In vitro antimicrobial and synergistic effect of essential oil from the red macroalgae Centroceras clavulatum (C. Agardh) Montagne with conventional antibiotics
Ahmed Nafis, Fatima El Khalloufi, Asmae Aknaf , Brahim Oudra, Najat Marraiki, Sarah Al-Rashed, Abdallah M.Elgorban, Asad Syed, Lahcen Hassani, Luísa Custódio
1Microbiology, Health and Environment Team, Faculty of Sciences Chouaïb Doukkali University, El Jadida, Morocco
2Laboratory of Chemistry, Modeling and Environmental Sciences, Polydisciplinary Faculty of Khouribga, Sultan Moulay Slimane University of Beni Mellal, B.P.: 145, 25000, Khouribga, Morocco
3Polydisciplinary Faculty of Nador, University Mohammed First, BP 300, 62700, Nador, Morocco
4Laboratory of Water, Biodiversity and Climate Change. Faculty of Sciences Semlalia Marrakech, Cadi Ayyad University, P.O. Box 2390, 40000,Marrakech, Morocco
5Department of Botany and Microbiology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia
6Laboratory of Microbial Biotechnologies, Agrosciences, and Environment (BioMAgE), Faculty of Sciences Semlalia Marrakech, Cadi Ayyad University, P.O. Box 2390, 40000, Marrakech, Morocco
7Center of Marine Sciences, Faculty of Sciences and Technology, University of Algarve, Ed. 7, Campus of Gambelas, 8005-139 Faro, Portugal
ABSTRACT Objective:To study the chemical profile, antimicrobial properties,and synergistic effect with known antibiotics of essential oil extracted from the marine red macroalgae Centroceras clavulatum (C.Agardh) Montagne, collected in Morocco.
KEYWORDS:Macroalgae; Centroceras clavulatum (C. Agardh);Antimicrobial potential; Microbial drug resistance; Marine natural products; Synergism with antibiotics
1. Introduction
The increasing microbial drug resistance occurring worldwide is a major worry of public health[1]. Approximately 700 000 persons die each year due to resistant microorganisms, while about 10 million lives will be threatened per year as a result of the increase in pharmaco-resistant infections[2]. Therefore, the development of new strategies to overcome microbial drug resistance becomes mandatory, such as the identification of new antimicrobial agents from natural sources, increasing the efficacy of existing antibiotics,or the combination of both[1,3]. In particular, the assessment of possible synergy between natural products and existing antibiotics is considered an emerging area in phytomedicine’s research[4].
Marine organisms are an exceptional source of secondary metabolites with unique chemical features[5]. Until now, more than 28 000 compounds were identified from different marine species, with potential applications in the energy, food, cosmetic,pharmaceutical, and agricultural areas[6]. Among marine organisms,seaweeds, i.e., marine macroalgae, have a long history of traditional uses either for direct human consumption or for medicinal purposes[7,8]. These uses are due to their richness in a wide variety of bioactive compounds, which include carrageenans, alkaloids,polysaccharides, and quinones[6,9], displaying important biological properties, such as antioxidants, anticancer, antimicrobial, and antiviral[10-12].
The Centroceras genus contains 17 species distributed worldwide[13],but only one, Centroceras clavulatum (C. clavulatum) (C. Agardh)Montagne (Ceramiaceae, Rhodophyta), is found in Morocco[14].C. clavulatum is a red macroalga and contains several bioactive molecules, such as (-)-loliolide, neophytadiene, phytol, phenolics, and phycobiliproteins[13,15]. This species shows relevant bioactivities, such as antioxidant, anti-trypanosomal, and antifungal[13,16]. In addition,Murugan et al.[16] reported that C. clavulatum-synthesized silver nanoparticles had significant activity against Aedes aegypti, the primary vector of dengue, with residual toxicity towards mammalian cells.Organic extracts of C. clavulatum also had significant inhibition against Proteus mirabilis[17] and Staphylococcus aureus (S. aureus)[18]. This work aimed to further explore an essential oil (EO) of C. clavulatum as a potential source of antimicrobial agents. For that purpose, EO was extracted in a Clevenger-type apparatus, and its chemical profile was established by gas chromatography coupled to mass spectroscopy(GC/MS). The EO was then evaluated for in vitro anti-bacterial and anti-fungal properties, and for synergistic effects with conventional antibiotics, namely ciprofloxacin and fluconazole.
2. Materials and methods
2.1. Algal sample collection
Samples of C. clavulatum (4 kg) were collected in the Marchica Mediterranean lagoon (35.156468” N; −2.90434200 W), in June of 2019. The alga was identified by one of the authors (Asmae AKNAF) and a voucher specimen (CECL-101) was deposited at the Laboratory of Microbial Biotechnologies, Agrosciences and Environment, Faculty of Sciences Semlalia, Cadi Ayyad University,Marrakech Morocco. The collected material was air-dried for one week at room temperature (circa 25 ℃), and reduced to powder.
2.2. Extraction and chemical composition analysis of the EO
The dried biomass was subjected to steam-distillation for 3 h until total recovery of EO was achieved, using a Clevenger-type apparatus, and the obtained sample was stored at 4 ℃ in darkness until use. The obtained EO yield in relation to the dry plant material was 0.6% (w/v). The quantitative and qualitative analyses of the chemical components of the EO were performed by GC/MS, as described previously[19]. The volatile constituents were identified by comparing their retention indices and mass spectra with reference libraries[20].
2.3. Assessment of the antimicrobial activity
2.3.1. Tested microorganisms
The following pathogenic bacterial species were used to evaluate the antibacterial potential of the EO: Pseudomonas aeruginosa (P.aeruginosa) (DSM 50090), Escherichia coli (E. coli) (ATCC 8739),Bacillus subtilis (B. subtilis) (ATCC 9524), Micrococcus luteus (ATCC 10240), S. aureus (CCMM B3) and Klebsiella pneumoniae (clinical isolate). The antifungal activity was tested against four pathogenic Candida strains, namely Candida albicans CCMM-L4, Candida glabrata CCMM-L7, Candida krusei CCMM-L10, and Candida parapsilosis CCMM-L18[21].
2.3.2. Disc diffusion method
The disk diffusion method was used to test the antimicrobial activities of the EO as described previously by the National Committee for Clinical Laboratory Standards[22]. Briefly, Whatman sterile disks (6 mm) were soaked with 10 µL of the EO (at the concentration of 981 mg/mL) and placed on the surface of the inoculated agar media using a cell suspension of bacteria (at 10CFU/mL) and yeasts (at 10CFU/mL). The Petri dishes were incubated for 2 h at 4 ℃ to promote the diffusion, and then further kept at 28 ℃ for 48 h and 37 ℃ for 24 h for yeasts and bacteria,respectively. The antimicrobial activity was determined by measuring the diameters of the clear inhibition zone. Discs of ciprofloxacin(5 µg) and fluconazole (10 µg) were used as positive standards for antibacterial and antifungal activity, respectively.
2.3.3. Determination of minimum inhibitory concentration(MIC), minimum microbicidal concentration (MMC), and synergistic effects
The two-fold serial dilution method in 96-well microplates, known as the microdilution assay, was used to evaluate the MIC of the algae EO[23]. In brief, 100 µL of each dilution of the sample (from 40 mg/mL to 0.019 5 mg/mL) were added to the same volume of an overnight microbial culture diluted at a ratio of 1/50. The plates were then incubated at the optimal conditions for each microorganism.The MMC was determined by spreading an inoculum, taken from each without visible growth microwell, on the agar media.
The synergistic effect of the EO, at a sub-inhibitory concentration(MIC/4), combined with the reference antibiotics (ciprofloxacin and fluconazole) was evaluated by the checkerboard method[24]. Aliquots(50 µL) of the EO were mixed with 50 µL of each antibiotic dilution and added to 100 µL of cell suspension. Then, the 96-microwell assay plates were incubated at the optimal temperature of each microorganism. The fractional inhibitory concentration (FIC) was calculated according to the following formula:
FIC of oil = MIC of EO in combination with antibiotic/MIC of EO alone; FIC of antibiotic = MIC of antibiotic in combination with EO/MIC of antibiotic alone; FIC index = FIC of EO + FIC of antibiotic
This assay allows for the determination of the fractional inhibitory concentration index (FICI) values which are then interpreted according to the following odds[25]: No interaction (FICI between 0.5 and 4), synergism (FICI ≤ 0.5), and antagonism (FICI ≥ 4).
The antibiotic MIC gains were calculated according to the following formula: MIC gain = MIC of antibiotic alone/MIC of antibiotic combined with EO.
2.4. Statistical analyses
Statistical analysis was made using one-way analysis of variance(ANOVA) with SNK test (Student, Newman, Keuls) of SPSS version 21.0. Data of all analyses, in triplicate, expressed as mean values ±standard deviations (SD) were calculated. Analysis of variance was performed on the basis of mean values to determine the significant difference among results at P < 0.05.
3. Results
3.1. Chemical composition of the EO
The steam distillation of the dried thallus of the red macroalgae C.clavulatum afforded a yellow liquid, EO, with an extraction yield of 0.6%. The chemical composition of the EO was established by GC/MS, and results are summarized in Table 1 and Figure 1. Thirty compounds were identified, representing 96.27% of the total oil composition. Monoterpenes were the predominant compounds(56.67%), followed by sesquiterpenes (24.03%). Other compounds accounted for 15.57% of the total oil. The major constituents were carvacrol (36.06%), caryophyllene (14.67%), endo-borneol (9.04%)pyroterebic acid (3.23%) and caryophyllene oxide (3.13%).
3.2. Antimicrobial activity
The antimicrobial activity of the EO was evaluated against pathogenic bacteria and clinical yeasts and obtained results are summarized in Table 2. The oil had a moderate antimicrobial activity with inhibition zone diameters ranging from 9.0 to 15.0 mm, and the MIC values ranged from 0.9 to 14.7 mg/mL. The bacteria B. subtilis and E.coli were more sensitive, with MIC values of 0.9 and 1.9 mg/mL,respectively. S. aureus, Klebsiella pneumoniae, and P. aeruginosa had the same sensitivity as Candida albicans and Candida krusei, with MIC values of 14.7 mg/mL. More importantly, the MIC values were often equal to the MMC values showing the microbicidal action of the tested oil, except for B. subtilis, E. coli, Candida glabrata, and Candida krusei.
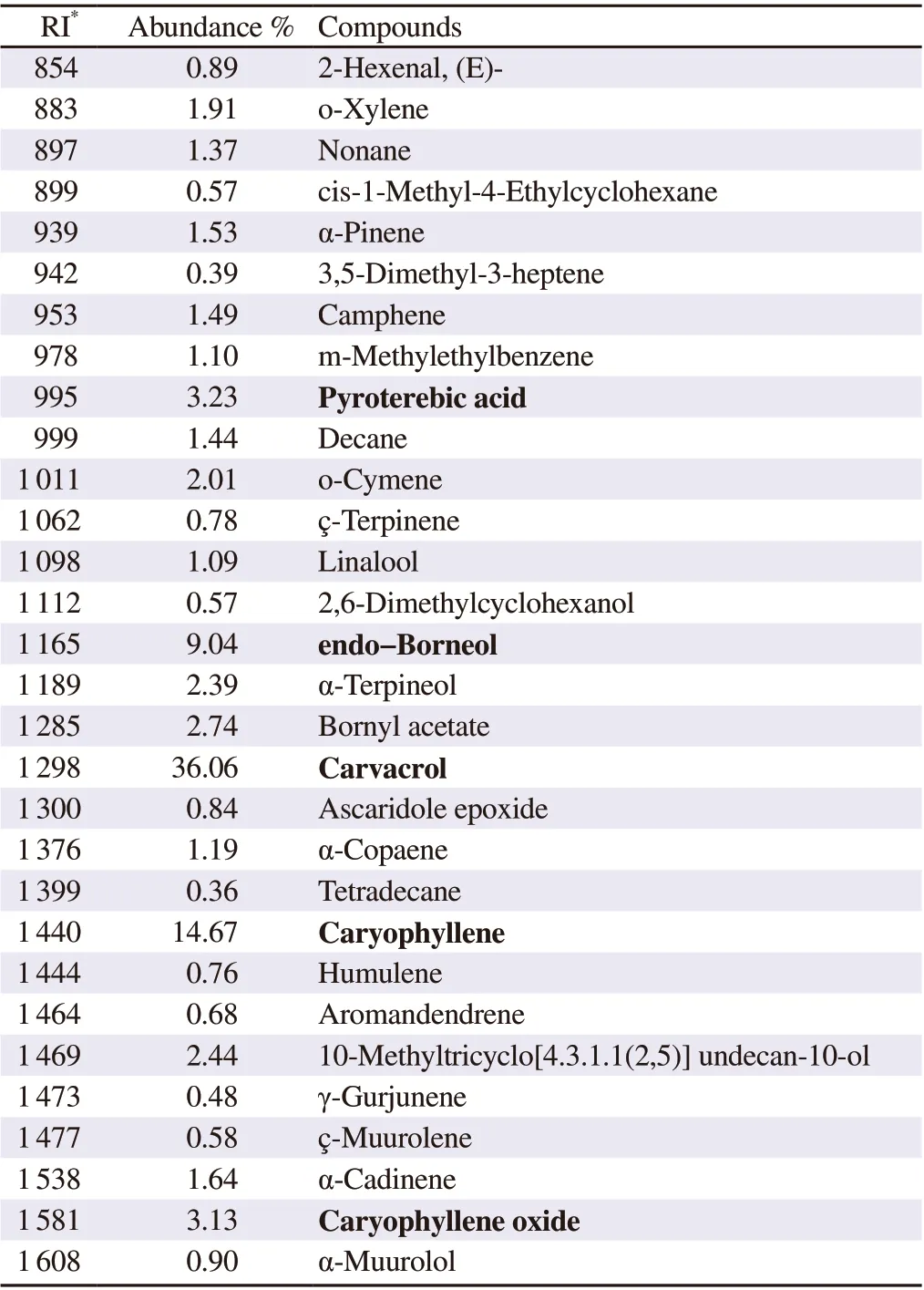
Table 1. Volatile components identified in the essential oil from Centroceras clavulatum (C. Agardh) Montagne by gas chromatography coupled to mass spectroscopy.
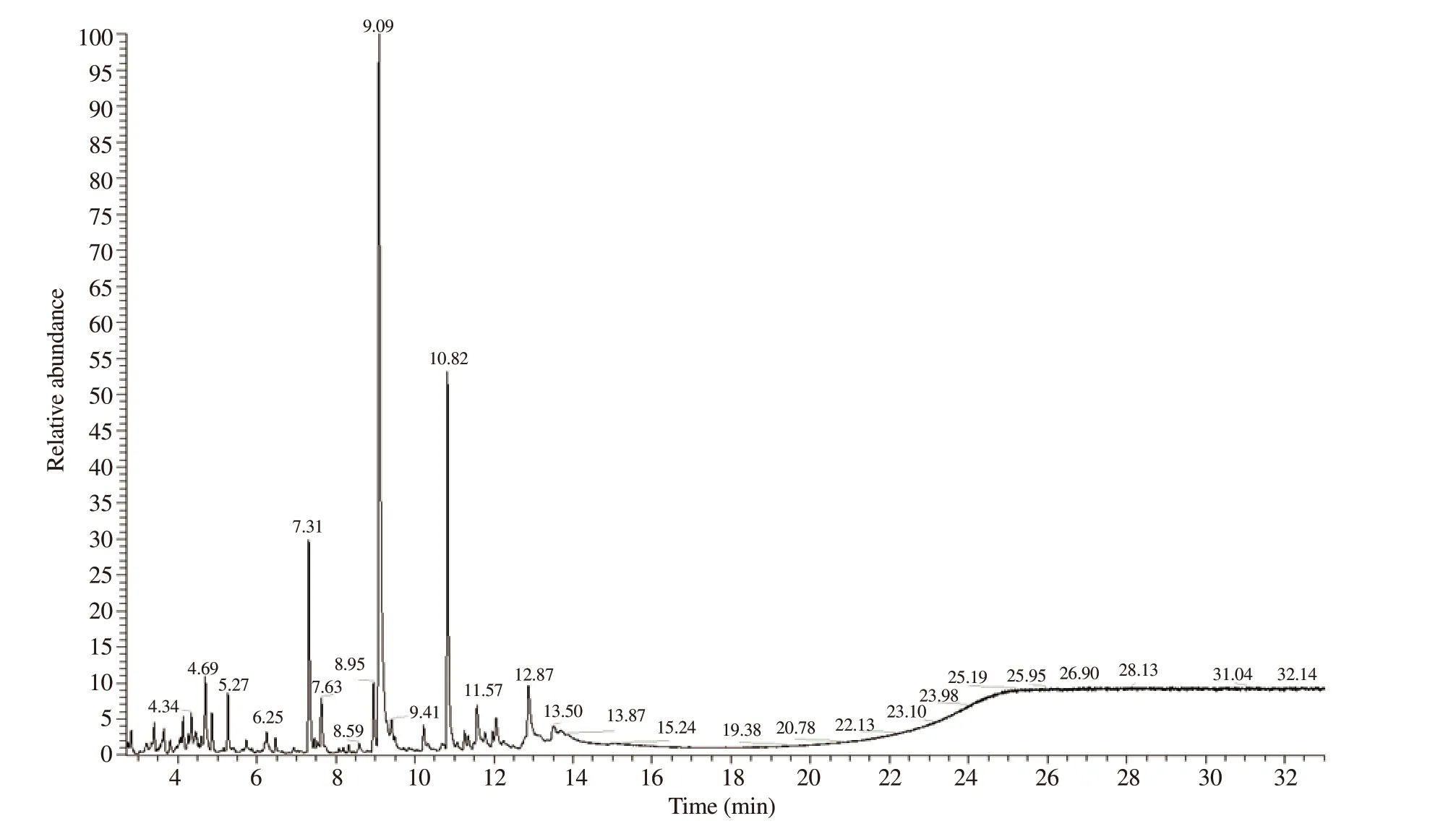
Figure 1. Gas chromatography/mass spectrometry chromatogram of Centroceras clavulatum (C. Agardh) Montagne essential oil.
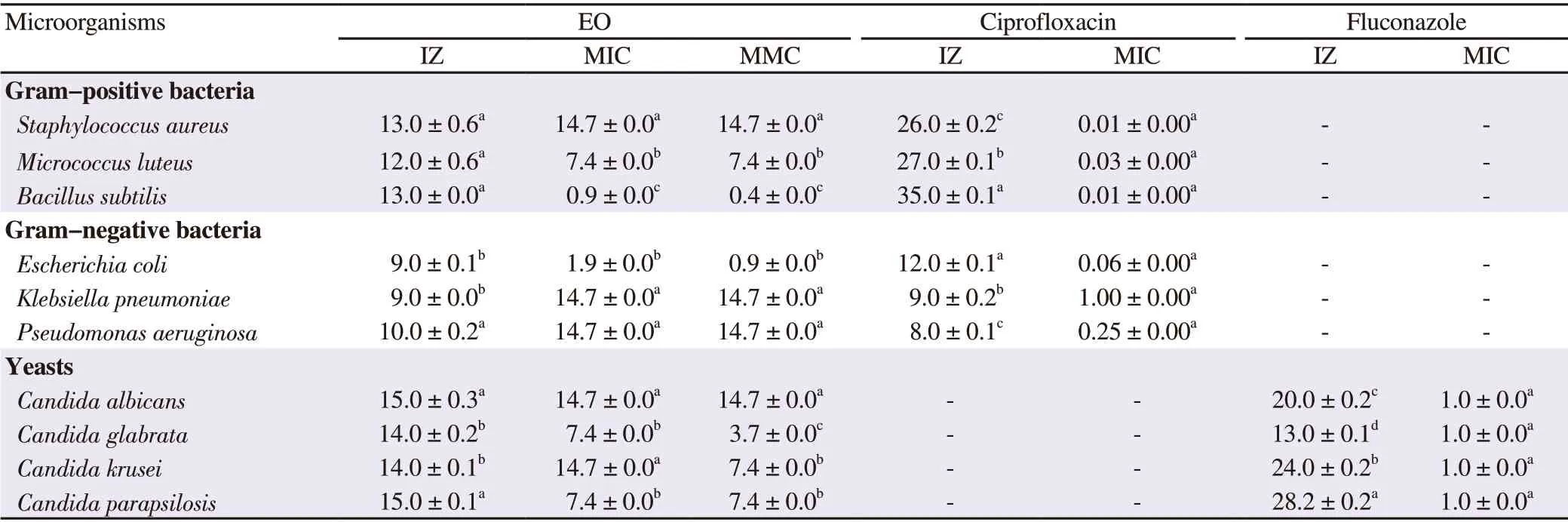
Table 2. Inhibition zone diameters, minimum inhibitory concentration, and minimum microbicidal concentration of essential oil extracted from the algae Centroceras clavulatum (C. Agardh) Montagne against pathogenic bacteria and yeasts using the disc diffusion and micro-well dilution assays.

Table 3. Synergistic interaction of Centroceras clavulatum (C. Agardh) Montagne essential oil and ciprofloxacin against resistant bacteria.
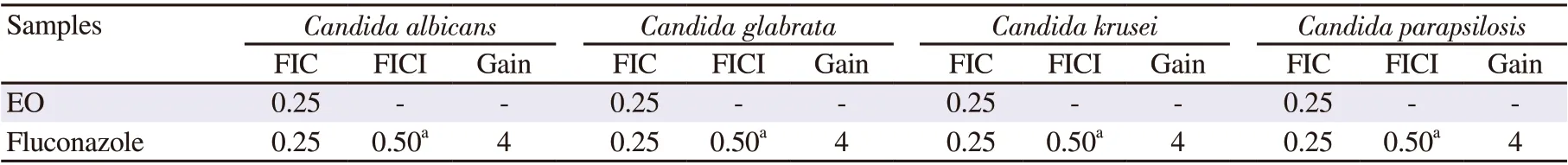
Table 4. Synergistic interaction of Centroceras clavulatum (C. Agardh) Montagne essential oil and fluconazole against clinical pathogenic yeasts.
3.3. Synergistic effects with antimicrobial drugs
The results obtained on the synergistic effects of C. clavulatum EO at a sub-inhibitory concentration (MIC/4) in combination with two standard antimicrobial drugs, ciprofloxacin, and fluconazole,are presented in Tables 3 and 4. Eighty percent of the tested combinations showed synergistic interaction regarding all tested strains with a considerable reduction of the antibiotic’s MICs,with FICI values varying between 0.31 and 0.50. No interaction was observed for the combination of ciprofloxacin and EO against Micrococcus luteus and P. aeruginosa, with a FICI value of 0.75. The EO reduced the MICs of the antibiotics by 2 to 16-fold, as gains.The highest synergism was observed in B. subtilis, with a 16-fold gain. The EO significantly reduced the sensitivity of S. aureus, E.coli and Klebsiella pneumoniae to ciprofloxacin, with an 8-fold gain.Concerning the yeast strains, a 4-fold gain was obtained for all tested Candida species.
4. Discussion
Few investigations have focused on EO from macroalgae. The obtained yield (0.6%) from the EO in this study is higher than that obtained from the red macroalgae Laurencia dendroidea J. Agardh(0.066%)[26]. Our results greatly differ from those previously obtained with other macroalgae species. For example, the main terpene components of the EO extracted from the cultivated red macroalgae Ochtodes secundiramea (Montagne) M. Howe were myrcene, 10Z-bromomyrcene, 10E-bromo-3-chloromyrcene,apakaochtodene B, and acyclic 1,3-dibromoadamantane[27].Moreover, Gressler et al.[26] identified two major tricyclic sesquiterpenes (SPF-1 and SPF-2) in EO from the red seaweed Laurencia dendroidea collected on the Brazilian coast. In another work, Jerković et al.[28] reported the volatile oil composition of the brown macroalgae Taonia atomaria (Woodward) J. Agardh and Padina pavonica (Linnaeus) Lamouroux, sampled from the central Adriatic Sea. The major compounds identified in the oil from Taonia atomaria were germacrene D, epi-bicyclosesquiphellandrene,β-cubebene, and gleenol, while higher aliphatic alcohols, such as trans-phytol and pachydictol A, were the chief molecules in Padina pavonica[28]. In another study, Patra et al.[29,30] identified high levels of tetradecanoic and hexadecanoic acids in the oil extracted from the edible seaweed species, namely Laminaria japonica L. (kombu,brown algae) and Undaria pinnatifida (Harvey) Suringar (wakame,green algae).
Macroalgae have a high potential to provide biomedical compounds,for example, antimicrobials to tackle antimicrobial resistance issues.The detected antimicrobial activity in the C. clavulatum EO may be due to the presence of carvacrol, which was identified as the major oil component. Essential oils from plants containing high levels of carvacrol presented high antimicrobial activity towards numerous pathogens[31]. In addition, several studies showed that carvacrol has antimicrobial properties and stated that it is extensively used to control pathogens by causing damage to the microbial membrane,particularly the reduction of energy-dependent cellular processes due to the subsequent reduction in adenosine triphosphate synthesis and dissolution of the proton motive force[32,33]. Furthermore, the antimicrobial activity might be also related to the presence of other components in the EO, such as caryophyllene and caryophyllene oxide that also exhibit antimicrobial properties[34,35].
Several studies reported the inhibitory activities of seaweed extracts against bacteria and yeasts, but few focused on EO[27,29,30,36]. The EO from Laminaria japonica was tested against three foodborne pathogens and displayed potent antibacterial activity, especially against S. aureus ATCC49444[29]. In another study, the EO from Undaria pinnatifida had an interesting activity against Salmonella typhimurium ATCC 43174 with a MIC value of 25 mg/mL[30].Furthermore, Patra and Baek[37] demonstrated the effectiveness of the EO extracted by microwave hydro-distillation method from Enteromorpha linza (Linnaeus) J. Agardh and Porphyra tenera Kjellman against Listeria monocytogenes, with a MIC value of 12.5 mg/mL for both species.
However, several recent works reported the interest in mixing carvacrol, which was the major volatile identified in the C.clavulatum EO, with several antibiotics[33]. For example, the combination of carvacrol with erythromycin was assessed by the checkerboard assay against several bacteria, and a 2 to 2 048-fold reduction of the MIC value of erythromycin was observed[31].Carvacrol could also significantly reduce the MIC values of tetracycline, ampicillin, novobiocin, penicillin, and erythromycin by 1/4 to 1/8[38]. However, many investigations have shown the synergistic effect of the combination of macroalgae extracts with antibiotics. The combination of a dichloromethane extract from Bifurcaria bifurcata R.Ross with rifampicin, ampicillin, gentamicin,and tetracycline, resulted in a significant decrease in the MIC values of these antibiotics against some bacterial strains[39]. Additionally,Eom et al.[40] evaluated the interaction between the hexane fraction of a methanol extract from Ecklonia cava Kjellman and two commercial antimicrobials, namely oxytetracycline and erythromycin, and observed that the antibiotic MICs were markedly reduced by 64-fold against the resistant strain Streptococcus parauberis. He et al.[41]also evaluated the synergistic effect of combining azithromycin and marine alginate-derived oligosaccharide against the resistant P.aeruginosa and a significant reduction factor of 2.5 was observed.A significant average reduction with a factor of four was obtained when fucoidan, a sulphated polysaccharide extracted from brown seaweeds, was combined with ampicillin and gentamicin against some oral pathogenic bacteria[42].
In conclusion, 30 components were found in the EO, with a predominance of carvacrol and caryophyllene. The oil displayed moderate antimicrobial activity towards tested bacteria and pathogenic yeasts, but a significant synergistic interaction was observed regarding most of tested strains, with a considerable reduction of the MICs of the used antibiotics. The antimicrobial properties of the EO may be likely due to the presence of carvacrol and caryophyllene, as their major compounds. Our findings suggest that the antimicrobial properties of the EO extracted from C.clavulatum should be further explored and clarified for its possible use to restore the activity of used drugs and to aid to the management of multi-drug resistant microorganisms.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/56), King Saud University, Riyadh,Saudi Arabia, and the Foundation for Science and Technology (FCT)and the Portuguese National Budget for the UIDB/04326/2019 project, and the FCT Scientific Employment Stimulus(CEECIND/00425/2017).
Funding
This study is supported by the Researchers Supporting Project number(RSP-2021/56), King Saud University, Riyadh, Saudi Arabia; the Foundation for Science and Technology (FCT) and the Portuguese National Budget for the UIDB/04326/2019 project; and the FCT Scientific Employment Stimulus (CEECIND/00425/2017).
Authors
’contributions
For the current article, individual author’s contributions are as follows: conceptualization, AN and FEK; sampling and taxonomy,AA and BO; methodology, AN; statistic and validation, SAR and AME; review of the article draft, NM; investigation, LH and LC;writing—original draft preparation, AN; writing—review and editing, LC; resources and funding acquisition, AME and LC.
 Asian Pacific Journal of Tropical Biomedicine2021年9期
Asian Pacific Journal of Tropical Biomedicine2021年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Improved performance of naringenin herbosomes over naringenin in streptozotocininduced diabetic rats: In vitro and in vivo evaluation
- Tylophora hirsuta L. leaf extract attenuates alloxan-induced diabetes in mice by suppressing oxidative stress and α-amylase
- Antibacterial activity and inhibition against Staphylococcus aureus NorA efflux pump by ferulic acid and its esterified derivatives
- Biopeptides of Pyropia yezoensis and their potential health benefits: A review
