Comparison of clinical characteristics in patients with coronavirus disease and infl uenza A in Guangzhou, China
Wen-qiang Jiang, Xu-sheng Li, Wen-hong Zhong, Lin-qiang Huang, Xiao-jun Lin, Miao-yun Wen, Yu-jun Deng, Xin Li, Hong-ke Zeng
Department of Emergency and Critical Care Medicine, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
KEYWORDS: Coronavirus disease; Infl uenza A; Clinical characteristics
INTRODUCTION
The disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been named coronavirus disease (COVID-19) by World Health Organization (WHO).[1-3]Thus far, the COVID-19 has been spreading quickly, and almost all countries and regions around the world have been affected.[4-6]WHO has declared the COVID-19 outbreak as a global pandemic on March 11, 2020.[7]
The influenza A (H1N1) pdm09 virus causes seasonal epidemics that result in severe illnesses and deaths almost every year in China.[8]Coincidentally, it was found clinically that a few patients were diagnozed with both COVID-19 and infl uenza virus in China.[9]In light of this, there is an urgent need to distinguish COVID-19 characteristics from H1N1, which may play an important role in clinical practice.
This study aims to compare the epidemiological, clinical, and laboratory characteristics between patients with COVID-19 and H1N1, and to develop a diff erentiating model and a simple scoring system.
METHODS
Study design and participation
For this single-center retrospective study, we recruited COVID-19 patients with age >14 years from January 18 to February 28, 2020 and H1N1 patients from December 21, 2009 to February 5, 2010 who visited the fever clinics in the Guangdong Provincial People’s Hospital. The requirement for informed patient consent was waived by the ethics committee.
Defi nitions
A confirmed case was defined as a positive result of SARS-CoV-2 or H1N1 pdm09 on real-time reverse transcription-polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swab specimens.
Data collection
Data, including age, gender, COVID-19 related exposure history, clinical symptoms and signs, coexisting illness, laboratory findings, and radiologic changes on admission, were collected. Computed tomography (CT) and all laboratory tests were performed according to the clinical care needs of each individual patient.
Laboratory assessments included complete blood count, blood chemical analysis, coagulation testing, liver and renal functions, electrolytes, C-reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase, and creatine kinase. To ensure the accuracy of diagnosis, a consultant radiologist, a member of the Guangdong Provincial People’s Hospital expert group for COVID-19, was arranged to review all CT scan images.
Virological investigations
Nasal or nasopharyngeal swab samples were obtained when the patient visited the fever clinic, and the test for SARS-CoV-2 was performed at the Guangdong Provincial People’s Hospital. Reverse transcription-polymerase chain reaction (RT-PCR) assays were performed following the protocol established by the WHO.[10]
H1N1 pdm09 virus was detected by real-time RTPCR assay that used fluorogenic hydrolysis probe technology. The real-time RT-PCR assays were in accordance with the protocol from Centers for Disease Control and Prevention recommended by the WHO. The manufacturer’s protocol (Roche) was followed.
Statistical analysis
Categorical variables were expressed as frequency (%), and continuous variables were expressed as median with interquartile range (IQR). To compare variables between groups, the non-parametric comparative test andχ2test were used for continuous data and categorical data, respectively. AP-value <0.05 was considered statistically significant. Multivariate logistic regression analysis was performed using the signifi cant diff erentiating factors (P<0.05) between COVID-19 patients and H1N1 patients. The adjusted odds ratio (OR) was calculated by constructing logistic regression models of two diff erent variable sets (clinical and laboratory). Age and gender were initially entered into these models. For each model, the variables that did not meet the signifi cance level of likelihood ratio test (P>0.05) were removed by reverse elimination until all the unimportant variables were removed. The remaining signifi cant variables from each of the two initial models were then entered into a final multivariate logistic regression model. Insignificant variables were again removed by backward stepwise elimination. In the final multivariate logistic regression model, the scoring model was matched with the weight of age as 1, and the scores were allocated to the remaining variables according to their logicalOR. When an adjustedORwas less than 1, the best cut-off was less than or equal to the cut-off point, and the score was the reciprocal of adjustedOR, with scores rounded. Receiver operating characteristic (ROC) was constructed for the scoring model. All statistical analyses were performed using SPSS Statistics version 21.0 software.
RESULTS
Clinical features and severity of illness
A total of 236 patients were enrolled, including 20 patients with COVID-19 and 216 patients with H1N1. The most commonly reported symptoms of H1N1 patients were fever (100%), sore throat (54%), sputum production (51%), myalgia (51%), chilly (50%), headache (43%). The symptoms of COVID-19 patients included fever (80%), cough (35%), and sore throat (25%). Compared with H1N1 patients, COVID-19 patients were significantly older in age (26 years vs. 59 years,P<0.001) with those between 60 and 69 years being more common (3/216 vs. 10/20,P<0.001), while patients’ age under 39 years (185/216 vs. 6/20,P<0.001) were more common in H1N1 patients. H1N1 patients were more likely to have fever (100% vs. 80%,P<0.001) and chilly (50% vs. 5%,P<0.001). Additionally, upper respiratory symptoms were more common in H1N1 patients, including nasal congestion (29% vs. 0%,P=0.003), sneeze (12% vs. 0%,P<0.001), rhinorrhea (39% vs. 0%,P<0.001), sore throat (54% vs. 25%,P=0.018), and sputum production (51% vs. 25%,P=0.034). For some vital signs, COVID-19 patients had lower heart rate (86 beats/minute vs. 106 beats/minute,P<0.001) and body temperature (37.3 ℃ vs. 38.5 ℃,P<0.001), higher respiratory rate (20 breaths/minute vs. 20 breaths/minute,P=0.024) and systolic pressure (126.5 mmHg vs. 119 mmHg,P=0.013, 1 mmHg=0.133 kPa) compared with H1N1 patients. The indicators regarding severity assessment, such as Confusion, Uremia, Respiratory rate, Blood pressure and age 65 years (CURB-65) score, and quick Sequential Organ Failure Assessment (qSOFA) score between COVID-19 patients and H1N1 patients were comparable (allP>0.05); however, COVID-19 patients were more likely to develop pneumonia (75% vs. 14%,P<0.001) and longer in time from symptom onset to hospitalization (1 day vs. 5 days,P<0.001). The prevalence of chronic cardiovascular disease (35% vs. 1%,P<0.001) was signifi cantly higher in patients with COVID-19 than in those with H1N1. There were no significant differences between the two groups in the underlying medical conditions.
Laboratory fi ndings
Compared with H1N1 patients, COVID-19 patients had lower leukocyte counts (6.495×109/L vs. 4.215×109/L,P<0.001), lower ratios and counts of neutrophil (ratio 0.743 vs. 0.615,P=0.001; counts 4.76×109/L vs. 2.86×109/L,P<0.001) and basophilic granulocyte (ratio 0.003 vs. 0.002,P=0.032; counts 0.02×109/L vs. 0.01×109/L,P=0.001), and a higher ratio of lymphocyte (0.173 vs. 0.235,P=0.001). Compared with H1N1 patients, levels of hemoglobin (135.5 g/L vs. 148 g/L,P=0.005) and lymphocyte to monocyte ratio (LMR, 1.38 vs. 1.86,P=0.036) were higher in COVID-19 patients, while neutrophil to lymphocyte ratio (NLR, 5.31 vs. 2.67,P<0.001) and platelet to lymphocyte ratio (PLR, 212.74 vs. 164.37,P=0.023) were lower. As for liver and renal functions, patients’ lactate dehydrogenase (199 U/L vs. 144 U/L,P<0.001) was higher and creatine kinase-myocardial band isoenzyme (CK-MB, 4.0 U/L vs. 10.7 U/L,P<0.001) was lower in COVID-19 patients than in H1N1 patients.
lndependent diff erentiating factors of COVlD-19 in suspected COVlD-19 patients
Multivariate logistic regression analysis was performed for identifying the risk factors of SARSCoV-2. The ROC curve analysis was used to distinguish the best cut-off value of these factors. The significant variables that remained in favor of SARS-CoV-2 infection were age >34 years (adjustedOR1.167, 95% confi dence interval [CI] 1.077-1.264), temperature >37.5 °C (adjustedOR0.054, 95%CI0.009-0.314), no sputum (adjustedOR12.591, 95%CI1.412-112.305), without myalgia (adjustedOR19.318, 95%CI1.480-252.214), lymphocyte ratios ≥20% (adjustedOR117.372, 95%CI1.825-165.330) and CK-MB >9.7 U/L (adjustedOR1.091, 95%CI1.033-1.152), which were independent diff erentiating factors. Each of these variables was scored according to itsOR(Table 1). The sum of the scores was the total score of predicted COVID-19.
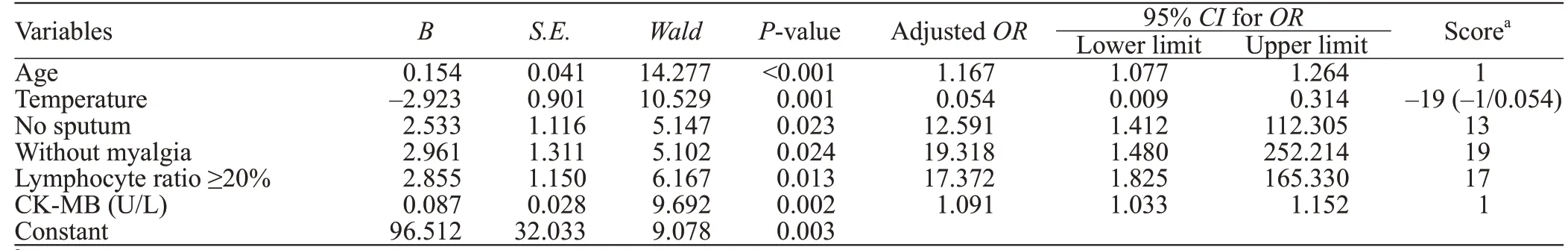
Table 1. Adjusted odds ratio (OR) for signifi cant features of COVID-19
A regression equation model of predicted probability was established, including the only independent risk factor, as follows:
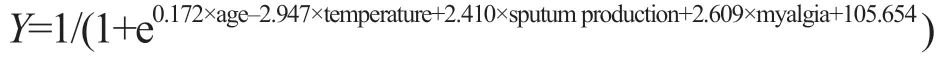
(without sputum production, sputum production=0; with sputum production, sputum production= -1; without myalgia, myalgia=0; with myalgia, myalgia= -1)
Diff erentiating performance of the prediction model and scoring system for COVlD-19
The area under curves (AUCs) of the prediction model and scoring system in differentiating COVID-19 patients from H1N1 were 0.988 (0.974-1.000) (cut-off value >0.1, sensitivity=95.0%, specificity=94.8%) and 0.962 (0.922-1.000) (cut-off value >18.0, sensitivity=90.0%, specifi city=90.3%), respectively (Figure 1).
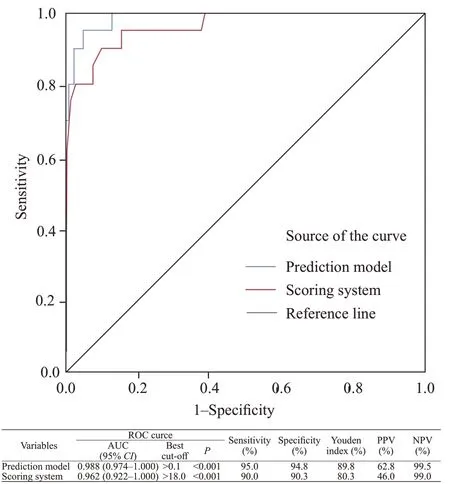
Figure 1. ROC analyses of the model prediction probability and scoring system for diff erentiating COVID-19. PPV: positive predictive value; NPV: negative predictive value; ROC: receiver operating characteristic; AUC: area under curve.
DISCUSSION
Our study retrospectively analyzed H1N1 patients in the Guangdong Provincial People’s Hospital diagnosed from 2009 to 2010 and COVID-19 patients diagnosed in 2020. We presented the diff erences between the early clinical features and laboratory fi ndings of both diseases. Besides, we have formulated a simple scale to distinguish the above two kinds of infectious diseases in order to better clinically identify and treat patients with related infections.
Similarities of clinical features between COVID-19 and H1N1 have been earlier noted.[9]The present fi ndings showed that there was no significant difference in the severity of the disease between the two groups by two clinical scores; however, the proportion of pneumonia in the COVID-19 group was significantly higher than that in the H1N1 group. Patients in the two groups mainly presented with fever, sore throat, and cough. However, very few patients with COVID-19 infection had symptoms as chilly, nasal congestion, sneeze, rhinorrhea, headache, or myalgia, indicating that the target cells may be located in the lower airway. This may be related to the difference in binding sites between the two viruses and their receptors.[11-13]It has been speculated that COVID-19 infected human respiratory epithelial cells through the spike protein on the outer surface of the virus and the mediation of angiotensin-converting enzyme-2 (ACE2) receptors on the surface of human cells.[14]Of note, ACE2 is expressed at higher levels in the lungs of the elderly,[15]which may offer an explanation on why older people are more vulnerable to be infected with COVID-19. Patients with COVID-19 have a lower heart rate than those with H1N1, which may refl ect diff erences in body temperature rather than the severity of pneumonia. It remains uncertain if there is any diff erence in heart damage between the two viruses. Results of the multivariate analysis indicated that sputum and myalgia were more common in H1N1 patients, which was not completely consistent with Tang’s report.[16]
For laboratory findings, we found that lymphopenia and low lymphocyte ratio were common not only in COVID-19 patients but also in H1N1 patients, as well as a reduced number of basophils, a feature that was consistent with most current clinical studies.[2-3,17-19]Interestingly, the lymphocyte ratio of COVID-19 patients was signifi cantly higher than that of H1N1 patients. The difference may be attributed to the higher neutrophils in the H1N1 group. However, there was no statistical difference in lymphocyte counts between the two groups. We found that the number of basophils of COVID-19 patients was significantly lower than that of H1N1 patients, but the reason was still unclear. The NLR, PLR, and LMR, as immune and infection-related biomarkers, had been widely reported in tumors and other infectious diseases;[20-22]increases in NLR and MLR in COVID-19 patients often indicate impaired immune function and can predict progression.[18,23-24]In the current study, NLR and PLR were elevated in both groups, but higher in H1N1 patients than in COVID-19 patients. However, in our cohort, patients of the two groups were equally severe, even more severe in COVID-19 patients. In other words, the diff erence between the two groups may not be due to the severity of the disease, but to the diff erent pathogenesis of the two viruses. The lung and the immune system are the main targets that the two viruses attack. From our data, the proportion of pneumonia was signifi cantly higher in the COVID-19 group than in the H1N1 group, so we speculated that SARS-CoV-2 may be more prone to the lungs, while H1N1 pdm09 virus to the immune system.
In addition, H1N1 patients had higher serum creatinine levels but lower CK-MB levels. This suggests that H1N1 pdm09 may be more likely to damage the kidney, but the effect on the heart was not as much as that of the COVID-19. A recent study[25]reported that pericytes with high expression of ACE2 might act as the target cardiac cell of SARS-CoV-2. The pericytes injury due to virus infection may result in capillary endothelial cells dysfunction, inducing microvascular dysfunction. It is interesting to note that patients with basic heart failure disease showed increased ACE2 expression at both mRNA and protein levels. This may be one reason why COVID-19 had a higher proportion of basic chronic cardiovascular disease and a higher level of CK-MB in our cohort.
Finally, we comprehensively analyzed the clinical characteristics and laboratory fi ndings, and screened out six diff erentiating factors for COVID-19: age >34 years, temperature ≤37.5 ℃, no sputum or myalgia, lymphocyte ratio ≥20% and CK-MB >9.7 U/L. Besides, based on theORof each factor in the regression model, we weighted each factor and developed a scoring scale, in which a patient with a score >18 was very likely to diagnose COVID-19. At present, viral nucleic acid detection may still be the most eff ective method to distinguish between different pathogen infections;[26,27]however, there is a certain false negative in nucleic acid detection, and there is a shortage of detection reagents in the outbreak of infectious disease. Although COVID-19 shares many similarities with H1N1, it has its own characteristics. In the present study, the developed prediction model and scoring system both have good differentiating performance. The clinical model scores based on the clinical features of H1N1 and COVID-19 may be a good complement to the clinical diagnosis. Meanwhile, in areas where two diseases coexist, the simple scoring system may be useful to identify patients with certain features. This strategy of hierarchical treatment of patients can relieve strained medical resources, especially during outbreaks of infectious disease.
This study analyzed and compared the differences between the COVID-19 and H1N1 among non-critical patients in the fever clinic. However, the current study had some limitations. Firstly, this was a single-center study, and the data for the two groups were not from the same period. In view of this, the applicability of our findings may vary depending upon the relative prevalence of the etiological agent during any particular period. Secondly, the majority of patients in the two groups were mild patients, and their clinical characteristics and laboratory data may not be applicable to patients with severe infection. Thirdly, CT imaging diagnosis is one of the important methods of the COVID-19.[28,29]However, the radiological assessment of influenza cases in 2009 was not as good as that of patients with COVID-19 in 2020. Therefore, we did not explore in depth in imaging. Finally, the power of the study was limited because of a small sample size; and the clinical data were incomplete, such as lack of CRP, PCT, and other infl ammatory indicators.
CONCLUSIONS
The present results indicate that there are clinical and laboratory differences between COVID-19 and H1N1. Compared with H1N1, COVID-19 patients are older, no sputum or myalgia, lymphocyte ratio ≥20%, and CKMB >9.7 U/L. The prediction model and scoring system based on these independent discriminant factors have a good diff erentiating performance. It may be a useful tool to identify patients who need further tests for SARS-CoV-2 and immediate medical isolation. However, it is of paramount importance to consider the epidemiological situation in each country and region when applying this model.
Funding:None.
Ethical approval:The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital.
Conflicts of interests:The authors declare that they have no competing interests.
Contributors:WQJ, XSL, and WHZ contributed equally to this paper. All authors have read and approved the fi nal manuscript.
 World journal of emergency medicine2021年4期
World journal of emergency medicine2021年4期
- World journal of emergency medicine的其它文章
- Fatal and non-fatal injuries due to suspension trauma syndrome: A systematic review of defi nition, pathophysiology, and management controversies
- Saddle pulmonary embolism is not a sign of high-risk deterioration in non-high-risk patients: A propensity score-matched study
- Two-point compression ultrasonography: Enough to rule out lower extremity deep venous thrombosis?
- High-fl ow nasal cannula oxygen therapy and noninvasive ventilation for preventing extubation failure during weaning from mechanical ventilation assessed by lung ultrasound score: A single-center randomized study
- Development of septic shock and prognostic assessment in critically ill patients with coronavirus disease outside Wuhan, China
- Protective eff ect of extracorporeal membrane pulmonary oxygenation combined with cardiopulmonary resuscitation on post-resuscitation lung injury
