Biopeptides of Pyropia yezoensis and their potential health benefits: A review
Sivakumar Allur Subramaniyan, Naziya Begum, Sung Jae Kim, Youn Hee Choi, Taek-Jeong Nam
1Institute of Fisheries Sciences, Pukyong National University, Busan 46041, South Korea
2Department of Chemistry, College of Natural and computational sciences, Debre Berhan University, Debye Berhan, P.O. Box-445 Ethiopia
3Department of Orthopaedic Surgery, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Republic of Korea
4Department of Marine Bio-Materials & Aquaculture, Pukyong National University, Busan 48513, South Korea
ABSTRACT Pyropia yezoensis (P. yezoensis) is a popular species of red algae that are commercially cultivated and consumed in East Asia, China,Japan, and Korea. The high protein content of P. yezoensis provides a source of multiple bioactive peptides exhibiting antioxidant,anti-inflammatory, antihypertensive, anticancer, tissue healing,immunomodulatory, and anticoagulant properties. Furthermore,many other biologically active substances in P. yezoensis, including carbohydrates, lipids, dietary fibers, and polyphenols, have shown potential health benefits and are important in both the food and agriculture industries. This review provides a detailed summary of researches over the last decade on the biological and medicinal properties of bioactive peptides. The information was extracted from various electronic resources, including Google Scholar, PubMed,MEDLINE, and Google Patents.
KEYWORDS:Red algae; Pyropia; Bioactive peptides; Human health
1. Introduction
Marine algae have been studied extensively over the last few decades due to their high content of natural, bioactive compounds[1].The tolerance of marine algae to severe climate change ensures their survival and continued availability for human use[2]. Macroalgae particularly Pyropia species (genus Porphyra) offer a broad range of bioactive compounds that are potentially beneficial to human health[3]. Among the 80 known species of Pyropia, Pyropia yezoensis(P. yezoensis) and Pyropia haitanensis are the best characterized in terms of chemical content (www.algaebase.org). Innumerable bioactive compounds such as polysaccharides, porphyrans, proteins,fatty acids, carotenoids, vitamins, and minerals have been identified in P. yezoensis[4]. P. yezoensis contains a minimal amount of moisture and ash, while its high protein content (12.5%-51.5% w/w) is a significant source of biopeptides[5]. Many have demonstrated antioxidant, anti-inflammatory, anti-aging, antihypertensive,anticancer properties and have been used as alternatives to conventional synthetic drugs[6-11].
The electronic, web-based databases Google Scholar, PubMed,and MEDLINE were used to extract relevant studies from research articles, review articles, and book chapters. Information was searched using keywords such as “health benefits of seaweeds”OR “pharmacological bioactive compounds of seaweeds” OR“biopeptides of seaweeds” OR “Pyropia yezoensis extracts” OR“bioactive compounds of Pyropia yezoensis” OR “protective effects of Pyropia yezoensis” OR “chemical structure of Pyropia yezoensis”OR “nutritional advantages of Pyropia yezoensis” OR “biopeptides of Pyropia yezoensis”. Table 1 shows data explicitly related to studies on biopeptides in P. yezoensis, and Figure 1 shows the schematic representation of P. yezoensis peptides’ health benefits. Patents related to P. yezoensis biopeptides were found using the “Google Patent” search engine (Table 2). All electronic searches were performed with a date range from January 1, 2010, to October 31,2020.
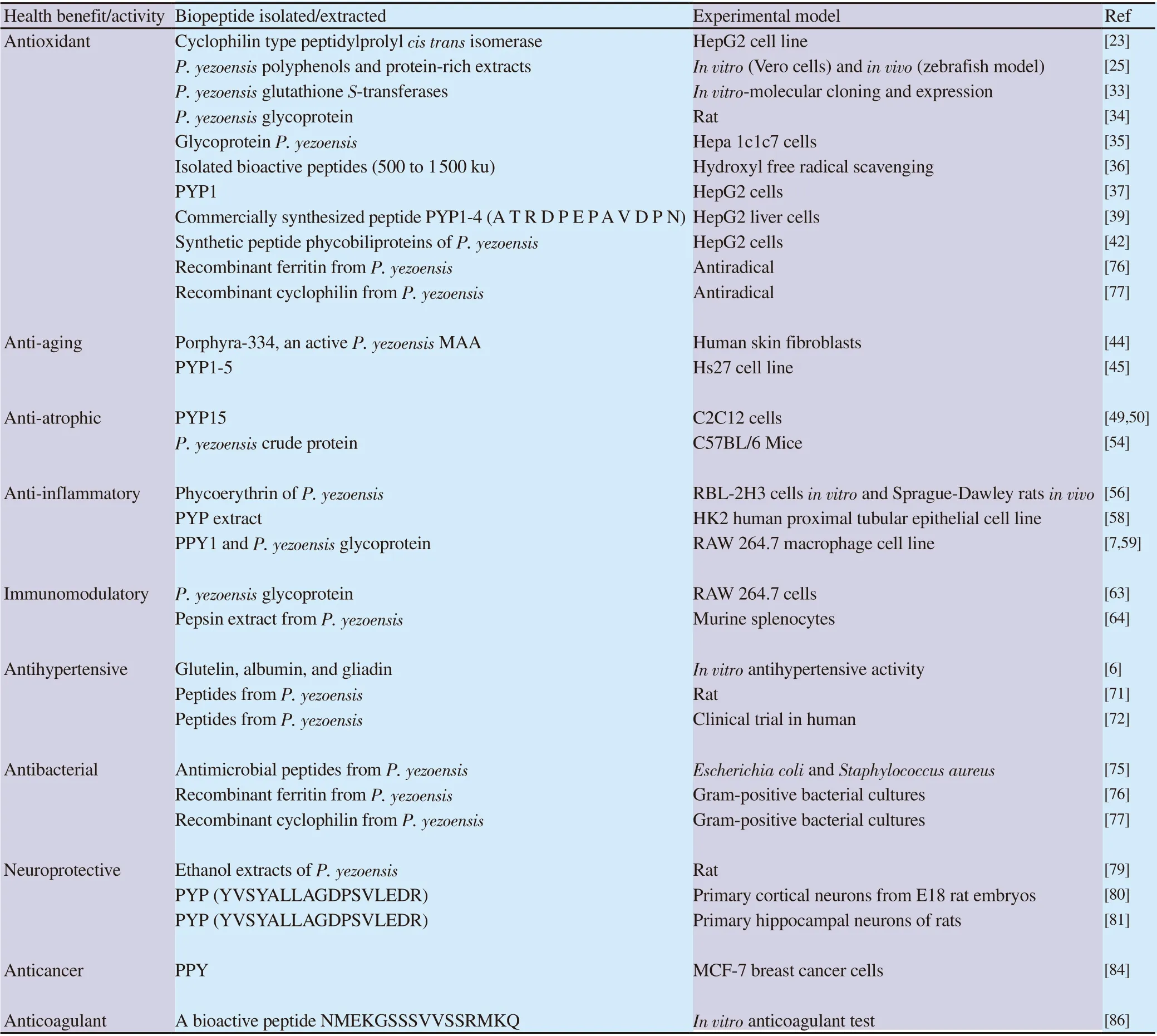
Table 1. Health benefits of bioactive peptides from Pyropia yezoensis (P. yezoensis).
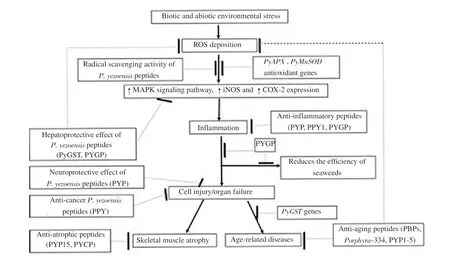
Figure 1. Schematic representation of P. yezoensis peptides' health benefits. ROS: reactive oxygen species; PyAPX: P. yezoensis ascorbate peroxidase;PyMnSOD: P. yezoensis manganese-superoxide dismutase; MAPK: mitogen-activated protein kinase; iNOS: inducible nitric oxide synthase; COX:cyclooxyganese; PyGST: P. yezoensis glutathione-S-transferase; PYGP: P. yezoensis glycoprotein; PYCP: P. yezoensis crude protein; PBP: phycobiliproteins.
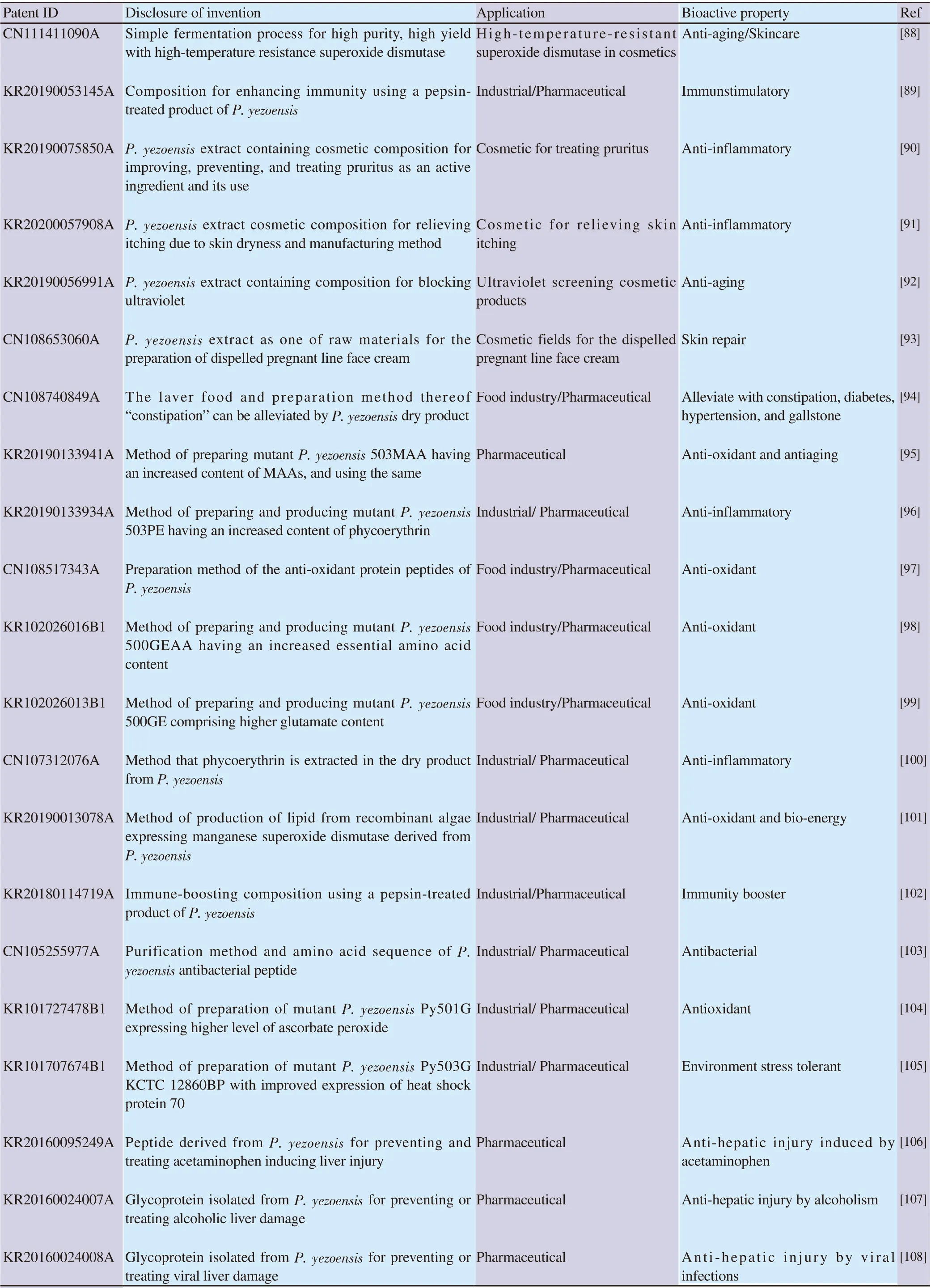
Table 2. Patents related with P. yezoensis crude extracts and peptides.
2. History of P. yezoensis cultivation and utilization
Globally, Pyropia species are one of the most cultivated seaweeds with an annual value of nearly $950 million in 2017[12]. The cultivation of seaweed in Korea started with Pyropia[13]. Pyropia is a genus of red algae in the Bangiaceae family cultivated to produce nori in East Asia and to a lesser extent in Southeast Asia[14]. Nori,an edible seaweed product made from P. yezoensis, is a staple food in Japan that is enjoyed with sushi and cha[15]. Cuisines featuring nori introduce diversity and feature unique flavors, nutrients, and medicinal benefits[14].
3. Biopeptides of P. yezoensis and their potential health benefits
3.1. Antioxidant peptides
P. yezoensis is an intertidal alga that frequently encounters environmental stressors with switching tides[16]. Such environmental(biotic/abiotic) stress can induce increases in levels of intracellular reactive oxygen species (ROS), ultimately impeding growth by decreasing pigment production efficiency and photosynthesis[17].Many researchers have studied the tolerance and adaptability of P. yezoensis in the face of biotic (pathogen infection) and abiotic stressors (high temperature, oxidative/osmotic stress, lead, Cuions, high salinity)[18-21]. Li et al. reported the photosynthetic and antioxidant physiological responses of P. yezoensis to desiccation stress[22]. Recombinant peptidyl-prolyl cis-trans-isomerase of P. yezoensis exhibits antioxidant activity by decreasing HO-mediated ROS formation in HepG2 cells[23]. Antioxidant protection mechanisms consisting of multiple molecular components appear to be necessary for the survival of P. yezoensis in the intertidal area[24]. Polyphenol and protein-rich extracts from six Korean strains of P. yezoensis demonstrated antioxidant activity by reducing levels of ROS species induced by 2, 2’-azobis (2-amidinopropane)dihydrochloride in Vero cells and zebrafish[25]. None of the extracts showed any cytotoxic effects, suggesting that they would be safe for use in food and pharmaceuticals. The antioxidant genes PyAPX (P. yezoensis ascorbate peroxidase) and PyMnSOD (P.yezoensis manganese-superoxide dismutase) of P. yezoensis were overexpressed when exposed to HO, salinity, and ultraviolet (UV)B stress[26]. Many intertidal habitats respond to external stimuli through signal transduction pathways and exploit controlling mechanisms in response to environmental stress[27]. Moreover, these systems are often interlinked with regulatory systems such as protein kinase signaling cascades[28]. Recently, Kong et al. identified 33 MAP3K genes (mitogen-activated protein kinase kinase kinase) in P. yezoensis, classifying them as rapidly accelerated fibrosarcomas,mitogen-activated protein/ERK kinases (MEKKs), or members of the ZIK family[29]. Among the 33 identified genes, several responded to environmental stressors (dehydration/rehydration, temperature,drought, and cold) by regulating expression patterns.
Drug-induced hepatotoxicity is the main obstacle for homeostasis and shifts metabolism toward ROS development, eventually contributing to possible organ failure[30]. Glutathione-S-transferase(GST), a pivotal phase Ⅱ enzyme, catalyzes the conjugation of glutathione to xenobiotic obstacle for drug approval by the United States Food and Drug Administration[31]. PhaseⅠandⅡ liver enzymes play a vital role in detoxifying medications and xenobiotics through the sulfhydryl group, thereby increasing the xenobiotic water solubility[32]. A close examination of the genomic sequence of P. yezoensis GST revealed a strong identity with most algal GSTs, and under lead stress (10 mg/L) significantly enhanced P.yezoensis GST expression, suggesting its detoxification efficacy[33].P. yezoensis glycoprotein (PYGP) from commercially available P.yezoensis was hepatoprotective in a rat model via upregulation of antioxidant enzymes and downregulation of the mitogen-activated protein kinase (MAPK) signaling pathway and inducible nitric oxide synthase/cyclooxygenase 2 expressions[34]. Choi et al. investigated the hepatoprotective properties of PYGP against D-GaIN-induced toxicity in Hepa 1c1c7 cells[35]. The hepatoprotection mechanism’s key was the antioxidant capacity of PYGP (SOD and GST) and decreased lipid peroxidation (TBARS). At the molecular level, it was observed that PYGP elevates the expression of extracellular signal-regulated kinase, c Jun N terminal kinase, and p38/MAPK phosphorylation induced by GaIN[35]. Besides, Yao et al. showed PYGP (molecular weight <1 500 ku) hydroxyl radicals activity generated by GaIN with inhibition of 80.6% and an ICof 0.744 mg/mL[36]. The chemical structure assessment of P. yezoensis protein (PYP) illustrated that it contains two proteins: PYP1(10 kDa, SDS resistant dimer) and PYP2 (10 kDa) and PYP1(ALEGGKSSGGEATRDPEPT) demonstrated a chemoprotective effect on Chang liver cells damage induced by acetaminophen[37].PYP1 (1-20) results showed its protective role by neither causing any cytotoxicity nor disturbing the proliferation in Chang liver cells.PYP1 showed a sequence homology similar to late embryogenesis proteins that protect against protein denaturation during desiccation,freezing, heat or salt exposure, and osmotic stress[38]. Kim et al. found that the commercially synthesized peptide PYP1-4(ATRDPEPAVDPN) of P. yezoensis decreased oxidative damage,growth inhibition, and apoptosis in HepG2 liver cells that were exposed to acetaminophen[39]. P. yezoensis strain was subjected to a mutation to increase its amino acid content and antioxidant activity. Choi demonstrated mutant Py500 G (the mutant strain of P. yezoensis irradiated at a dose of 500 Gy) had a high overall phenolic and free amino acid content with higher antioxidant activity compared to the wild form, which could be useful for the cultivation of such an essential mutant strain for human wellbeing[40].
3.2. Anti-aging peptides
The free radical theory of aging speculates that aging characteristics are triggered by the production and release of various ROS induced by environmental factors[41]. Non-toxic phycobiliproteins (PBP) (1–13)in P. yezoensis showed antioxidant activity by inhibiting the HO-induced production of ROS in HepG2 cells in vitro[42]. PBP2 peptide was recognized as a possible antioxidant among several PBP peptides.This down-regulated ROS and increased superoxide dismutase (SOD)and glutathione peroxidase appeared to operate along p-Nrf2/SOD pathways; p-nrf2, a conventional antioxidant stress reliever, may be beneficial for aging prevention. Another factor that affects aging is exposure to UV rays that increases collagenase activity of the skin through collagen degradation in the dermal extracellular matrix[43].Mycosporine-like amino acids (MAAs) provide a substantial chemoprotective effect against photo-induced skin senescence[43].Porphyra-334, an active P. yezoensis MAA, inhibits UVA-induced cellular senescence in human skin fibroblasts by regulating matrix metalloprotease expression while altering typeⅠcollagen, mRNA elastin, and protein levels in a dose-dependent manner[44]. Hence,Porphyra-334 is a possible candidate for use in anti-photoaging therapeutics. Kim et al. explored the anti-aging effects of the P.yezoensis peptide, PYP1–5, in human dermal fibroblast cell line Hs27[45]. Their findings suggest that PYP1–5 facilitates collagen synthesis by suppressing the matrix metalloprotease-1 protein and enhancing the expression of tissue inhibitor of metalloproteinase-1 and-2. Furthermore, PYP1–5 has been shown to activate the transforming growth factor-β/Smad signaling pathway, which subsequently stimulates collagen synthesis in Hs27 cells.
3.3. Skeletal muscle anti-atrophic peptides
Skeletal muscle atrophy is characterized by insufficient protein synthesis and excessive muscle protein degradation[46]. Bioactive peptides are typically composed of 3-20 amino acid residues[1].The protein content of P. yezoensis is reportedly more remarkable than that of soya beans[47]. Moreover, it offers a large pool of potential biologically active peptides. Atrogin1/muscle atrophy F box (MAFbx) and muscle RING finger 1(MuRF1) are known muscle atrophy genes and are expressed before muscle mass loss[48]. The P. yezoensis peptide PYP15 significantly decreased the expression of atrogin1/MAFbx and MuRF1 in dexamethasoneinduced C2C12 cells[49]. These observations were elaborated upon by a similar group of researchers who demonstrated the antiatrophic mechanism of PYP15 in insulin-like growth factor (IGF)-I-mediated Akt-mTOR and Akt-FoxO signaling pathways[50].PYP15 enhances dexamethone (DEX)-induced IGF-IR and insulin receptor substrate-1 phosphorylation, which induces skeletal muscle hypertrophy[50]. Thus, PYP15 helps protect DEX-induced C2C12 myotubes through the activation of IGF-I signaling. Mammalian target of rapamycin (mTOR) is a serine/threonine kinase, also referred to as nutrient-sensing protein kinase, that provokes agerelated diseases[51]. Hence, mTOR inhibitors may be useful for treating several age-associated conditions. Another serine/threonine kinase, Akt, is involved in multiple regulatory mechanisms and biological functions associated with human disease[52]. Activation of the Akt-mTORC1 signaling pathway stimulates protein synthesis by activating p70S6K and inhibiting 4E-BP1[53]. PYP15 treatments have been shown to increase the phosphorylation of p70S6K, S6, and 4EBP1 and the expression of eIF4E[50]. The phosphorylation of FoxO(forkhead box transcription factors) was significantly enhanced by treatment with PYP15[50]. These results indicate that PYP15 protects C2C12 myotubes by activating the Akt-mTORC1 pathway via activation of IGF-I signaling and regulating the signaling pathways of Akt-mTORC1 and Akt-FOXO. Mechanistic investigations into P. yezoensis crude protein (PYCP) revealed that PYCP inhibits ubiquitin-proteasome and autophagy-lysosome pathways in DEXadministrated C57BL/6 mice[54]. These findings showed that PYCP prevents muscle atrophy by enhancing protein synthesis while preventing protein degradation.
3.4. Anti-inflammatory peptides
The inflammatory response of bioactive material is directly linked to its antioxidant potential. Among the natural substances obtained from macroalgae, several compounds extracted from P.yezoensis have exhibited potent antioxidant activity[21,25,26]. For example, phycoerythrobilin from Porphyra species has been proved to have antioxidative activity[55]. Further, Sakai et al. showed anti-inflammatory efficacy of phycoerythrin and its pigment moiety phycoerythrobilin from P. yezoensis by suppressing mast cell degranulation in rat basophilic leukemia (RBL-2H3) cells and Sprague-Dawley rats[56]. The dose of phycoerythrin and phycoerythrobilin used in that study was equivalent to 5-12 g dried nori prepared from P. yezoensis for human consumption. Thus, daily intake of nori can be useful for preventing inflammation caused by allergens. Acute kidney injury can impair renal function due to multiple structural and functional changes within the kidney due to aging[57]. Treatment with a PYP extract effectively prevented cisplatin-induced nephrotoxicity in HK2 human proximal tubular epithelial cells[58].
P. yezoensis peptide (PPY), PYGP and PPY1 both demonstrated potent anti-inflammatory and antioxidant activities in lipopolysaccharide (LPS)-activated RAW 264.7 macrophage cells[7,59]. MAPK is a potential anti-inflammatory therapeutic, as its expression regulates the production of inflammatory products at the molecular level[60]. PPY1 consists of five amino acids (K, A, Q, A, D)and exhibits anti-inflammatory activity through a MAPK-signaling mechanism[59]. This inhibits the expression of inducible nitric oxide synthase and cyclooxygenase 2, which are inflammatory mediators in LPS-stimulated RAW 264.7 macrophages, without affecting cell viability[59]. Transmembrane protein toll-like receptor 4 (TLR4)induces pro-inflammation under LPS-induced oxidative stress and activates both MAPK and NF-κB[61]. The anti-inflammatory impact of PYGP occurs by modulating TLR4 signaling, thereby inhibiting NF-κB and MAPK[7]. PYGP also suppresses the LPSinduced formation of TLR4-interleukin receptor associated kinase 4 complexes in a dose-dependent manner. In addition, PYGP has been shown to reduce the binding of TIR-domain-containing adapterinducing interferon-β to TLR4 in a MyD88-independent manner.Thus, PYGP inhibits the ability of LPS to activate TLR4 signaling pathways, regardless of MyD88, thereby limiting the activation of MAPK and NF-κB. However, further investigation is needed to verify the use of these P. yezoensis biopeptides as therapeutic agents for inflammatory-related disorders.
3.5. Immunomodulatory peptides
Cytokines mediate their reactions by activating the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway,which plays a significant role in regulating immunomodulatory function[62]. Choi et al. showed that PYGP is a potent antiinflammatory agent by monitoring the phenotypic shift between M1 and M2 through STAT3 and STAT6[63]. In RAW 264.7 cells pre-treated with PYGP, the phosphorylation levels of STAT3 and STAT6 increased in a dose-dependent manner. Besides, a pepsin extract of P. yezoensis demonstrated an immunomodulatory effect on murine splenocytes by increasing macrophages and dendritic cells’activation[64]. However, very limited studies are carried out as an immunomodulatory efficacy of P. yezoensis peptides.
3.6. Antihypertensive peptides
The morbidity and mortality of cardiovascular diseases are among the most critical public health issues of the 21st century[65].The renin-angiotensin system and the kallikrein-kinin system are two mechanisms responsible for regulating blood pressure in humans[66,67]. Angiotensin-converting enzyme (ACE) can disturb the balance between renin-angiotensin system and kallikrein-kinin system, leading to hypertension[68]. Antihypertensive peptides can inhibit ACE by chelating Znat its active site[69]. Among the seven commercial enzymes that have been evaluated as antihypertensive therapeutics, alcalase from P. yezoensis was the most potent ACE inhibitor with an ICof 1.6 g/L[6]. In comparison, alcalase from Pyropia columbina attained only 45% inhibition of ACE[70]. Peptides from P. yezoensis displayed their considerable ACE inhibitory ability in rat[71]. Furthermore, it demonstrated a positive effect when tested on humans[72]. P. yezoensis-peptides, when studied in hypertensive patients at a dosage of 1.8 g/day, substantially decreased blood pressure without harming other clinical parameters, which proves safe to use as an alternative to the existing antihypertensive medicines.
3.7. Antibacterial peptides
Emergent bacterial resistance to conventional antibiotics is a significant challenge to public health worldwide[73]. In 2013 alone,more than 1 000 pharmacologically active marine compounds were identified with potential effectiveness against viruses, bacteria,and/or fungi[74]. Jiang et al. were the first to isolate antimicrobial compounds from P. yezoensis and exhibited an effect similar to that of a penicillin solution (100 μg/mL) against Escherichia coli and Staphylococcus aureus[75]. Ulagesan et al. recently examined the antibacterial activity of purified recombinant ferritin from P. yezoensis against Gram-negative and Gram-positive bacterial cultures[76]. Recombinant cyclophilin from P. yezoensis also showed significant antibacterial activity[77]. These findings demonstrate the potential of antimicrobial proteins from P. yezoensis as potential therapeutic agents, although further study is needed to fully explore their antimicrobial properties.
3.8. Neuroprotective peptides
Antioxidant, anti-inflammatory compounds are also expected to exhibit neuroprotective effects[78]. Ethanol extracts of P. yezoensis demonstrated neurogenesis by modulating synaptogenesis and supporting neurons by scavenging ROS in rat hippocampal neurons[79]. P. yezoensis (PYP) Glu-derived peptide facilitated the survival of frontal cortical neurons by triggering tyrosine kinase B receptor-ERK1/2 signaling and attenuating estrogen receptor stress against perfluorooctane sulfonate (PFOS) and glutamate exposure in primary hippocampal neurons of rats[80,81]. Data also suggest that PYP decreased the PFOS-mediated susceptibility to calcium deregulation, potentially alleviating cognitive deficiencies and behavioral problems associated with chronic organic pollutants. These results could be appropriate to use P. yezoensis peptides as a possible therapeutic benefit for treating aging brain neurodegenerative diseases.
3.9. Anticancer peptides
Owing to its high potential for the presence of essential bioactive compounds in marine flora, its research into anticancer agents is growing day by day[3]. The role of apoptosis in preventing cell proliferation is significant, which is regulated by the p53 and Bcl-2 families in the mTOR pathway and is triggered in malignant tumors[82]. Autophagy is another signaling pathway that contributes to the degradation of cellular components due to lysosomal activity[83]. The relationship between autophagy and cancer has been of interest for many years. Toward a means of controlling autophagy,Park et al. investigated mTOR pathway activation in MCF-7 breast cancer cells treated with PPY[84]. Their study showed that the p53/NF-kB and mTOR pathways were influenced by PPY, which contributed to understanding the functional relationship between the Bcl-2 family and mTOR under apoptotic conditions in MCF-7 cells.These studies indicate that the bioactive components of Porphyra have possible anti-cancer applications.
3.10. Anticoagulant peptides
Despite its high concentrations of bioactive substances, very few studies have examined the anticoagulant properties of P. yezoensis.Porphyrans exhibit an anticoagulation activity by enhancing the activated partial thromboplastin time range[85]. The bioactive peptide NMEKGSSSVVSSRMKQ, derived from enzymatically hydrolyzed P. yezoensis, showed a dose-dependent prolongation of activated partial thromboplastin time[86]. Anticoagulation measured by microtiter plate reader was found to be non-cytotoxic and had a similar activity order as that of heparin. Thus, compared to conventional medications, algae-derived bioactive compounds offer various advantages, including therapeutic action and high bioactivity[87].
4. Conclusions
P. yezoensis is abundantly cultivated in East Asia, China, Japan,and Korea because of its commercial, biological, and medicinal significance. Multiple studies have demonstrated the importance of Porphyra species as functional foods due to their high protein and polysaccharide content. An increasing number of studies have shown the usefulness of bioactive compounds from Porphyra for various applications. Biopeptides from P. yezoensis can exhibit antioxidant,anti-aging, anti-inflammatory, immunomodulatory, antihypertensive,and/or anticoagulant properties. Among the various Porphyra species that have been evaluated, P. yezoensis has been extensively exploited for its nutritional benefits. However, further research and clinical studies are required to understand these metabolites and their biological activities better.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
The Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education(Grant No. 2012R1A6A1028677), supported this research.
Authors
’contributions
SAS and NB made a concept of subject, drafted the manuscript,and revised it critically for scholarly content. SJK created figure content. YHC was involved in gathering patent related information.TJN corrected typogramatical errors and approved final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年9期
Asian Pacific Journal of Tropical Biomedicine2021年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Improved performance of naringenin herbosomes over naringenin in streptozotocininduced diabetic rats: In vitro and in vivo evaluation
- Tylophora hirsuta L. leaf extract attenuates alloxan-induced diabetes in mice by suppressing oxidative stress and α-amylase
- Antibacterial activity and inhibition against Staphylococcus aureus NorA efflux pump by ferulic acid and its esterified derivatives
- In vitro antimicrobial and synergistic effect of essential oil from the red macroalgae Centroceras clavulatum (C. Agardh) Montagne with conventional antibiotics
