Protective eff ect of extracorporeal membrane pulmonary oxygenation combined with cardiopulmonary resuscitation on post-resuscitation lung injury
Ji-yang Ling, Chun-sheng Li, Yun Zhang, Xiao-li Yuan, Bo Liu, Yong Liang, Qiang Zhang
1 Department of Emergency Medicine, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China
2 Department of Emergency Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
3 Department of Emergency Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China
KEYWORDS: Cardiac arrest; Swine; Alveolar surface-active protein; Oxidative stress injury; Pulmonary edema
INTRODUCTION
Cardiac arrest (CA) is a critical condition that is a concern to healthcare workers. Cardiopulmonary resuscitation (CPR) is widely used in CA of various etiologies and is considered to be the only effective method.[1]Moreover, when the duration of CA exceeds 10 minutes, the survival rate declines rapidly; if the duration of CA exceeds 20 minutes, patients rarely survive.[2]
Extracorporeal membrane oxygenation (ECMO) is a technology that relies on instrumentation to support the basic needs of the human body. Since the 1990s, ECMO combined with CPR technology (extracorporeal cardiopulmonary resuscitation [ECPR]) has been increasingly applied in attempts to improve the success rate of recovery following CA.[3]Several studies have analyzed and summarized relevant cases, and comparatively studied ECPR and conventional cardiopulmonary resuscitation (CCPR) technologies and have indicated that ECPR is superior to CCPR.[4-7]
However, the current literature mainly comprises retrospective studies or case analyses, and there is a lack of multicenter randomized controlled clinical trials that compare the protective effects of the two resuscitative methods on organs. In particular, there have been no reports on the effects of early oxygenated blood perfusion in relieving post-resuscitation lung injury in experimental animals such as swine.[8-10]
In this study, using a swine model of ventricular fibrillation-induced CA, we aim to evaluate whether early application of ECPR has advantages over CCPR in the lung injury and to explore the protective mechanism of ECPR on the post-resuscitation pulmonary injury.
METHODS
Study animals
We used 40-day-old male long-white swine (n=16; weight 35.13±5.57 kg) in this study. The experimental animals were randomly divided into two groups: CCPR group (CCPR after CA,n=8) and ECPR group (ECPR after CA,n=8).
At the beginning, anesthesia was induced using intramuscular injections of ketamine (10 mg/kg) and midazolam (0.5 mg/kg). Then, propofol (2.0 mg/kg) was intravenously administered. Propofol (9.0 mg/[kg·h]) and fentanyl (1.0 μg/[kg·h]) were continuously infused intravenously. Endotracheal intubation was carried out with a 6.5-mm tracheal catheter, and then the animals were placed on mechanical ventilation (Servo 900 c; Siemens, Berlin, Germany) with volume-controlled ventilation (tidal volume 15.0 mL/kg, respiratory rate 12-20 breaths/minute, and oxygen concentration 35%).
Subsequently, a 5-Fr deep venous catheter was inserted into the right external jugular vein of the experimental animal for intravenous infusion and fibrillation access. A 16-Fr (Dragon Laifu Medical Products Co., Ltd., China) intravenous catheter was inserted into the right atrium, and a 14-Fr arterial catheter was inserted into the ascending aorta. These two catheters were connected to the ECMO machine. All catheters were pretreated with normal heparin saline (5.0 U/mL).
电网调度技术在电气工程中是借助相关服务器和自动化系统来实现的,具有稳定性、高效性、安全性的特点。运用电气自动化技术可以有效维持电网运行的稳定性,利用自动监控体制代替人工监控,实现了电网调度自动化。即电气自动化技术利用自身的自动化特点,有效监控了电气系统的运行状态,并根据所采集信息在电网发生故障时进行调动,有效避免了人力维修的低效繁琐,保障了人们的正常生活。
Preparation of ECMO
The ECMO equipment consisted of arterial and venous catheters, Levitronix CentriMag control system (Sarns Healthcare/3M, Ann Arbor, USA), a centrifugal force pump (MAQUET Cardiopulmonary AG, Germany), a coated porous biofi lm lung (MAQUET Holding B.V. and Co. KG, Germany), and gas mixers (Thoratec Corporation, USA). The catheters and ECMO machine were prewashed with normal heparin saline and filled with heparinized colloid solution (250 U/kg) oxygenated up to 50% oxygen through the oxygenator. An arterial catheter (5 Fr; Terumo, Japan) and Swan-Ganz catheter (7 Fr; Edwards Life Sciences, USA) were placed in the left femoral artery and left femoral vein, respectively, and connected to an HP monitor (M1165; Hewlett-Packard Co., USA) to continuously monitor the heart rate (HR) and the mean arterial pressure (MAP). Furthermore, the thermal dilution method was used to detect extravascular lung water (EVLW), pulmonary vascular permeability index (PVPI), and other relevant data.
Experimental procedure
The swine were placed under observation for 30 minutes. Care was taken to ensure that the animals were in a stable state before the onset of artificial ventricular fi brillation.
A programmed medical stimulator (GY-600A; Kaifeng Huanan Instrument Co., China) was used to induce fibrillation (parameters: mode, S1/S2 [300/200 ms]; output voltage, 40 V; 8:1 ratio; step-size, -10 ms continuous program-controlled stimulation until ventricular fi brillation). The success of the ventricular fibrillation was determined by the rapid decrease in arterial pressure and the observance of ventricular fibrillation on the ECG monitor. Both the CCPR and ECPR groups were observed for 12 minutes, during which no treatment or intervention was administered in either group. Thereafter, CPR without any defibrillation treatment was conducted in both groups. Fourteen minutes after shock, the ECPR group was treated with ECMO using continuous CPR.
Both the CCPR group and ECPR group received their first defibrillation at 18 minutes after shock. The defi brillator was applied with a two-way wave of 4.0 J/kg for defibrillation. Subsequent compressions, defibrillation, and medication use were in accordance with the 2015 guidelines for CPR.[11]
The standard criteria for the return of spontaneous circulation (ROSC) are as follows: MAP greater than 60 mmHg (1 mmHg=0.133 kPa) or systolic pressure greater than 80 mmHg for more than 20 minutes.[12]
The endpoints were the status of the animal at 6 hours after ROSC, or the death of the animals. After 6 hours of ROSC, the swine were intravenously injected with 60 mg propofol before administered with 20 mL of 10% potassium chloride and sacrifi ced.
Data collection and testing
Hemodynamic parameters including HR, MAP, and end-expiratory carbon dioxide were monitored continuously. EVLW and PVPI were measured by the thermal dilution method at baseline in both groups. These were measured in the CCPR group at 6 hours after ROSC, and in the ECPR group the ECMO machine was stopped at 6 hours after ROSC.
Blood samples were collected at baseline, ROSC, and 1, 2, 4, and 6 hours after ROSC. The samples were tested by enzyme-linked immunosorbent assay (ELISA) using ELISA kits (Shanghai Sangon Biotechnology Company, Ltd., China) for pulmonary surfactant protein A (SP-A), pulmonary surfactant protein D (SP-D), Clara cell protein 16 (CC16), malondialdehyde (MDA), and superoxide dismutase (SOD).
Lung tissue samples (lower lobe of the right lung) were collected immediately after the swine were euthanized. The samples were also tested for myeloperoxidase (MPO), MDA, SOD, SP-A, and SP-D using ELISA (Shanghai Sangon Biotechnology Company, Ltd., China).
The ultrastructure of the tissue was observed using electron microscopy (JEM-1010; JEOL, Japan).
Statistical analysis
All the data were statistically analyzed using SPSS 22.0 (IBM, NY, USA). Continuous variables were expressed as mean±standard deviation (SD), and the differences between CCPR and ECPR groups were determined by the Student’st-test. Survival rates between the two groups were estimated by Kaplan-Meier analysis. TheP-value <0.05 was considered statistically signifi cant.
RESULTS
Comparison of survival rates
All swine were successfully induced into ventricular fibrillation. In the CCPR group, two swine died at 3.7 and 5.3 hours after ROSC, while the remaining survived up to 6 hours (6/8, 67.5%). All animals in the ECPR group successfully survived to ROSC for 6 hours (8/8, 100%). The survival rates between the two groups were not statistically signifi cant (P>0.05).
Comparison of blood and tissue biomarkers
There were no significant differences in serum SP-A, SP-D, CC16, MDA, and SOD at baseline between the two groups (P>0.05). Serum SP-A, SP-D, CC16, and MDA were found in the ROSC and 1, 2, 4, 6 hours after ROSC. The levels of the biomarkers mentioned at the above time points were statistically higher in the CCPR group than in the ECPR group (P<0.05), whereas serum SOD at the above mentioned fi ve time points in the CCPR group was lower than that of the ECPR group (P<0.01) (Figures 1A-E). The comparison of tissues from the two groups showed that the levels of MDA (11.05±1.07 nmol/mL vs. 5.67±1.62 nmol/mL,P<0.01) and MPO (634.66±54.62 ng/mL vs. 274.08±99.78 ng/mL,P<0.01) were significantly higher in the CCPR group than in the ECPR group, whereas the levels of SP-A (32.06±4.13 ng/mL vs. 71.87±18.88 ng/mL,P<0.01), SP-D (45.08±3.40 ng/mL vs. 131.29±22.80 ng/mL,P<0.01), and SOD (82.92±32.02 U/mL vs. 158.65±20.68 U/mL,P<0.01) were signifi cantly lower in the CCPR group.

Figure 1. Comparison of serum markers at each time point between the two groups. Data were expressed as mean±standard deviation (n=8); CCPR: conventional cardiopulmonary resuscitation; ECPR: extracorporeal cardiopulmonary resuscitation; SP-A: surfactant protein A; SP-D: surfactant protein D; CC16: Clara cell protein 16; MDA: malondialdehyde; SOD: superoxide dismutase; ROSC: return of spontaneous circulation; ROSC1h/2h/4h/6h: 1, 2, 4, and 6 hours after ROSC; compared with baseline, *P<0.01, **P <0.05.
Signifi cant diff erences in EVLW and PVPl between the two groups
Table 1 compares the EVLW and PVPI between the two groups. At baseline, there were no significant differences between the two groups (P>0.05). The EVLW at 6 hours after ROSC (ROSC6h) and the PVPI values at ROSC6h in both groups showed statistically significant differences (P<0.01). Moreover, the EVLW values at ROSC6h compared with the baseline in both groups also showed statistically significant differences (P<0.05). The PVPI at ROSC6h in the CCPR group was statistically higher than that at baseline (P<0.01), while there was no diff erence for the ECPR group (P>0.05).
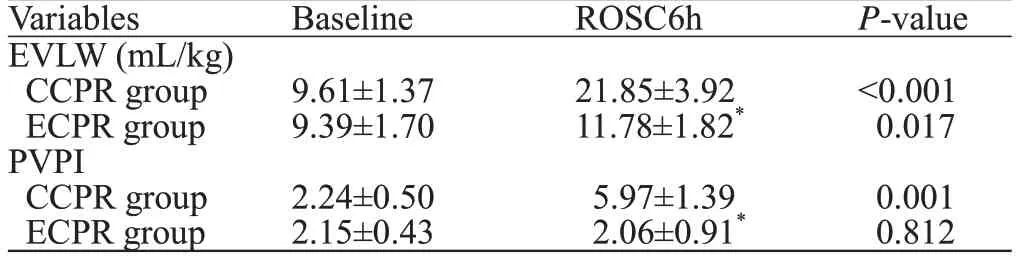
Table 1. Comparison of EVLW and PVPI between the two groups
Electron microscopy results between the two groups
In the CCPR group, the broadening of the blood-gas barrier was observed. Furthermore, in the epithelial cells, we observed empty vacuoles in type II lamellar bodies. In the ECPR group, the blood-gas barrier was clear without any broadening, and the epithelial cells showed non-empty type II lamellar bodies (Figure 2).
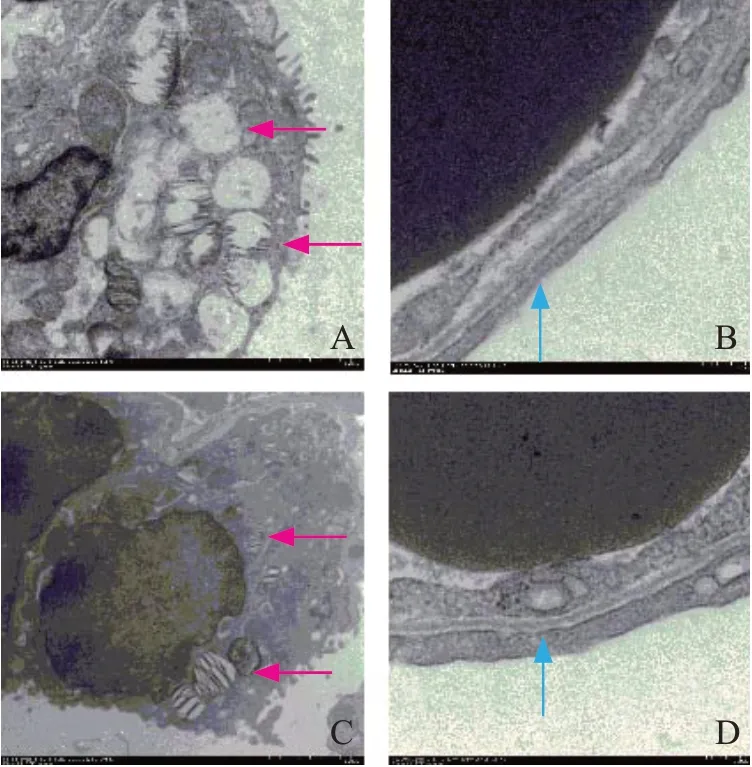
Figure 2. Organizational structure on electron microscopy. Red arrow: type II lamellar (×2,500); blue arrow: blood-gas barrier (×12,000); A, B: CCPR group; C, D: ECPR group; CCPR: conventional cardiopulmonary resuscitation; ECPR: extracorporeal cardiopulmonary resuscitation.
DISCUSSION
This study showed that ECPR had a better pulmonary protective effect than CCPR. Compared with the ECPR group, the CCPR group experienced more severe oxidative stress injury, and had a worse scavenging ability for oxygen free radicals. In the ECPR group, the more protective active proteins were present on the alveolar surface, the blood-gas barrier was intact, and there was greater abundance of the alveolar surface-active protein in the lamellar body and less pulmonary edema.
SP-A and SP-D can minimize lung injury by reducing the production of inflammatory factors, and can clear various pathogens.[13]Meanwhile, SP-A and SP-D have strong antioxidant functions, and these two proteins can alleviate and prevent the oxidative stress response of the lung induced by various etiologies.[14]Furthermore, SP-A can control apoptosis and stabilize alveolar epithelial cells.[15]Low SP-A expression in lung tissues will lead to decreased stability of alveolar epithelial cells and increase the possibility of pulmonary edema, pulmonary infection, and pulmonary injury.[16]The SP-D secretion in lung tissues has a clear protective effect on lungs against infection and maintains the stability of alveolar epithelial cells.[17,18]Lung injury will lead to the reduction of SP-A and SP-D in lung tissue.[13,19]The injury to the blood-gas barrier caused by lung injury will lead to the increased secretion of serum SP-A and SP-D.[20]Serum SP-D concentration is positively correlated with the degree of lung injury[21]as well as mortality.[22]When Clara cells are damaged or there are changes in alveolar epithelial permeability, the synthesis and secretion of CC16 will change, which is of great value in the diagnosis of lung injury.[21]Increased serum concentrations of CC16 can clearly indicate the lung injury, and are positively correlated with the degree of lung injury.[23-25]SOD is a key enzyme for scavenging oxygen free radicals. Cells are protected from superoxide damage by catalysis, competitive binding of superoxide radicals, and increased bioavailability of nitric oxide (NO).[26,27]MDA is the fi nal product of the lipid peroxidation of the main structure of the cell membrane and occurs because of the degradation of polyunsaturated lipids. Lipid peroxidation is a mature mechanism of cell damage in plants and animals, and it is used as an indicator of oxidative stress in cells and tissues.[28]Several experiments have shown that serum examination of MDA and SOD can refl ect the degree of oxidative damage to tissues.[29,30]MPO is an important enzyme released by neutrophils, and promotes the formation of hypochlorous acid, a powerful oxidant associated with bactericidal effects and tissue destruction through induction of necrosis and apoptosis.[31,32]The increased tissue MPO has been associated with aggravated oxidative damage of tissues.[29,33]Oxidative damage leads to the destruction of lipid structure of tissue cells, and subsequent increase of MDA in tissues.[30,33]This process will excessively consume SOD, resulting in a sharp decline in tissue SOD levels.[33]
The levels of serum and tissue SOD in the CCPR group decreased signifi cantly, however, the levels of serum SP-A, SP-D, CC16, and MDA, and the levels of tissue MDA and MPO were significantly increased, suggesting more severe lung injury and more severe damage from oxidative free radicals. The diff erences between the two groups after ROSC indicated that ECMO began to protect the lungs during the resuscitation process. Lung injury occurred in both groups and gradually decreased after resuscitation, but there was less injury and damage from oxidative free radicals in the ECPR group.
From the EVLW and PVPI results, we found that the pulmonary edema in the CCPR group was more serious when comparing the changes of EVLW and baseline values in the CCPR group and the ECPR group at ROSC6h. The PVPI value of the CCPR group showed an abnormal increase at ROSC6h, whereas the PVPI value of the ECPR group remained in the normal range and did not diff er from the baseline PVPI value. The increase in PVPI is attributable to permeable pulmonary edema, and the increased EVLW in the CCPR group was found to be mainly caused by permeable pulmonary edema. The serum and tissue SP-A and SP-D levels showed that the loss of SP-A and SP-D was serious in the CCPR group because of the release of more surface-active materials through damage to the bloodgas barrier rather than by secretion into the alveoli, which subsequently led to changes in the lung microstructure, increased alveolar tension, and abnormal changes of permeability, and contributed to the severity of the permeable pulmonary edema. Through this between-group comparison, ECMO was found to have a positive effect on reducing pulmonary fluid extravasation and also on stabilizing the vascular permeability of the lungs. Moreover, it indicated that the ECPR group had significantly less pulmonary edema compared with the CCPR group, which indicated the protection of lung function.
F urthermore, histology with electron microscopic examination showed that the CCPR group had severe alveolar type II lamellar body cell loss and inadequate storage of alveolar surface-active protein, which increased the damage to the blood-gas barrier, indicating an inability to guarantee the stability of the alveolar membrane permeability and increased permeability leading to pulmonary edema. However, the ECPR group retained better organized microstructure and showed alveolar type II cells with plenty of stored alveolar surface-active materials, which can be secreted as needed into the alveolar space. There was no serious damage to the blood-gas barrier, and therefore the optimal functioning of the barrier was maintained. Based on the histological changes between the two groups, it is reasonable to assume that the ECPR group can have greater resistance to resuscitated pulmonary exogenous infection than the CCPR group when the duration of treatment is prolonged.
The limitations of our experimental study are as follows. First, it is nearly impossible to carry out large-scale animal experiments; therefore, the sample size was small and there existed bias. Second, in order to enable ECMO to be added to the previous treatment process of ROSC, the catheterization for ECMO was completed well in advance. The timely placement and operation of ECMO are extremely difficult to be completed quickly in practical circumstances.
CONCLUSIONS
Early application of ECPR may have a better protective effect on post-resuscitation lung injury than CCPR. Its mechanism may be partly related to the regulation of the alveolar surface-active proteins and the improvement of oxidative stress response after resuscitation.
Funding:There was no funding support for this study.
Ethical approval:This study was approved by the Animal Care Committee of Capital Medical University Institutional and the Animal Care and Use Committee of Beijing Chaoyang Hospital, Capital Medical University.
Conflicts of interests:The authors declare that they have no confl icts of interest regarding this article.
Contributors:JYL proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
 World journal of emergency medicine2021年4期
World journal of emergency medicine2021年4期
- World journal of emergency medicine的其它文章
- Fatal and non-fatal injuries due to suspension trauma syndrome: A systematic review of defi nition, pathophysiology, and management controversies
- Saddle pulmonary embolism is not a sign of high-risk deterioration in non-high-risk patients: A propensity score-matched study
- Two-point compression ultrasonography: Enough to rule out lower extremity deep venous thrombosis?
- High-fl ow nasal cannula oxygen therapy and noninvasive ventilation for preventing extubation failure during weaning from mechanical ventilation assessed by lung ultrasound score: A single-center randomized study
- Comparison of clinical characteristics in patients with coronavirus disease and infl uenza A in Guangzhou, China
- Development of septic shock and prognostic assessment in critically ill patients with coronavirus disease outside Wuhan, China
