCs and recombinant HSP70-HER-3 protein complexes produces stronger immunological activity than autogenous HSP70-PCs alone and increase the killing capacity of autogenous CD8+T cells targeting HER-3-positive breast cancer cells.
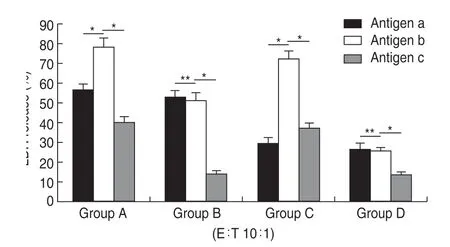
Fig.3 Immunological activity using the recombinant human HSP70-HER-3 protein complex and autogenous HSP70-PCs.Group A is HER-2(+) and HER-3 (+),group B is HER-2 (+) and HER-3 (-),Group C is HER-2(-) and HER-3 (+),Group D is HER-2 (-) and HER-3 (-).Antigen a is the autogenous HSP70-PC alone.Antigen b is a 1:10 ratio of the autogenous HSP70-PCs and the recombinant human HSP70-HER-3 protein complex.Antigen c is the recombinant human HSP70-HER-3 protein complex alone.Assays were performed in triplicate and results are expressed as the mean ± SD (n =6).*P <0.05,**P >0.05.
Discussion
DCs are powerful APCs,with an almost 1000 fold higher antigen-presenting capacity when compared to other APCs,such as macrophages and B lymphocytes.Many studies have shown that DCs effectively stimulate initial T cell activation,which is considered the main initiator of the body’s anti-tumor immune response.This means that DC-based cellular immunotherapy is an important component in tumor biotherapy.For this reason,this strategy is widely used in China when producing comprehensive treatments for a variety of malignant tumors,including breast cancer,and has been shown to achieve good therapeutic effect[12–13].
HSP70 is an important intracellular molecular chaperone and acts as an important cytoprotective agent via its binding of misfolded proteins preventing their denaturation during cellular stress.HSP70 is highly expressed in multiple kinds of tumor cells and can bind to a large number of tumor antigenic peptides via a noncovalent bond.Immunization with autogenous HSP70-PCs purified from cancer cells provides protection against tumors derived from the same type of cancer cells from which they were purified.The anti-tumor immunogenic mechanism underlying these effects relies on the fact that tumor-derived HSP70-PCs may produce specific antigens for processing by APCs such as DCs,which may then induce antigen-specific cytotoxic CD8+T cells which target and eradicate these specific tumor cells[14–15].
The human epidermal growth factor receptor (EGFR)family consists of four members:HER-1,HER-2,HER-3 and HER-4.All four family members are transmembrane glycoproteins and closely associated with the development and progression of breast cancer.Among these proteins the role of HER-2 in cancer pathogenesis is the best established.Previous studies have shown that the overexpression of HER-2 in breast cancer patients is associated with estrogen receptor and progesterone receptor negativity,increased histological grade,high rates of cell proliferation,lymph node involvement and poor prognosis.HER-2 has thus become an important target in the targeted treatment and cell therapy protocols developed for breast cancer[16].
In our previous study,we established a new method using CHAPS to purify HSP70-PCs containing more efficient tumor peptides with an increased proportion of membrane tumor-associated peptides from human breast cancer cells.CD8+cells induced with DCs pulsed using these products demonstrate better anti-tumor activity against the HER-2 overexpressing breast cancer cells from which the complex is derived.However,this method did not achieve good results for HER-2-negative breast cancers because of the absence of an accurate target.Many studies have shown that overexpression of HER-3 is also linked to HER-2 positivity and lymph node involvement.These results suggest that HER-3 may be a potential target for breast cancer treatment,although this hypothesis remains controversial[17].
Given this,we used immunohistochemical methods to determine the expression status of HER-3 in breast cancer in the samples for this study.We identified 37 cases (53%) of HER-3-positive tumors in this cohort and demonstrated that the expression of HER-3 was positively correlated with tumor size,histological type,lymph node metastasis and HER-2 expression status.These results suggest that HER-3 may be a potential target for breast cancer patients,although the correlations between HER-3 expression and various prognostic factors,such as progression-free survival (PFS) and overall survival (OS),are not yet confirmed.
We went on to produce autogenous HSP70-PCs from HER-3-overexpressing breast cancer cells.However,thein vitrocytotoxicity results showed that these HSP70-PCs did not exhibit good anti-tumor activity against the HER-3-overexpressing breast cancer cells from which the complexes were purified.Given this we speculated that the HER-3 protein content in these autogenous HSP70-PCs (as quantified by ELISA) was too low to induce a sufficient anti-tumor response.Several aspects might explain the low levels of HER-3 protein content,including a loss of HER-3 protein during the purification process or denaturation of the HER-3 protein during the purification process.
Thus,to address this issue,we prepared recombinant human HSP70-HER-3 protein complexes and combined these with the autogenous HSP70-PCs in an effort to circumvent the negative effects of reduced HER-3 expression.We compared the immune activity of these new products to those of the autogenous HSP70-PCs or recombinant human HSP70-HER-3 protein complexes on their own.The new protein complex had stronger immunocompetence and induced both autogenous DCs and CD8+T cells which were able to specifically kill HER-3-overexpressing breast tumor cells whether they were HER-2 positive or negative.This result suggests that the HER-3 protein can be used as a target for HSP70-DCbased cellular immunotherapy and that the anti-tumor immune activity of autogenous HSP70-PCs from breast cancer patients with increased HER-3 expression can be significantly increased usingin vitromodifications.
In summary,we demonstrated that HER-3 protein can act as a target for cellular immunotherapies against breast cancer.We also demonstrated a new treatment method for HER-3-overexpressing breast cancers.Our future research will focus on the following aspects:(1) we plan to follow the HER-3-positive patients to determine the relationship between HER-3 expression and OS and PFS;(2) we would like to conduct larger clinical trials to evaluate the therapeutic efficacy of this approach and(3) we want to examine the relationship between each of the HER family proteins and breast cancer.We firmly believe that our evaluations will facilitate more effective individualized immunotherapy for breast cancer patients in the future.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年4期
Oncology and Translational Medicine2021年4期
- Oncology and Translational Medicine的其它文章
- The expression of vascular endothelial growth factor (VEGF)/ endostatin (ES) and VEGF receptor 2(VEGFR2)/ES is associated with NSCLC prognosis *
- Relationship between molecular changes in epidermal growth factor receptor (EGFR)and anaplastic lymphoma kinase (ALK)mutations in lung adenocarcinoma *
- Efficacy and adverse reactions of apatinib as secondline or later-line treatment in advanced lung cancer
- The clinical efficacy of percutaneous ethanol-lipiodol injection (PEI) combined with high-intensity focused ultrasound (HIFU) for small hepatocellularcarcinoma in special or high-risk locations*
- Relationship between miR-7-5p expression and 125 I seed implantation efficacy in pancreatic cancer and functional analysis of target genes*
- Mechanism of tumor synthetic lethal-related targets
