Tylophora hirsuta L. leaf extract attenuates alloxan-induced diabetes in mice by suppressing oxidative stress and α-amylase
Muhammad Furqan Akhtar, Arsalan Shagufta, Ammara Saleem✉, Mirza Muhammad Faran Ashraf Baig, Ali Sharif , Azhar Rasul, Mohamed M. Abdel-Daim
1Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore Campus, Lahore, Pakistan
2Department of Pharmacology, Faculty of Pharmaceutical Sciences, Government College University Faisalabad, Faisalabad, Pakistan
3Laboratory of Stem Cells Research and Biomedical Engineering for Novel Biofunctional, and Pharmaceutical Nanomaterials, Faculty of Dentistry,Prince Philip Dental Hospital, The University of Hong Kong, Hong Kong 999077, China
4Institute of Pharmacy, Faculty of Pharmaceutical and Allied Health Sciences, Lahore College for Women University, Jail Road, Lahore, Pakistan
5Department of Zoology, Government College University Faisalabad, Faisalabad, Pakistan
6Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt
ABSTRACT Objective:To evaluate the antidiabetic potential of leaf extracts of Tylophora hirsuta (T. hirsuta).
KEYWORDS:Tylophora hirsuta; Diabetes mellitus; Oxidative stress; Alpha-amylase; Phenolic compounds; Pancreas
1. Introduction
Diabetes mellitus (DM) is a malfunction of carbohydrate, lipid,and protein metabolism due to insufficient secretion or action of insulin[1-3]. Life-long therapy is needed for its management[4]. A long-term and poor management causes extensive damage to body organs such as blood vessels, the heart, nerves, the liver, the kidney,and eyes[5]. DM had affected about 463 million (9.3%) people globally in 2019, while the incidence of DM is anticipated to reach 578 million in 2030[6]. It is the 9th major cause of death around the globe based on an investigation of one million causalities in 2017[7,8].
The progression of DM and related complications can be prevented or delayed by strict glycemic control. Presently, DM is controlled by medications such as insulin, oral hypoglycemic drugs,weight control, and modification in lifestyle and dietary habits[9].Antidiabetic drugs cause severe hypoglycemia, lethargy, general weakness, nausea, vomiting, weight loss, diarrhea, liver damage,lactic acidosis, kidney failure, and hepatotoxicity. These adverse reactions and high cost of antidiabetic drugs lead to poor patient adherence[10].
In addition to current medical practice, traditional and folkloric herbal remedies are effectively used to manage chronic diseases including but not limited to DM, cancer, and rheumatoid arthritis.Herbs efficiently control DM because of their multiple actions such as stimulation of insulin secretion, rejuvenation of damaged pancreatic β-cells, mimicking the activity of insulin, preventing insulin resistance, and reducing the absorption and breakdown of carbohydrates into glucose[11].
The plant Tylophora hirsuta (T. hirsuta) Wight, commonly called“Tylophora”, “Panja booti” or “Glow”, is a climbing herb of 1-2 m and belongs to Apocynaceae family (Subfamily: Asclepiadoideae).Apocynaceae family has 180-185 genera and 1 900 species, which are mainly distributed in tropical and subtropical areas of the world[12]. Earlier phytochemical studies have demonstrated the presence of alkaloids, sugars, saponins, steroids, and triterpenes in the methanolic extract of the aerial parts of the plant[12,13].Various alkaloids such as hirsutinidine, tylohirsutinidine, and hirsutine derivatives have been isolated from it[14,15]. Tylophorine has been obtained from its aerial parts along with gymnorhizol and β-sitosterol. It is traditionally used for the treatment of DM, asthma,diarrhea, cancer, allergy, jaundice, and arthritis[16-18]. It is also used as an emetogenic agent. The previous study has also reported the antispasmodic and antimicrobial activity of alpha-amyrin acetate and heptaeicosanol isolated from the aerial parts[19,20]. Methanolic,chloroform, and other extracts of the plant have demonstrated antileishmanial, antifungal, hypoglycemic, and antibacterial activities[21]. Furthermore, methanolic extract of T. hirsuta (THME)was mostly nontoxic as found in phytotoxicity against Lemna minor(25% growth inhibition at 1 mg/mL concentration) and brine shrimp toxicity [IC= (492.33±8.08) mg/mL] studies[20]. The present study was designed to establish the scientific basis and demonstrate the probable mechanism of action for the antidiabetic use of T. hirsuta.Therefore, the methanolic and ethyl acetate extracts of the plant leaves were prepared and analyzed by High Performance Liquid Chromatography (HPLC). Moreover, the plant extracts were tested for in vitro antioxidant and α-amylase inhibitory activities, and assessed for potential hypoglycemic activity in diabetic mice.
2. Materials and methods
2.1. Reagents and instruments
Methanol, ethyl acetate (RDH, Germany), alcohol, sulfuric acid,hydrochloric acid, Folin-Ciocalteau reagent, sodium carbonate,sodium nitrite, aluminum chloride, sodium hydroxide, alloxan monohydrate, HPLC (Shimadzu, Japan), vitamin E and ascorbic acid, acetonitrile and methanol HPLC grade (Sigma-Aldrich,USA), formalin, lipid profile diagnostic kits (Merck, Germany),glimepiride (Prudence PharmaChem, India) and glucometer(Acon laboratories Inc. USA), oxidative stress kits (Elabscience Biotechnology, China) were used in the study.
2.2. Collection, identification, and extraction procedure
Leaves of the plant T. hirsuta L. were collected from northern Punjab. The identification of plant material was done by a taxonomist from University of Agriculture, Faisalabad who assigned voucher number 515-1-2018. The plant material was deposited in the university herbarium for future reference.
The plant leaves (3 kg) were washed, shade dried, and ground to a coarse powder that was filled in a thimble for extraction by Soxhlet apparatus using methanol as solvent. The THME was concentrated at 40 ℃ under reduced pressure with a rotary evaporator. Similarly,T. hirsuta ethyl acetate extract (THEA) was prepared. The plant extracts were kept at 2-8 ℃.
2.3. Total phenolic and flavonoid contents
Total phenolic content (TPC) of both extracts was estimated by Folin-Ciocalteau reagent assay[22]. Total flavonoids content (TFC)was determined by aluminum chloride method[23].
2.4. HPLC analysis
The reverse-phase HPLC analysis of THME and THEA was carried out to quantify phenolic acids and flavonoids. Samples were prepared by dissolving in methanol and filtering through 0.2 µm syringe filters[24]. A gradient mobile phase comprising acetonitrile and acetic acid was passed at a 1 mL/min flow rate. The HPLC was equipped with a UV-visible detector to show HPLC spectrum of plant constituents at 280 nm[25].
2.5. Antioxidant activity
2.5.1. 1, 1-diphenyl-2-picrylhydrazyl (DPPH) assay
Antioxidant activity of the plant extracts was determined by DPPH method[26]. Ascorbic acid served as a standard antioxidant. A 0.1 mM DPPH and various concentrations (0.5-8 mg/mL) of plant extracts were prepared in methanol, admixed, and incubated at 25 ℃ for 30 min. The absorbance was determined at 517 nm. The experiment was repeated in triplicate and percentage inhibition of DPPH scavenging was calculated.
2.5.2. Reducing power
The reducing power of both extracts for ferric ion was determined by the ferric ion reducing method using trichloroacetic acid according to the previous method[25]. The plant extracts were tested at 0.5-8 mg/mL concentration for reducing power activity.For determining the reducing power, 100 µL of the test solution was added to 5 mL sodium phosphate buffer (pH: 6.6) which was followed by addition of 5 mL 0.1% potassium ferricyanide and incubation for 20 min. Then 5 mL 10% trichloroacetic acid was admixed and the mixture was centrifuged at 1 000 rpm for 5 min.Supernatant layer (5 mL) was separated and mixed with an equal volume of distilled water and 0.1 mL 1% ferric chloride. Absorbance of the solutions was recorded at 700 nm.
2.5.3. Hydrogen peroxide scavenging assay
The HOscavenging potential was also estimated according to the previous method[25]. Butyl hydroxyl anisole served as a standard antioxidant. The plant extracts were tested at 0.5-8 mg/mL concentrations for estimating HOscavenging activity. The test solution (1 mL) was incubated with 0.6 mL of 40 mM HO(prepared in phosphate buffer of pH 7.4) for 10 min and absorbance was determined at 230 nm wavelength.
2.6. α-Amylase inhibition
It was performed on 0.2, 0.4, 0.8, 1.6 and 3.2 mg/mL concentrations of the plant extracts according to a previous method[27]. Acarbose was used as a standard α-amylase inhibitor. Dinitro salicylic acid reagent was prepared by dissolving 2.36 g of dinitro salicylic acid in 80 mL of 0.5 mol/L NaOH followed by addition of 30 g sodium potassium tartrate. The pH of starch solution (1% w/v) was adjusted to 6.9 with 0.02 M phosphate buffer. For determining α-amylase activity, equal volumes of 1% w/v α-amylase and extract solutions were mixed and kept at 25 ℃ for half an hour. Then, 1 mL of starch solution was added and the solution was incubated again at 25 ℃for 10 min. One mL of dinitro salicylic acid reagent was poured into each test tube after heating at 90 ℃ on a water bath for 30 min. The volume of solution was adjusted to 10 mL in each test tube with water. The absorbance of each sample was determined at 540 nm.The experiment was repeated thrice and percentage inhibition was calculated[27].
2.7. In vivo study
2.7.1. Experimental animals
Albino mice of both sexes weighing 25-30 g were acquired and acclimatized in the animal house of Government College University, Faisalabad (GCUF) under standard laboratory conditions[(25±3) ℃, 12 h dark/light and 65% humidity]. All animals had free access to standard food pellets and water.
2.7.2. Induction of diabetes
Alloxan monohydrate solution was prepared in normal saline and injected into mice by an intraperitoneal route at a single dose of 150 mg/kg. Moreover, glucose solution (2 g/kg) was orally administered to avoid hypoglycemia. Blood glucose level of mice was checked by glucometer 3 days post alloxan administration. Animals with fasting blood glucose (FBG) of more than 200 mg/dL were included in the antidiabetic study[27].
2.7.3. Experimental design
Thirty-six mice were randomly divided into six groups. The normal control group comprised of healthy mice and while the disease control group contained diabetic mice. Both groups received normal saline only. The THME exhibited higher in vitro antioxidant activity and α-amylase inhibition activities than the THEA and was therefore selected for in vivo testing. Diabetic animals in the standard therapy group (drug control) were orally treated with 0.2 mg/kg glibenclamide. Diabetic mice in the remaining three groups received THME at 250, 500 and 750 mg/kg/day orally from day 1 to 14.Doses were selected on the basis of a previous study on T. hirsuta[21].The FBG level was determined on daily basis for 14 d. Body weight was determined on day 1, 7, and 14[28]. There was no mortality or signs of toxicity among diabetic mice at the treated dose levels.
2.7.4. Oral glucose tolerance test (OGTT)
It was carried on diabetic mice in fasting conditions. Glucose (2 g/kg) was orally given to diabetic mice. Blood glucose level was determined in animals just before and 30, 60, 90, 120, and 180 min post-administration of glucose solution[27].
2.7.5. Hematological, biochemical and histopathological evaluation
Experimental animals were anesthetized with diethyl ether to avoid undue animal sufferings. Blood was collected in plain and ethylene diamine tetraacetic acid vacutainers to determine liver functions, including alkaline phosphatase, alanine aminotransferase,and aspartate transaminase, and kindey function biomarkers, such as creatinine and blood urea. Lipid profile such as low density lipoproteins (LDL), very low density lipoproteins (VLDL),triglycerides and cholesterol was also determined in mouse serum.The pancreas, liver, and kidney were removed from the animals and preserved in 10% formalin solution. Tissues were embedded in paraffin wax, sectioned at 5 µm thickness with a microtome,and subjected to hematoxylin and eosin staining for histological examination. The slides were observed under inverted light micrope(Olympus Corporation, Japan) at ×40 magnification of objective lense[29].
2.7.6. Oxidative stress biomarkers
Various oxidative stress biomarkers were estimated in the liver tissue homogenates (10% w/v) of the mice. Activity of superoxide dismutase was determined by xanthine oxidase method[30]. The activities of catalase and peroxidase were determined by hydrogen peroxide and 4-methylated catechol methods, respectively[30].Moreover, the amount of malondialdehyde in mice tissue homogenates was determined by thiobarbituric acid method[31].
2.8. Statistical analysis
All data were expressed as mean ± standard deviation (SD). Area under curve (AUC) was calculated in OGTT assay. GraphPad Prism version 6 was used for statistical analysis. Two-way analysis of variance (ANOVA) was performed followed by Dunnett’s multiple comparison tests on in vitro activities. One-way ANOVA was carried out followed by Dunnett’s multiple comparison test to determine the statistical significance among hematological and biochemical parameters. The results were considered statistically significant at P<0.05.
2.9. Ethical statement
Guidelines of the National Institute of Health and Animal Ethical Committee were strictly followed for animal care and safety.Approval of animal study was obtained from Institutional Ethical Committee, GCUF (Approval No. GCUF/ERG/2045) in 2018.
3. Results
3.1. Phenolic and flavonoid contents
The THME exhibited higher amounts of phenolic and flavonoid compounds [TPC: (86.25±2.22) mg/g GAE, TFC: (66.80±2.15)mg/g CE] than the corresponding ethyl acetate extract [TPC:(29.59±3.84) mg/g GAE, TFC: (44.74±2.15) mg/g CE].
3.2. Quantification by HPLC
The HPLC analysis of THME indicated the maximum amount of gallic acid (2.43 ppm) followed by chlorogenic acid (2.17 ppm),while THEA contained the highest amount of benzoic acid (8.32 ppm) followed by chlorogenic acid (5.83 ppm). The quantity of quercetin, gallic acid, syringic acid, and p-coumaric acid was higher in THME than THEA. The content of chlorogenic acid, m-coumaric acid, and benzoic acid was higher in THEA than THME. The components of the plant extracts detected by HPLC along with their relevant retention times and percent areas are shown in Table 1.
3.3. Antioxidant activities
3.3.1. DPPH scavenging potential
Antioxidant activity of both extracts was remarkably less than the control at the corresponding concentrations. The THME[(88.24±2.56)%] and THEA [(80.03±3.05)%] extracts showed the highest DPPH scavenging activities at 8 mg/mL, which were significantly lower than the standard [(95.01±3.56)%] at the same concentration. The THME also showed greater DPPH scavenging activity than the respective concentrations of THEA (Figure 1A).
3.3.2. Reducing power
The percentage increase in reducing power of THME was significantly (P<0.001) different from corresponding concentrations of butylated hydroxytoluene (BHT) and THEA. The plant extracts and BHT exhibited a dose-dependent increase in reducing power.The highest reducing power was shown by BHT followed by THME at 8 mg/mL (Figure 1C).
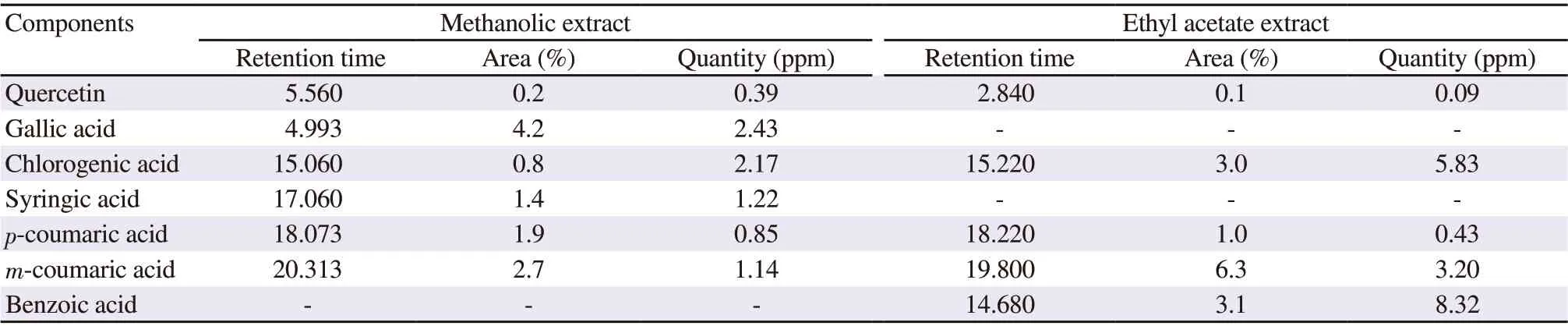
Table 1. Phytochemical components of Tylophora hirsuta extracts detected by HPLC.
3.3.3. Scavenging of HO
At 0.5, 1, 2, 4 mg/mL concentrations, HOscavenging activity of THME and THEA was significantly different from BHT. Moreover,the HOscavenging activity of both extracts increased dose dependenty (Figure 1B).
3.4. In vitro α-amylase inhibition
The α-amylase inhibitory potential of THME was higher than that of THEA at all tested concentrations. However, percentage inhibition of α-amylase exhibited by THME [(83.90±1.56)%] was significantly (P<0.01) lower than acarbose [(88.31±1.63)%] at 3.2 mg/mL concentration. There was a concentration-dependent increase in α-amylase inhibition activity of both extracts (Figure 1D). The ICvalues of THME and THEA were 0.18 and 0.43 mg/mL,respectively.
3.5. In vivo study
3.5.1. FBG
The THME showed a considerable decrease in FBG of diabetic mice at all dose levels which were more pronounced during the second week of study. All mice treated with THME showed a significant (P<0.05) reduction in FBG as compared to diabetic control on the 14th day as shown in Figure 2A.
3.5.2. OGTT on diabetic mice
Following glucose administration in OGTT, it was observed that the blood glucose level was significantly high at 30 and 60 min after administration of glucose. There was a decline in blood glucose level as time passing. Comparison of AUC of OGTT showed that the disease control group exhibited higher AUC (880.20 mg/dL•h)than the normal control group (513.52 mg/dL•h). Treatment with the standard drug (664.91 mg/dL•h) and the THME at 250 mg/kg(705.40 mg/dL•h), 500 mg/kg (689.52 mg/dL•h) and 750 mg/kg(661.60 mg/dL•h) significantly reduced the AUC of diabetic mice in comparison to the disease control group. It revealed that the 750 mg/kg dose of THME exhibited the maximum decrease in blood glucose level as evident in Figure 2B.
3.5.3. Bodyweight of diabetic mice
Weight loss in the disease control group was observed as compared to normal control after 14 days of treatment. Treatment with the plant extract for two weeks prevented weight loss in diabetic animals at all dose levels. The trend in weight changes in diabetic animals was evident in Figure 2C.
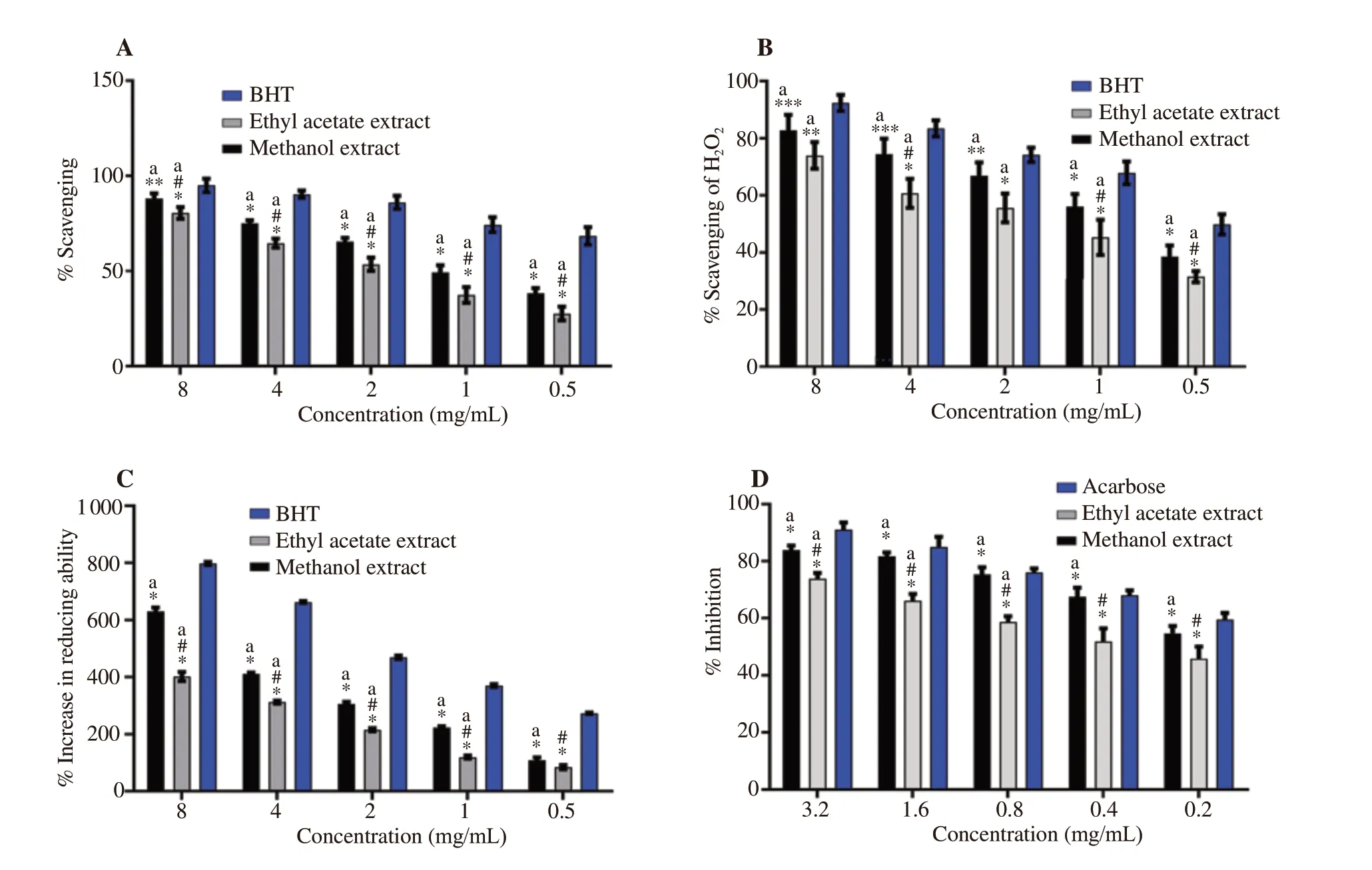
Figure 1. Antioxidant and α-amylase inhibitory activities of Tylophora hirsuta leaf extracts. A: DPPH radical scavenging; B: H2O2 radical scavenging; C:Ferric reducing power; D: α-amylase inhibitory activity. Data are presented as mean±SD (n=3) and analyzed by two-way ANOVA followed by Dunnett’s test.*P<0.05, **P<0.01 and ***P<0.001 indicate statistically significant difference in comparison to control. #P<0.05 indicates statistical significance in comparison to methanolic extract. aP<0.05 indicates significant effect of different concentrations. BHT: butylated hydroxytoluene.
3.5.4. Lipid profile in diabetic animals
Serum cholesterol level was slightly elevated (P<0.001) in the disease control group as compared to the normal control group.Treatment with the plant extract reduced the level of cholesterol in diabetic animals in contrast to the disease control group. Cholesterol level in THME at 500 and 750 mg/kg treated group was significantly(P<0.001) lower as compared to the disease control group.Moreover, a significantly raised level of triglycerides was found in the disease control group [(145.02±6.34) mg/dL] as compared to the normal control group [(88.30±2.12) mg/dL]. The level of triglycerides was notably (P<0.001) alleviated by the treatment,which was more prominent with THME 750 mg/kg. Furthermore,there was a substantially (P<0.001) increased level of LDL and VLDL [(134.08±1.30) and (46.60±3.82) mg/dL respectively] in the disease control group in comparison to the normal control group[LDL: (78.34±2.73) mg/dL, VLDL: (22.18±4.31) mg/dL]. THME successfully decreased the LDL and VLDL in diabetic mice with the most prominent effect found at 750 mg/kg [LDL: (85.34±2.52) mg/dL and VLDL: (24.02±2.04) mg/dL] in comparison to the diabetes control as shown in Figure 3A-D.
3.5.5. Glycated hemoglobin (HbA1c) and serum amylase
The results showed an increased HbA1c (P<0.001) in the disease control group as compared to the normal control group. However,administration of THME at 250, 500, and 750 mg/kg significantly(P<0.001) improved the glycemic control in diabetic animals by decreasing the HbA1c level. Moreover, the treatment with THME also significantly reduced the level of serum amylase as compared to the disease control group. The effect of THME at 750 mg/kg was the most pronounced as shown in Figures 3E and 3F.
3.5.6. Liver function biomarkers
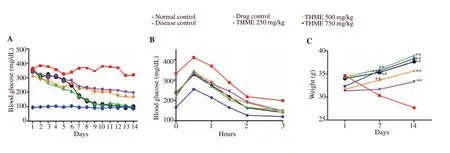
Figure 2. Effects of methanolic extract of Tylophora hirsuta on fasting blood glucose (A), oral glucose tolerance test (B), and body weight (C) of diabetic mice.**P<0.01 indicates statistical significance in comparison to the disease control. THME: methanol extract of Tylophora hirsuta.
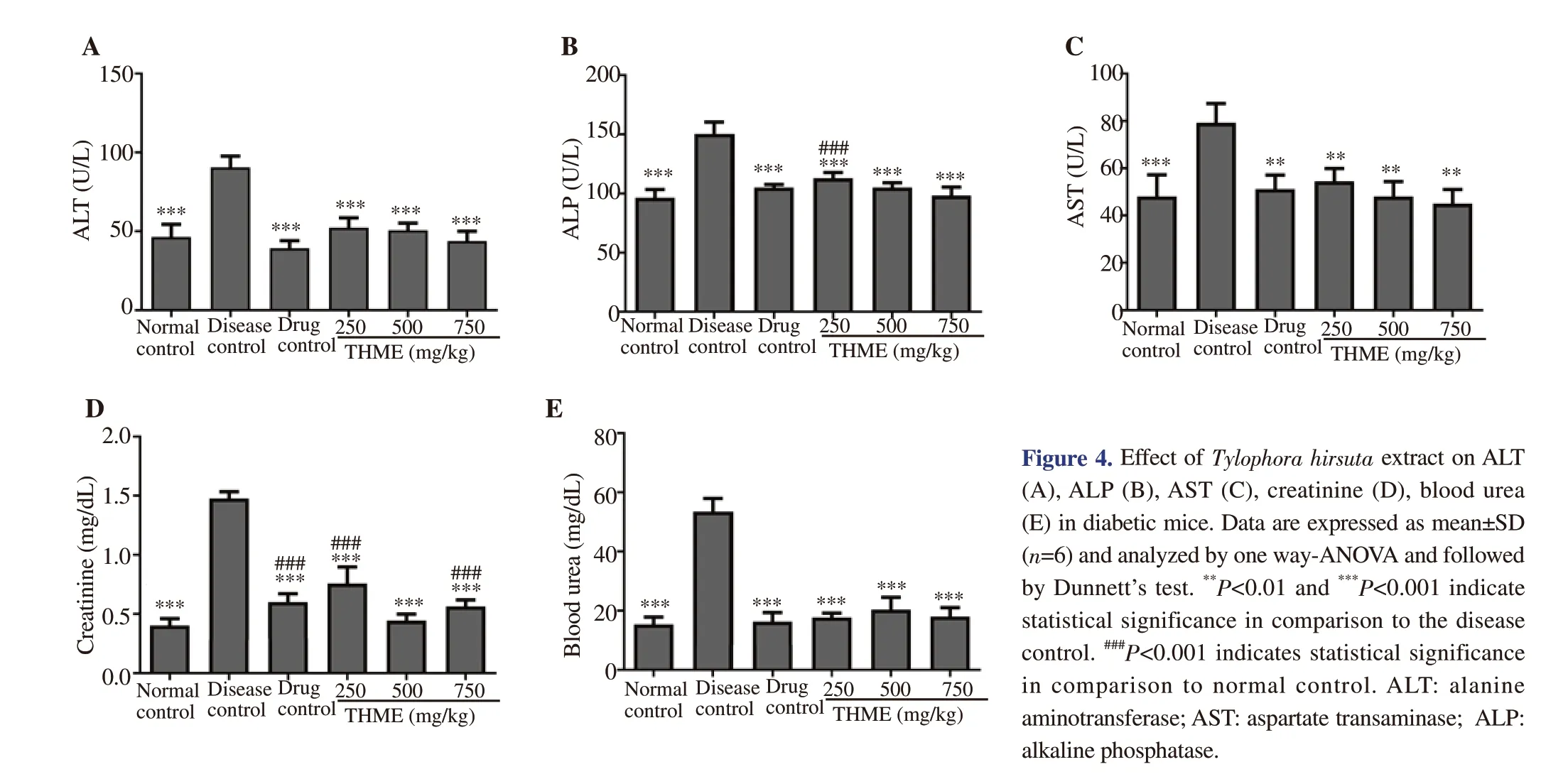
The results showed that the treatment with the plant extract had significantly ameliorated the alloxan-induced elevation in alkaline phosphatase, alanine aminotransferase, and aspartate transaminase.The most pronounced effect (P<0.05) was exerted by THME at 750 mg/kg dose [alkaline phosphatase: (98.03±7.54) U/L, alanine aminotransferase: (43.72±6.32) U/L, aspartate transaminase:(45.04±6.12) U/L] as shown in Figure 4.
3.5.7. Renal function parameters
The serum creatinine and urea levels of diabetic mice subjected to THME therapy were estimated after 14 d. It was found that diabetic mice showed a significant decline (P<0.05) in the kidney function as compared to the normal control group as evident by a rise in creatinine and urea levels. Diabetic mice treated with all doses of the plant extract exhibited a significant decline (P<0.05) in creatinine and urea levels as compared to the disease control group. The ameliorating effect of 750 mg/kg dose on kidney function was the most pronounced as shown in Figure 4.
3.5.8. Effect on oxidative stress
In vivo antioxidant activities were carried out on homogenate of the livers. It was found that disease control animals demonstrated significantly increased levels of peroxidase and malondialdehyde as well as reduced levels of superoxide dismutase and catalase as compared to the normal control. Treatment with THME ameliorated alloxan-induced oxidative stress. The effect of THME at 750 mg/kg dose was the most pronounced in ameliorating oxidative stress as shown in Figure 5.
3.5.9. Histopathology of the pancreas, liver and kidney
Histopathological examination of diabetic control mice showed partial damage to islets of Langerhans. Pancreatic acini showed an infiltration of eosinophils in untreated diabetic animals. Diabetic mice treated with THME at 250 mg/kg dose showed a slight hemorrhagic exudation in lymphatic channels and mild inflammation around acinar cells. Islets of Langerhans and acinar cells of the diabetic animals treated with different doses of THME were mostly normal in contrast to disease control group (Figure 6).
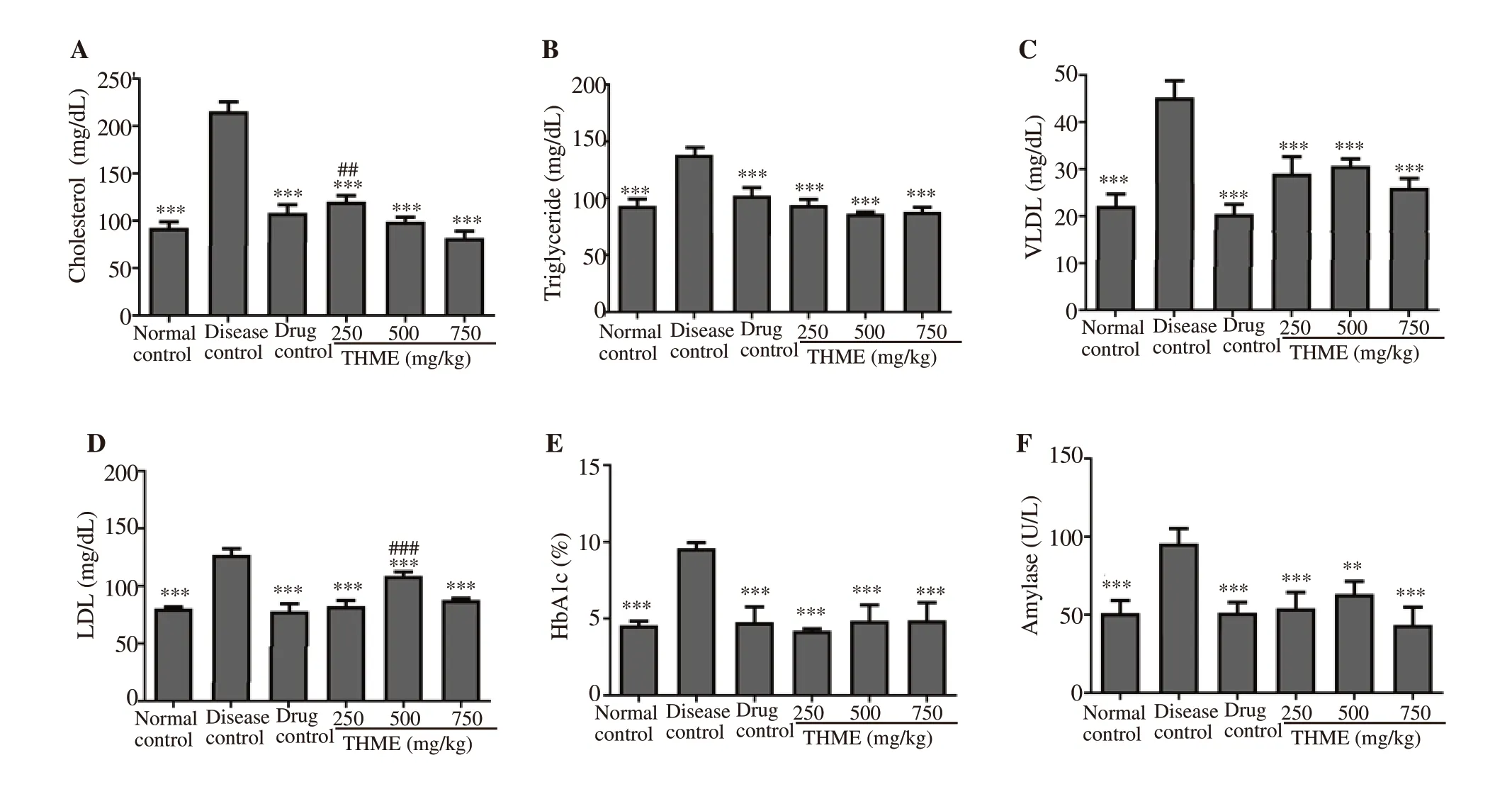
Figure 3. Effect of Tylophora hirsuta extract on serum cholesterol (A), serum triglyceride (B), very low density lipoprotein (VLDL) (C), low density lipoprotein(LDL) (D), glycated hemoglobin (HbA1c) (E), and serum amylase (F) in diabetic mice. Data are expressed as mean±SD (n=6) and analyzed by one way-ANOVA followed by Dunnett’s test. **P<0.01 and ***P<0.001 indicate statistical significance in comparison to the disease control. ##P<0.01 and ###P<0.001 indicates statistical significance in comparison to normal control.
Shrunken hepatocytes, slight steatohepatitis, pyknosis with condensed chromatin, and mild inflammation were seen in the liver of diabetic mice treated with 250 and 500 mg/kg dose of THME.However, treatment with 750 mg/kg dose of THME significantly improved the histology of the liver and the treated animals did not show periportal inflammation or steatosis in contrast to the disease control group (Figure 6).
Histology of normal mouse showed intact glomeruli with normal nuclei and collecting tubules. Administration of alloxan resulted in severe damage to the kidney as diabetic mouse showed necrotic Bowman’s capsule, tubular hyalinization, and perivascular inflammation. Treatment of diabetic animals with 250 mg/kg dose of THME showed slight thrombosis and clustered lymphocytes with mild to moderate inflammation. Diabetic animals treated with 750 mg/kg dose of THME showed normal glomeruli and blood vessels without any evidence of thrombosis as shown in Figure 6.
4. Discussion
In this study, we found that the THME had improved the glucose tolerance in diabetic mice as compared to negative control in OGTT.Results also indicated that the level of FBG in treatment groups was significantly lower as compared to the disease control after two-week therapy. The ameliorating effect of THME was more evident during the 2nd week of therapy. Previous studies on the antidiabetic plants also showed glucose-lowering effect in OGTT, which displayed an improved glucose tolerance[32]. This hypoglycemic effect of THME might be attributed to some active antidiabetic constituents such as quercetin[33]. HPLC analysis of methanol and ethyl acetate extracts of T.hirsuta showed the presence of various quantities of phenolic acids and flavonoids in our study. Similar research work on Gymnema sylvestre,subfamily Asclepiadoideae, showed a comparable hypoglycemic effect in animals[34]. Some phytochemicals detected in THME such as cinnamic acid and quercetin had previously exhibited the antidiabetic potential[35].
Alloxan, an analogue of glucose, is cytotoxic in nature. It was proposed that the dialuric acid produced from alloxan had generated ROS through inhibition of enzyme glucose kinase and inflicted damage to the pancreas,kidney and liver[34]. Biochemical analysis of blood serum indicated a reduced level of serum cholesterol, LDL, VLDL, and triglycerides in comparison to the disease control group. It is suggested that THME was effective in reducing hyperlipidemia in diabetic animals because of phenolic compounds i.e. quercetin[36]. The ameliorating effects of THME on liver function markers of diabetic mice were consistent with the histopathological findings, which suggested the possible hepatoprotective potential of the plant extract.
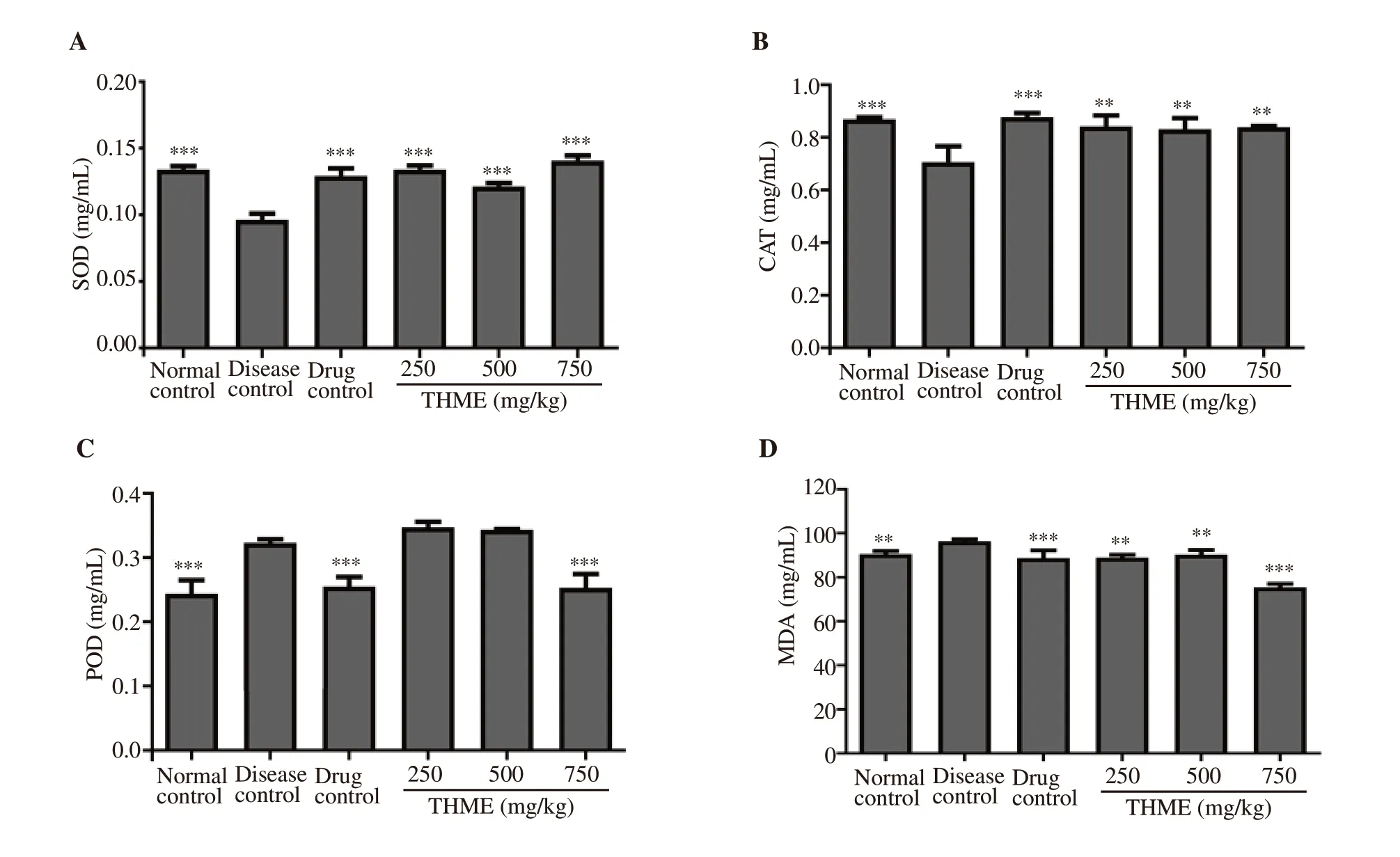
Figure 5. Effect of Tylophora hirsuta extract on superoxide dismutase (SOD) (A), catalase (CAT) (B), peroxidase (POD) (C), and malondialdehyde (MDA)(D) in the liver of diabetic mice. Data are expressed as mean±SD (n=6) and analyzed by one way-ANOVA followed by Dunnett’s test. **P<0.01 and ***P<0.001 indicate statistical significance in comparison to the disease control.
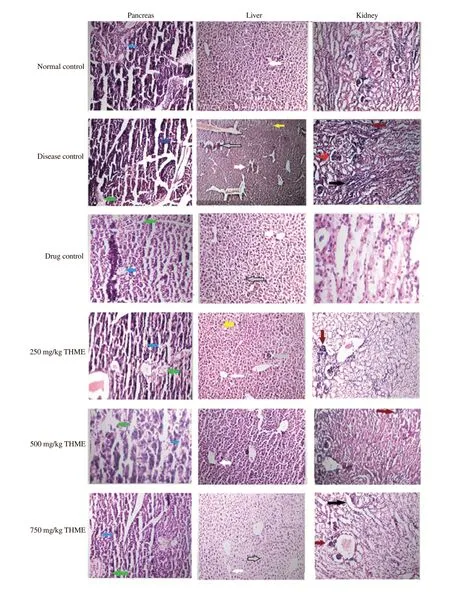
Figure 6. Effect of plant extract on the pancreatic, liver and kidney histology in diabetic mice at ×40 magnification. The pancreas in the disease control shows partially damaged islet’s of Langerhans and acini showing mild inflammation; the liver in the disease control group showed fragmented and detached hepatocytes and clumping of sinusoids, periportal inflammation, and inflammatory lymphocytes; the kidney in the disease control group showed inflamed Bowman’s capsule, tubular hyalinization, perivascular and interstitial inflammation, which were improved by treatment with THME extract. Arrows in blue and green colors show pancreatic acini and islet’s of Langerhans, respectively. Arrows of white, grey and yellow colors show sinosoids, hepatocytes and inflammation, respectively. Arrows of red, dark red and black colors show widened glomerulus, inflammation and tubular hyalinization, respectively.
Results of the current study indicated the damage to pancreatic acini and islets of Langerhans, inflammatory hepatocytes, shrinkage of glomeruli and tubules caused by alloxan. The THME restored the normal histology of the pancreas, liver, and kidney at a 750 mg/kg dose. Furthermore,treatment with THME significantly improved the body weight of diabetic animals, which suggested the improvement in metabolic dysfunction associated with DM[37].
Reactive oxygen species (ROS) activate several inflammatory mediators within the body such as chemokines and cytokines that lead to the inflammation of pancreatic acini and β-cells of islets of Langerhans,impair insulin secretion, and cause the resistance to the action of insulin.The oxidative stress parameters served as biomarkers in managing various diseases including DM[38]. Alloxan, used for induction of diabetes, is an analogue of glucose and cytotoxic through various mechanisms. It was proposed that the dialuric acid had generated ROS through inhibition of glucose kinase and infliction of damage to the pancreas, kidney, and liver.Dialuric acid produces free radicals such as superoxide and hydrogen peroxide[39]. Measurement of in vivo oxidative stress parameters indicated that the THME had exhibited ameliorating effect comparable to that of a standard drug. It is suggested that the reduction in oxidative stress was attributed to the presence of antioxidant phenolic acids and flavonoids. Furthermore, the phenolic acids and flavonoids increased the activity of in vivo antioxidant enzymes. Possible antidiabetic mechanism of action of the THME was reduction in oxidative stress and scavenging of ROS generated during DM. Similar findings on reduction of oxidative stress by Gymnema sylvestre were reported previously[39]. A previous study reported the antidiabetic activity of T. hirsuta through reduction in inflammatory modulators[40]. Current study showed that the THME had reduced the oxidative stress that led to modulation of inflammatory biomarkers.
It was found that THME exhibited higher levels of TPC and TFC, and higher antioxidant activities and α-amylase inhibition activities than THEA. The in vitro activities of THME might be attributed to higher levels of TPC and TFC than the THEA[40]. The THME had shown more inhibition percentage of glucosidase inhibitor as compared to THEA.Moreover, the α-amylase inhibitory activity of THME was comparable to acarbose. Similar findings were also reported by previous studies[41].
The present study has some limitations. HPLC analysis showed the concentrations of phenolic acids and flavonoids but failed to demonstrate the other potential antidiabetic phytochemicals in the plant extract such as alkaloids. Furthermore, the current study merely demonstrated the nephroprotective and hypolipidemic effects of THME in diabetic mice without any mechanistic insight.
Conflict of interest statement
The authors declare no conflict of interest.
Authors
’contributions
MFA, A Saleem, and AR conceptualized and designed the study. A Shagufta, A Saleem, and A Sharif carried out the experimental work.MFA, A Saleem, MMFAB and MMA analyzed and interpreted data.MFA, A Saleem and MMA worte the manuscript. All the authors read and approved the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年9期
Asian Pacific Journal of Tropical Biomedicine2021年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Improved performance of naringenin herbosomes over naringenin in streptozotocininduced diabetic rats: In vitro and in vivo evaluation
- Antibacterial activity and inhibition against Staphylococcus aureus NorA efflux pump by ferulic acid and its esterified derivatives
- In vitro antimicrobial and synergistic effect of essential oil from the red macroalgae Centroceras clavulatum (C. Agardh) Montagne with conventional antibiotics
- Biopeptides of Pyropia yezoensis and their potential health benefits: A review
