The expression of vascular endothelial growth factor (VEGF)/ endostatin (ES) and VEGF receptor 2(VEGFR2)/ES is associated with NSCLC prognosis *
Yuan Yang ,Baohua Lu (Co-first author),Baolan Li (✉),Weiying Li ,Mei Jiang ,Wentao Yue ,Qunhui Wang ,Tongmei Zhang
1 General Department of Medicine,Beijing Chest Hospital,Capital Medical University,Beijing 101149,China
2 Laboratory of Cell Biotechnology,Beijing Tuberculosis and Thoracic Tumor Research Institute,Beijing 101149,China
Abstract Objective The aim of our study was to detect the expression of angiogenesis inhibitory proteins and angiogenesis promotive proteins in the postoperative tumor tissue of non-small cell lung cancer (NSCLC)patients.We also investigated the relationship of protein expression with clinical characteristics and prognosis.Methods We examined the expression of vascular endothelial growth factor (VEGF),VEGF receptor 2(VEGFR2),and endostatin (ES) proteins in 255 specimens resected from NSCLC patients,using immune histochemistry (IHC).We then evaluated the relationships between the expression of the three proteins and clinical characteristics such as stage,histological type,differentiation,gender,tobacco use,and age.According to the value of VEGF/ES,we divided the cohort into angiogenesis-promoting group A,angiogenesis-inhibiting group A,and balance group A.The survival differences in the three groups were evaluated to determine the prognostic value of VEGF/ES.Similarly,we tested the prognostic value of VEGFR2/ES.Results VEGF-positive expression was observed in 93 patients (36.4%).VEGF expression was not correlated with the clinical characteristics.VEGFR2-positive expression was observed in 103 patients(40.4%).The expression of VEGFR2 was correlated with the clinical stage (χ2=21.414,P=0.045) and histological type (χ2=26.911,P=0.008).ES-positive expression was observed in 140 patients (54.9%).The expression of ES was correlated with the clinical stage (χ2=26.504,P=0.009).When evaluating the prognostic values of VEGF/ES and VEGFR2/ES,the prognosis of the angiogenesis balance group was similar to that of the angiogenesis-inhibiting group.The minimum survival time was observed in the angiogenesis-promoting group.Conclusion VEGF/ES and VEGFR2/ES in resected tumors have prognostic value in postoperative NSCLC patients.The survival time of the population with predominant angiogenic factors was short.
Key words: non-small cell lung cancer (NSCLC);angiogenesis;clinical characteristics;prognosis
Lung cancer is the most common cancer worldwide.In both sexes combined,lung cancer is the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths)[1].Lung cancer is the most life-threatening cancer in China[2].Non-small cell lung cancer (NSCLC) is the most common type of lung cancer.
As early as 1971,Folkman proposed that the growth and metastasis of tumors depended on neovascularization within tumors[3].Since then,many studies have been devoted to the treatment of tumors by blocking neovascularization.
Tumor angiogenesis is regulated by both antiangiogenic and pro-angiogenic factors.The growth and metastasis of tumors require sustained angiogenesis to provide oxygen and nutrients and to excrete metabolites.Tumor tissue induces an“angiogenesis shift,”which is why pro-angiogenic factors are often overexpressed in tumor tissue,while the expression of anti-angiogenic factors is limited[4].
Vascular endothelial growth factor-A (VEGF-A,also known as VEGF) is the most important pro-angiogenic factor that promotes the proliferation,migration,infiltration,and survival of endothelial cells.A study showed that serum VEGF levels might be a predictor of disease prognosis in different types of cancers[5].VEGF receptor 2 (VEGFR2) is the most important VEGF receptor[4].Many drugs are used in clinical practice to effectively inhibit the downstream signaling of the VEGFVEGFR2 pathway,such as bevacizumab (the monoclonal antibodies targeting VEGF) and ramucirumab and apatinib[6](the receptors of VEGFR2).
Endostatin (ES) is an important anti-angiogenesis factor that was isolated from mouse endothelial cell tumors for the first time in 1997[7].Endostatin has an intense and complete inhibitory effect on tumor-induced angiogenesis,showing strong anti-tumor activity.Endostar is an artificially modified vascular endothelial inhibitor that was extracted by Chinese scholars through theE.coliexpression system in 1999[8].Endostar combined with chemotherapy showed good antitumor effects[9].
We hypothesized that the expression of angiogenesispromoting and angiogenesis-inhibiting factors is imbalanced in patients with NSCLC.To date,many angiogenic promoters and inhibitors have been found to be involved in the regulation of tumors;however,VEGF-VEGFR2 and ES are amongst the most important factors;they play positive and negative regulatory roles in angiogenesis,respectively.
We designed this retrospective cohort study to evaluate the balance between VEGF and the inhibition of endothelial growth factor in resectable NSCLC.
Materials and methods
Patients and tissue specimens
We included 255 patients who underwent surgery for NSCLC at the Beijing Chest Hospital,Capital Medical University,China,between July 2008 and October 2009.Pathological surgical staging was performed according to the 8th edition TNM staging classification for lung cancer.Our study included patients with stage IA–IIIA disease,a small proportion of stage IIIB patients who were diagnosed locally or after surgery,and stage IV patients with single brain or adrenal metastasis.Previous studies have reported that the expression of VEGF,VEGFR2,and ES is significantly higher in tumor tissues and plasma than in normal tissues[8–13];therefore,we did not establish a healthy control group.None of the patients received radiotherapy,chemotherapy,orangiogenesis inhibitors before surgery.Patients who died during the perioperative period were excluded.
The experiment was completed in 2010,and the deadline for follow-up was from May 2018 to June 2018.The median observation period of these patients was 10 years.
Reagents
Primary VEGF antibody and primary VEGFR2 antibody were purchased from Fuzhou MaiXin Biotechnology Company,China.Primary ES antibody was purchased from Beijing Biosynthesis Biotechnology Company,China.The NovolinkTMpolymer detection system was purchased from Novocastra Laboratories,USA.
Immunohistochemical analysis
Tumor tissue sections (4–6 µm thick) were fixed in formalin,paraffin-embedded,and dried for 1 h at 60°C.The sections were washed with phosphate buffered Saline,incubated with hydrogen peroxide for 5 min at room temperature,washed again with phosphate buffered Saline,incubated with protein blocker for 5 min at room temperature.Primary antibodies against VEGF,VEGFR2,and ES were incubated with the same tissue sections at 4 °C.The sections were washed,incubated with Post Primary Block for 15 min,and then incubated with NovolinkTMpolymer for 15 min.The sections were stained with DAB (3,3-diaminobenzidine) working solution for approximately 5 min and counterstained with hematoxylin for 5 min.
Evaluation of immunohistochemical analysis
We used as emi-quantitative method to assess the expression of VEGF,VEGFR2,and ES.We estimated a weighted average of the percentage of tumor cells stained on whole tumor slides (0=positive staining in 0–10%of the tumor cells,1=positive staining in 11%–25% of the tumor cells,2=positive staining in 26%–50% of the tumor cells,and 3=positive staining in more than 51% of the tumor cells).The staining intensity was also evaluated in a semi-quantitative manner,representing the average intensity of the tumor cells (0=no staining,1=weak staining,2=moderate staining,and 3=strong staining,equivalent to the positive control,which showed clear,well-defined,and strong staining).The intensity and proportion scores were then multiplied to obtain a total score ranging from 0 to 9,with 0=no staining (–),1–3=weak positive (+),4–6=moderate positive (++),and 7–9=strong positive (+++).We defined (–,+) as negative expression,which could also be defined as low expression,and we defined (++,+++) as positive expression,which could also be defined as high expression.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (IBM Corp.,Armonk,NY,USA),and the groups were compared using Chi-Square tests.The survival rate was calculated using the Kaplan-Meier method and compared using the log-rank test.We used the Cox step-back regression model to understand the multivariate analysis related to overall survival,which yielded hazard ratios(HRs) ofP<0.05,which were considered significant in all of our analyses.
Results
The expression of VEGF,VEGFR2,and ES
The demographic features of the patients included in this study are presented in Table 1.The expression levels of VEGF,VEGFR2,and ES are summarized in Table 2.
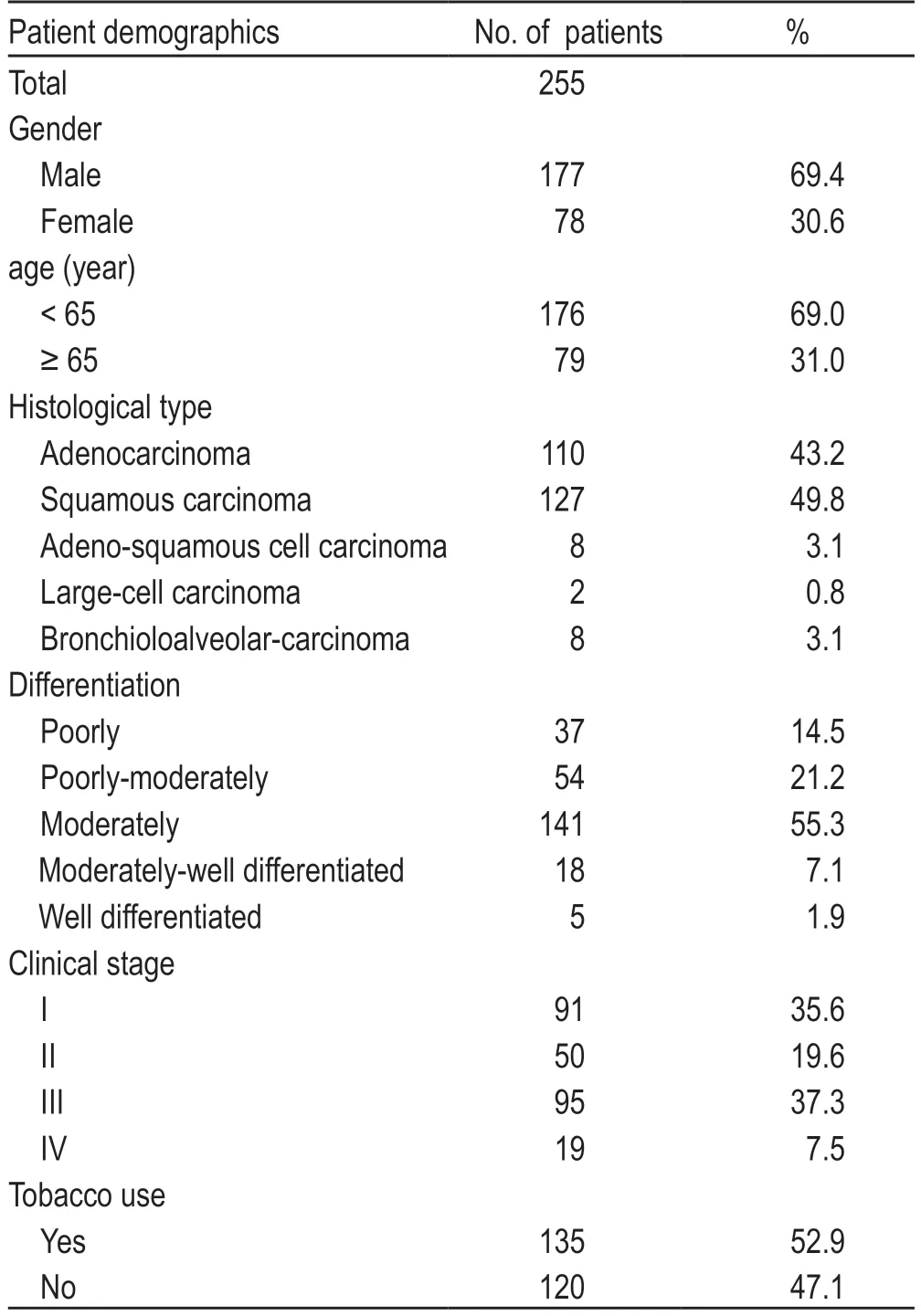
Table 1 The demographic features of these patients
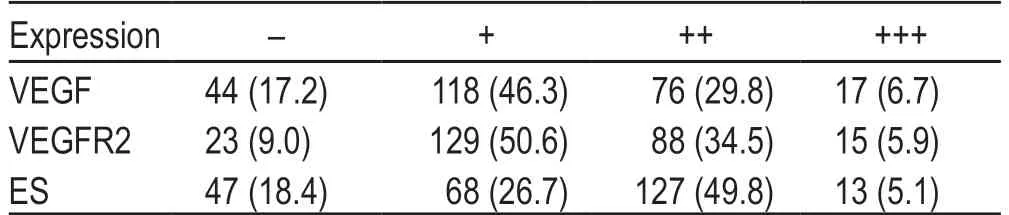
Table 2 The IHC expression of VEGF,VEGFR2 and ES [n (%)]
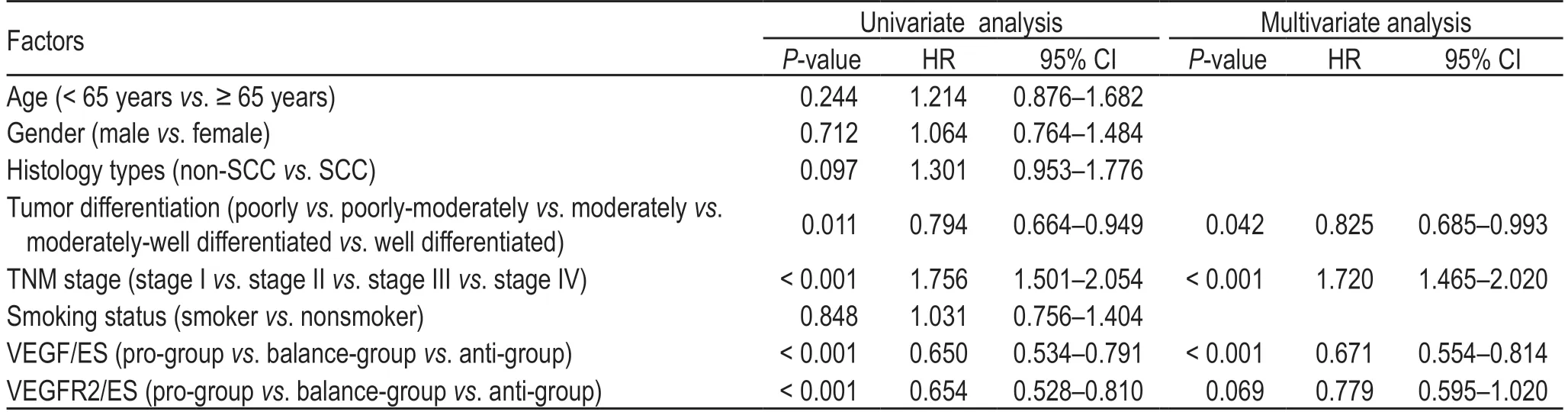
Table 3 Univariate and multivariate analysis of overall survival
Correlations between VEGF staining and clinical features
VEGF staining of the cytoplasm of malignant cells accounted for 36.4% of high expression,and 93 of the 255 specimens showed moderate/strong staining,as shown in Fig.1a (adenocarcinoma cells) and 1d (squamous cell carcinoma cells).Regarding the percentage of positively stained malignant cells in the tumor tissue,the expression of VEGF did not show significant differences in terms of the clinical stage (χ2=10.083,P=0.609),histological type(χ2=17.425,P=0.134),differentiation (χ2=12.175,P=0.432),sex (χ2=2.182,P=0.902),tobacco use (χ2=5.735,P=0.453),and age (χ2=1.872,P=0.765).
Correlations between VEGF2 staining and clinical features
VEGFR2 staining of the cytoplasm of malignant cells accounted for 40.4% of high expression,and 103 of the 255 specimens showed moderate/strong staining.Staining was mainly observed in the cytoplasm,and a few tumors stained positively on the cell membrane,as shown in Fig.1b (adenocarcinoma cells) and 1e (squamous cell carcinoma cells).
The percentage of positive tumor cells was different between the clinical stage (χ2=21.414,P=0.045)and histological type (χ2=26.911,P=0.008),and the cells did not show a significant difference in terms of differentiation (χ2=11.616,P=0.478),sex (χ2=7.619,P=0.267),tobacco use (χ2=5.056,P=0.537),and age (χ2=1.971,P=0.715).
Correlation between ES staining and clinical features
ES staining of the cytoplasm of malignant cells accounted for 54.9% of high expression,and 140 of the 255 specimens showed moderate/strong staining.The cytoplasm was stained,as shown in Fig.1c(adenocarcinoma cells) and 1f (squamous cell carcinoma cells).
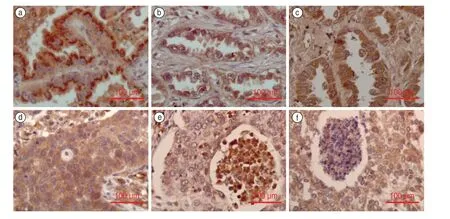
Fig.1 (a) positive VEGF staining in adenocarcinoma;(b) positive VEGFR2 staining in adenocarcinoma;(c) positive ES staining in adenocarcinoma;(d) positive VEGF staining in squamous cell carcinoma;(e) positive VEGFR2 staining in squamous cell carcinoma;(f) positive ES staining in squamous cell carcinoma.Note: VEGF,Vascular endothelial growth factor;VEGFR2:VEGF receptor 2;ES,Endostatin (Immunohistochemistry staining,40 ×optical microscopy)
The percentage of positive tumor cells differed among the clinical stages (χ2=26.504,P=0.009),and there was no significant difference observed in terms of the histological type (χ2=8.344,P=0.758),differentiation(χ2=11.839,P=0.459),sex (χ2=3.024,P=0.806),tobacco use (χ2=3.378,P=0.760),and age (χ2=3.946,P=0.139).
The survival differences among the antiangiogenesis group,pro-angiogenesis group,and balance group
According to the IHC score,when the VEGF score was greater than the ES score,it was placed in the proangiogenesis group A (pro-A,including 72 patients);when the VEGF score was equal to the ES score,it was placed in the balance group A (bal-A,including 83 patients);and when the VEGF score was less than the ES score,it was placed in the anti-angiogenesis group A (ant-A,including 100 patients).
Similarly,when the VEGFR2 score was greater than the ES score,it was placed in the pro-angiogenesis group B (pro-B,including 74 patients);when the VEGFR2 score was equal to the ES score,it was placed in the balance group B (bal-B,including 105 patients);and when the VEGFR2 score was less than the ES score,it was placed in the anti-angiogenesis group B (ant-B,including 76 patients).
The median survival time of the pro-A group was 24 months (first quartile,9 and third quartile,49 months).The median survival time of the bal-A group was 68 months (first quartile,26 and third quartile,115 months).The median survival time of the ant-A group was 84 months (first quartile,26 and third quartile,116 months).The difference was statistically significant (P<0.001).The survival curves are shown in Fig.2.
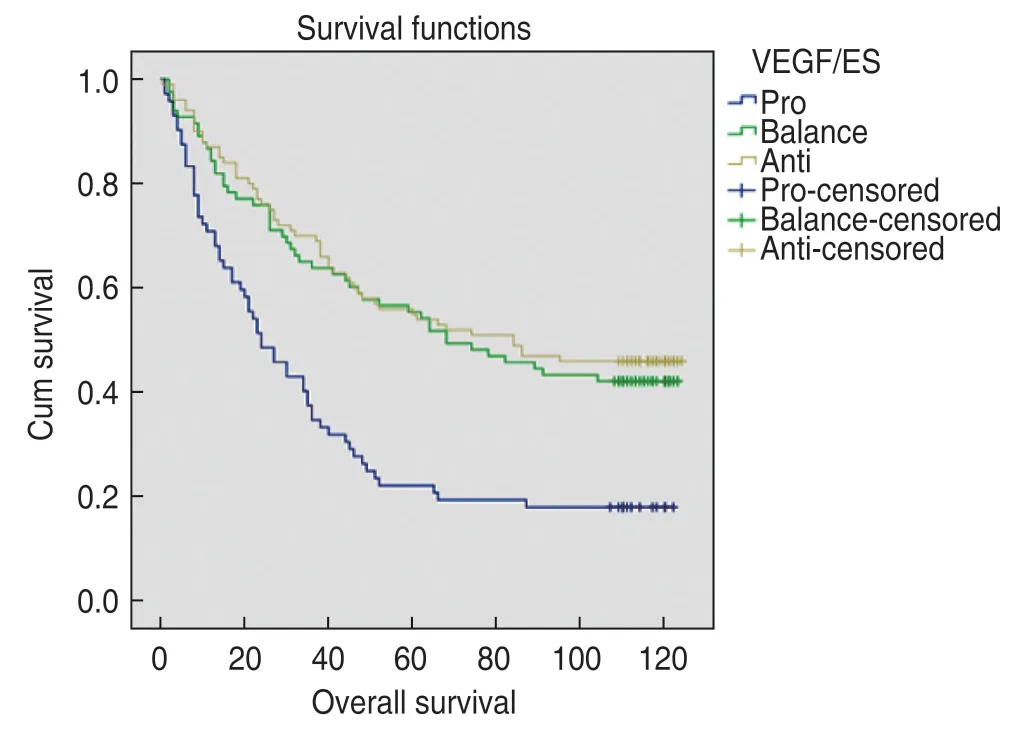
Fig.2 Survival curves in pro-angiogenesis group A,balanceangiogenesis group A and anti-angiogenesis group A
The median survival time of the pro-B group was 24 months (first quartile,10 and third quartile,51 months).The median survival time of the bal-B group was 104 months (first quartile,26 and third quartile,118 months).The median survival time of the ant-B group was 76 months (first quartile,27 and third quartile,114 months).The difference was statistically significant (P<0.001).The survival curves are shown in Fig.3.
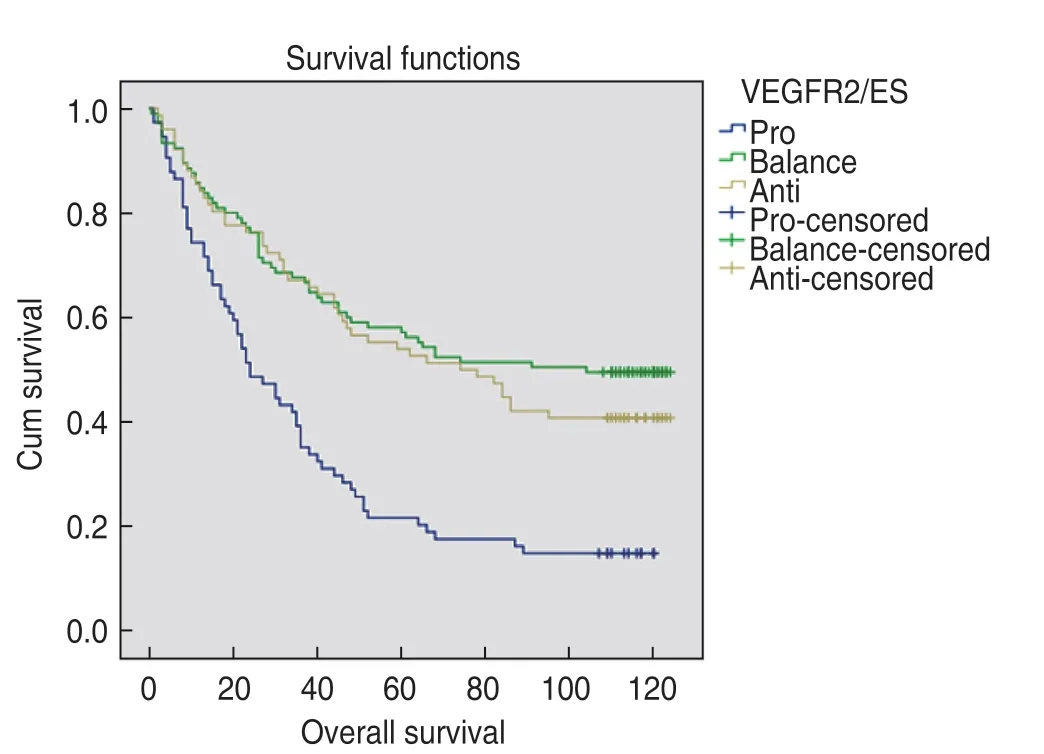
Fig.3 Survival curves in pro-angiogenesis group B,balanceangiogenesis group B and anti-angiogenesis group B
The Cox proportional hazard model was used to determine multiple factors related to OS.The results showed that smoking,sex,age,and histological type were not correlated with survival,while the disease stage (P<0.001),degree of differentiation (P=0.042),and VEGF/ES(P<0.001) were independent factors related to survival(Table 3).
Discussion
Since Folkman proposed the theory of tumor angiogenesis[13–14],“starvation of tumors”has become the fourth major treatment for tumors,in addition to surgery,chemotherapy,and radiotherapy.
From the molecule to the cell and then to the organ– there is a process of regulation in organisms to achieve system balance.Angiogenesis is also a process of confrontation between promoters and inhibitors.Angiogenesis and inflammation are involved in the process of wound healing.Tissue injury activates angiogenesis factors that promote the growth of granulation tissue and tissue repair.This process is strictly regulated by angiostatin,to avoid excessive scar formation[15].Tissue wounds are ultimately repaired or scarred;however,the proliferation and invasiveness of tumors are different processes compared to the tissue/wound healing process.Tumors are chronic stimuli that result in“never-healing wounds”[16].
Our research showed that postoperative NSCLC patients with higher levels of anti-angiogenic factors had longer survival times than patients with pro-angiogenic factors,according to the expression of VEGF/ES and VEGFR2/ES.
The balance group included patients with antiangiogenic factors that were similar to pro-angiogenic factors.Patients in the balance group had a survival time that was close to or even longer than those in the antiangiogenesis group.We infer that in the process of tumor angiogenesis,when angiogenesis factors are strong,feedback leads to an increase in the levels of angiogenesisinhibiting factors.When the factors promoting and inhibiting angiogenesis reach a relatively stable state,tumor growth slows down.Systemic homeostasis prolongs the overall survival of patients.This may also explain the accelerated progression of tumors when anti-angiogenic drugs are withdrawn.
We further hypothesized that there would be a more suitable population for anti-angiogenesis therapy,which may benefit more from anti-angiogenesis therapy with a high expression of the angiogenesis promoter.The effect of anti-angiogenesis therapy may be limited in patients with strong tumor inhibitors and in patients with a balance of promotion and inhibition factors.This is an inference that needs to be confirmed by further research.This phenomenon may also explain why some multicenter clinical studies have shown that antiangiogenesis therapy can prolong the survival of patients,but its efficacy is limited[17].
At present,many pro-angiogenic and anti-angiogenic factors have been identified.VEGF and ES are the most important factors.VEGF/VEGFR2 promotes endothelial cell proliferation and migration,which increases vascular permeability and angiogenesis.When VEGF is combined with VEGFR2,it activates signaling pathways,including the MAPK,ERK,and ATK pathways,leading to endothelial cell proliferation and angiogenesis.ES can inhibit angiogenesis through many signaling pathways,including the VEGF-triggered signaling pathway[18].In contrast,ES leads to the up-regulation of VEGF.In a murine lung cell line transfected with the ES gene,ES protein was secreted,and the expression of VEGF protein was increased[19].The test showed that ES upregulates the VEGF protein.Thus,we concluded that we should combine these two important indicators (VEGF and ES)to evaluate the status of angiogenesis.
Abdollahiet al[20]established the concept of“direct”angiogenesis inhibitor ES and“indirect”antiangiogenic inhibitor VEGFR2-TKI.The difference between direct and indirect agents is whether their target is microvascular endothelial cells.VEGFR2 is the most important and specific receptor for VEGF.They confirmed that the combination of low-dose ES and VEGFR2-TKI could remarkably reduce tumor growth when compared tomono-agent therapy in diverse human xenograft models.Niuet al[21]established an animal model and treated subcutaneously-implanted tumors in mice with ES,bevacizumab,or a combination of the two drugs.They found that combining bevacizumab with ES resulted in better results than the use of a single drug.
Based on these studies,we speculated that the combined use of direct angiogenesis ES and indirect anti-angiogenic bevacizumabin NSCLC could achieve better efficacy.We chose patients with strong angiogenic factors and then treated them with bevacizumab and ES to achieve better results.
However,more clinical trials are needed to confirm this as our research is retrospective,and we only observed that people with high levels of angiogenesis-promoting factors have the worst prognosis.Therefore,more prospective clinical studies are required to verify our findings.
Ethics approval and consent to participate
All procedures performed in this study that involved human participants were in accordance with the ethical standards of the Institutional and National Research Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.Informed consent was obtained from all the participants enrolled in this study.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年4期
Oncology and Translational Medicine2021年4期
- Oncology and Translational Medicine的其它文章
- Mechanism of tumor synthetic lethal-related targets
- Relationship between miR-7-5p expression and 125 I seed implantation efficacy in pancreatic cancer and functional analysis of target genes*
- The clinical efficacy of percutaneous ethanol-lipiodol injection (PEI) combined with high-intensity focused ultrasound (HIFU) for small hepatocellularcarcinoma in special or high-risk locations*
- Enhancing the treatment effects of tumor cell purified autogenous heat shock protein 70-peptide complexes on HER-3-overexpressing breast cancer *
- Efficacy and adverse reactions of apatinib as secondline or later-line treatment in advanced lung cancer
- Relationship between molecular changes in epidermal growth factor receptor (EGFR)and anaplastic lymphoma kinase (ALK)mutations in lung adenocarcinoma *
