Antibacterial activity and inhibition against Staphylococcus aureus NorA efflux pump by ferulic acid and its esterified derivatives
Patrícia Gonçalves Pinheiro, Gilvandete Maria Pinheiro Santiago, Francisco Erivaldo Freitas da Silva, Ana Carolina Justino de Araújo, Cícera Rejane Tavares de Oliveira, Priscilla Ramos Freitas, Janaína Esmeraldo Rocha, José Bezerra de Araújo Neto, Maria Milene Costa da Silva, Saulo Relison Tintino, Irwin Rose Alencar de Menezes✉,Henrique Douglas Melo Coutinho✉, José Galberto Martins da Costa
1Departamento de Química Biológica, Programa de Pos-Graduação em Química Biológica, Universidade Regional do Cariri – URCA, Crato – CE,Brazil
2Programa de Pós-Graduação em Química, Centro de Ciências, Universidade Federal do Ceará – UFC, Fortaleza – CE, Brazil
ABSTRACT Objective:To evaluate the inhibitory activity of ferulic acid and four of its esterified derivatives (methyl, ethyl, propyl, and butyl)against resistance mechanisms in Staphylococcus aureus strains.
KEYWORDS:Ferulic acid; Esterified derivatives; Efflux pump;Staphylococcus aureus; Resistance mechanisms
1. Introduction
The increase in mortality rate as a consequence of bacterial infections is ascribed, in particular, to bacterial resistance, which can be associated with the abusive and indiscriminate use of antibiotics[1-3]. Bacteria become resistant to drugs by obtaining genes, usually contained in plasmids and transposons, and by mutations that produce changes in the active site of antibiotics[4,5].
The mechanisms of bacterial resistance, although not common for all antibiotics, act in three principal ways: antibiotic inactivation, by hydrolysis or chemical modification; antibiotic target modification,by genetic mutation or post-translational modification; and reduced antibiotic intracellular concentrations, as a result of poor penetration or efflux mechanisms[6-8].
The decrease in intracellular concentration of an antibiotic may be due to an extrusion mechanism, by which the drug is expelled out of the cell through an energy-dependent process, called active efflux, mediated by efflux pumps[9]. Such pumps are ubiquitous,transmembrane proteins, capable of actively expelling or exchanging toxic compounds out of the bacterial cell[6]. These systems can provide resistance to a given drug or a class of drugs, however, the main problem is caused by so-called multi-resistant efflux pumps(MDR pumps) that can extrude a wide variety of structurally unrelated compounds[9].
The most studied bacteria among the microorganisms that present an efflux pump are the Gram-positive bacteria from the Staphylococcus genus, which are associated with infections of the skin, wound, and soft tissues, in addition to being identified as a cause of endocarditis and infections associated with implant devices,such as valves and catheters[10]. The antibiotic resistance severity of this species lies in the fact that these bacterial strains are not only highly virulent but also resistant to commonly available antibacterial drugs[11-13]. Moreover, the number of Staphylococcus aureus (S.aureus) strains present in clinical isolates resistant to various drugs is increasing[14].
The NorA pump, specific to S. aureus, is responsible for the efflux of various drugs such as fluoroquinolones, quinolones, verapamil,and omeprazole, in addition to dyes such as acridine and ethidium bromide[15,16]. Many studies are being carried out to find new substances capable of reversing bacterial resistance from the NorA pump and other pumps promoted by efflux pumps. Various isolated plant compounds, as well as extracts and essential oils, have shown good results in terms of inhibiting this mechanism[17,18].
Phenolic compounds have been widely reported among these substances as promising alternatives in the search for new sources of treatment against infections caused by MDR bacteria[19,20]. Studies indicate that these compounds can contribute satisfactorily as new sources of adjuvant treatment, since they can modify bacteria,impairing their locomotion, as well as surface adhesion, biofilm formation, and the formation of virulence determinants[21].
Ferulic acid is a phenolic compound from the hydroxycinnamic acid class, generated from the metabolism of the phenylalanine and tyrosine amino acids, which can be found in the most diverse natural sources, such as corn, rice, wheat, beet, artichoke, coffee, and red fruits, either in a free form or in conjunction with cell wall proteins and polysaccharides[22-24].
A study evaluated the influence of ferulic acid on the antibacterial activity of quinolone-based antibiotics against Acinetobacter baumannii and showed that ferulic acid potentiated the antibacterial activity of quinolones[24]. According to the results obtained by Takahashi et al.[25], ferulic acid has a very strong antibacterial activity against Listeria monocytogenes, inhibiting its growth with minimal risk of developing compound resistance.
Given the above, the objective of this study was to evaluate the antibacterial activity and possible NorA efflux pump inhibition of ferulic acid and its four esterified derivatives against S. aureus strains and to establish relationships between the structures and activities of these compounds.
2. Materials and methods
2.1. Chemical products
Ferulic acid, 4-hydroxy-3-methoxycinnamic acid, trans-4-hydroxy-3-methoxycinnamic acid, absolute methyl alcohol content (200,99.8%), absolute ethyl alcohol content (200, 99.5%), absolute propyl alcohol content (200, 99.7%), n-butanol alcohol, absolute butyl alcohol content (200, 99.8%), and carbonyl cyanide m-chlorophenyl hydrazone (CCCP) were used in this study. All chemical substances were obtained from Sigma Aldrich.
2.2. Substance preparation and microorganisms
For the microbiological tests, the substances norfloxacin and ethidium bromide were initially solubilized in dimethyl sulfoxide and then diluted in water. The efflux pump inhibitor used was CCCP, following its dissolution with methylene and concentration adjustment to 1 024µg/mL. The 1199B strain and all the substances used were carried out according to the study of Santos et al[26]. The S. aureus strains used in the present study were kindly provided by Prof. Gibbons(University of London).
2.3. Acquisition of the esterified derivatives
The derivatives were synthesized by esterifying ferulic acid with methyl, ethyl, propyl, and butyl alcohols, following the classic Fischer esterification mechanism. Ferulic acid (60 mg, 0.309 2 mmol), was dissolved in the respective alcohols (10 mL: MeOH, EtOH, PropOH,and ButOH) and dicyclohexylcarbodiimide (DCC, 54 mg, 0.262 1 mmol), at catalytic quantities of 4-N,N-dimethylaminopyridine. The reaction mixture was continuously refluxed, under magnetic stirring with heating at 50 ℃ for 4 h. Afterward, the N,N-dicyclohexylurea formed was filtered away, the solvent was removed on a rotary evaporator at room temperature, and the crude residue was purified by column chromatography (silica gel, hexane/EtOAc:70/30). Ferulic acid, used as a substrate, and the four esterified derivatives were characterized byH andC NMR, including the DEPT 135° technique.
2.4. Structural characterization
TheH andC NMR spectra were recorded on a Bruker Avance DPX 300 spectrometer, operating at the frequency of 300 MHz forH and 75 MHz forC. The spectra were obtained in CDOD solvent and chemical displacements (δ
) were expressed in ppm, with tetramethylsilane as the internal standard. The multiplicities of theH NMR signals were indicated following the convention: s (singlet),sl (broad singlet), d (doublet), dd (double doublet), t (triplet), q(quartet), and m (multiplet). All derivates obtained were completely used in the NMRC andH analysis to confirm the reaction, and in the biological assays performed.2.5. Minimum inhibitory concentration (MIC) assays
Tests for MIC of ferulic acid and its esterified compounds were performed according to the methods of Tintino et al[27]. The bacterial strain was incubated at 37 ℃ for 24 h. After that, the procedures for standardization and dilution of the sub-branches were performed according to the base study. After 24 h, the plates were read by visualizing the color change of the medium, characterized by the addition of 20 µL of resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide). The characteristic color change of the medium from blue to red in this experiment indicates the presence of bacterial growth,while its permanence in blue indicates the absence of growth. The above experiments were carried out in triplicate.
2.6. Efflux pump inhibitory study
As previously performed, the methodology of Tintino et al.[27]was adopted to assess the antibacterial activity of the compounds,verifying the reduction effect on MICs of ethidium bromide and norfloxacin. In the test, the substance was used in subinhibitory concentration. For the control, the conditions were similar to the test,but without the addition of substances. Then, these substances were transferred to 96-well microdilution plates, with vertical distribution,characterized by the addition of 100 µL of Eppendorf content in each well. After this stage, micro dilutions of ethidium bromide and norfloxacin were performed[27]. After 24 h, the plates were read by visualizing the color change of the medium, as previously performed. The experiments were carried out in triplicates.
2.7. Statistical analysis
Antibacterial assays were performed in triplicates and results were expressed as the average of replicates. The results from the tests were expressed as the mean ± standard error of mean and evaluated by analysis of variance (unidirectional ANOVA), followed by Tukey test using the GraphPad Prism v software. 6.01, with a level of statistical significance set at 5% (P < 0.05). Principal component analysis was used to achieve better visualization of the different data sets and a more distinct view of the relationship among the variables. This method is based on matrix linking percentages of the major components to provenance stations and the variability of physicochemical parameters and antimicrobial activity. These multivariate analyses were performed by the Minitab Software.
2.8. NorA structure prediction and molecular docking
All dockings were carried out using the Schrodinger Suite 2015 molecular modeling software. A two-dimensional Rip-B structure was built using Maestro. This structure was converted into its threedimensional form, including various tautomers, conformers, and ionization states using LigPrep and ConfGen modules. A threedimensional model of NorA was generated using I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/). The best scoring model was used as a receptor for ligand-receptor docking. The receptor was prepared for docking by employing the Protein Preparation Wizard and generating the binding site grid. Ligand-receptor docking was performed using the Glide module, and flexible docking was carried out for all the conformers to determine the ligand-binding mode. The Glide Extra Precision scoring function was used.
2.9. Measure of drug likeliness, Lipinski rule analysis and ADMET predictions
ADME computational methods are used to predict pharmaceutical potential, optimize new lead candidates, and evaluate pharmacological properties. The therapeutic actions are dependent on pharmacokinetic parameters (Absorption, Distribution, Metabolism, and Excretion).A large number of structural modifications can be an influence of diverse physicochemical parameters. Then, understanding of this helps screen weak candidates in the early stage of drug development that finds potential drug candidates. The prediction of physicochemical descriptors and determination of the pharmacokinetic properties and drug likeliness, and other ADME properties are done by the swissADME (http://www.swissadme.ch) server.
3. Results
3.1. Esterified ferulic acid derivatives
The derivatives (Figure 1) were obtained through the Fischer esterification reaction, where the structural changes occurred specifically in the ferulic acid carboxylic group with the insertion of alkyl groups, ranging from carbons 1 to 4, depending on the alcohol used. The compounds were identified by interpreting the respectiveC NMR,H NMR, and DEPT 135° spectra.
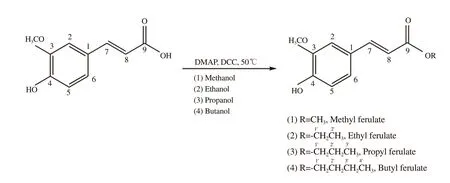
Figure 1. Ferulic acid and esterification reaction products. DCC: Dicyclohexylcarbodiimide, DMAP: 4-Dimethylaminopyridine.
3.1.1. Ferulic acid (MW = 194.18 g/mol)
White solid:H NMR (300 MHz, CDOD) 3.78 (s, OCH), 6.21(1H, d, J=16.0 Hz, H-8), 6.71 (1H, d, J=8.0 Hz, H-5), 6.93 (1H, dd,J=8.0 and J=1.7 Hz, H-6), 7.06 (1H, d, J=1.7 Hz, H-2), 7.49 (1H, d,J=16.0, H-7);C NMR (75.5 MHz, CDOD) 56.56 (CHO), 111.83(C-2), 116.04 (C-5), 116.60 (C-8), 124.12 (C-6), 127.93 (C-1),147.06 (C-7), 149.48 (C-3), 150.61 (C-4), 171.13 (C=O).
3.1.2. Methyl ferulate (MW = 209.21 g/mol)
Yellow solid (63%):H NMR (300 MHz, CDOD) 3.75 (s, OCH),3.87 (s, OCH), 6.32 (1H, d, J=16.0 Hz, H-8), 6.80 (1H, d, J=8.0 Hz,H-5), 7.04 (1H, dd, J=8.0 and J=1.7 Hz, H-6), 7.14 (1H, d, J=1.7 Hz, H-2), 7.58 (1H, d, J=16.0, H-7);C NMR (75.5 MHz, CDOD)52.14 (OCH*), 56.56 (CHO), 111.82 (C-2), 115.31 (C-5), 116.59(C-8), 124.20 (C-6), 127.77 (C-1), 146.92 (C-7), 149.46 (C-3),150.72 (C-4), 169.83 (C=O).
3.1.3. Ethyl ferulate (MW = 223.34 g/mol)
Yellow solid (73%):H NMR (300 MHz, CDOD) 1.30 (t, 3H),3.87 (s, OCH), 4.21 (q, 2H), 6.32 (1H, d, J=16.0 Hz, H-8), 6.80(1H, d, J=8.0 Hz, H-5), 7.04 (1H, dd, J=8.0 and J=1.7 Hz, H-6), 7.15(1H, d, J=1.7 Hz, H-2), 7.58 (1H, d, J=16.0, H-7);C NMR (75.5 MHz, CDOD) 14.78 (CH), 56.55 (CHO), 61.56 (OCH), 111.80(C-2), 115.75 (C-5), 116.59 (C-8), 124.16 (C-6), 127.82 (C-1),146.73 (C-7), 149.47 (C-3), 150.69 (C-4), 169.41 (C=O).
3.1.4. Propyl ferulate (MW = 237.27 g/mol)
Yellow solid (47%):H NMR (300 MHz, CDOD) 0.98 (t, 3H),1.70 (m, 2H), 3.87 (s, OCH), 4.12 (t, 2H), 6.34 (1H, d, J=16.0 Hz, H-8), 6.80 (1H, d, J=8.0 Hz, H-5), 7.05 (1H, dd, J=8.0 and J=1.7 Hz, H-6), 7.16 (1H, d, J=1.7 Hz, H-2), 7.58 (1H, d, J=16.0, H-7);C NMR (75.5 MHz, CDOD) 10.90 (CH), 23.30 (CH), 56.56(CHO), 67.20 (OCH), 111.78 (C-2), 115.68 (C-5), 116.60 (C-8),124.19 (C-6), 127.80 (C-1), 146.76 (C-7), 149.48 (C-3), 150.72(C-4), 169.49 (C=O).
3.1.5. Butyl ferulate (MW = 251.29 g/mol)
Yellow solid (73%):H NMR (300 MHz, CDOD) 0.95 (t, 3H),1.42 (m, 2H), 1.65 (m, 2H), 3.87 (s, OCH), 4.15 (t, 2H), 6.32(1H, d, J=16.0 Hz, H-8), 6.80 (1H, d, J=8.0 Hz, H-5), 7.03 (1H, dd,J=8.0 and J=1.7 Hz, H-6), 7.14 (1H, d, J=1.7 Hz, H-2), 7.56 (1H, d,J=16.0, H-7);C NMR (75.5 MHz, CDOD) 14.22 (CH), 20.35(CH
), 32.06 (CH), 56.55 (CHO), 65.41 (OCH), 111.79 (C-2),115.70 (C-5), 116.58 (C-8), 124.16 (C-6), 127.80 (C-1), 146.72(C-7), 149.43 (C-3), 150.66 (C-4), 169.45 (C=O).
3.2. Microbiological tests
The microdilution method was used to determine the MIC of ferulic acid and its respective esterified compounds against multi-resistant S. aureus strains carrying the NorA efflux systems. All esterified compounds and ferulic acid obtained MIC values ranging between 101.6-128.0 µg/mL against S. aureus.
3.3. Effects on S. aureus NorA proteins
Ethidium bromine is used as a marker for the NorA efflux pump activity because the extrusion of this compound is possible only with the action of this pump. The ethyl ferulate compound at subinhibitory concentrations inhibited the NorA efflux pump and reduced the MIC of ethidium bromide, which was similar to that observed when using CCCP, a known efflux pump inhibitor (Figure 2).
Moreover, CCCP, as well as the ethyl and propyl ferulate compounds enhanced the effectiveness of norfloxacin against S. aureus 1199B strains that express the NorA pump with reduced MICs (Figure 3).
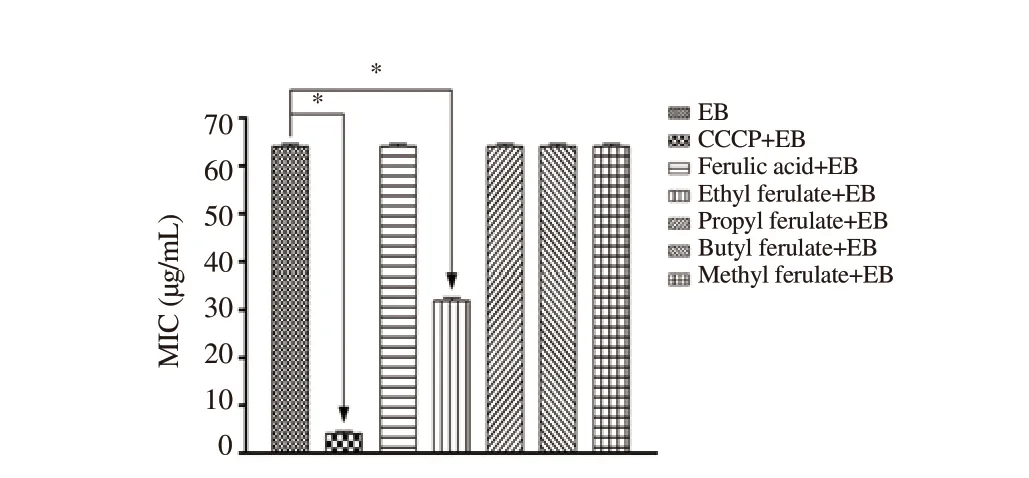
Figure 2. Minimum inhibitory concentration (MIC) of ferulic acid and its four esterified compounds in association with ethidium bromide against Staphylococcus aureus 1199B. Statistical significance was determined by oneway ANOVA and Tukey’s post hoc test. * - indicates statistically significant differences between groups with P<0.05. EB: Ethidium bromide, CCCP:carbonyl cyanide m-chlorophenyl hydrazone.
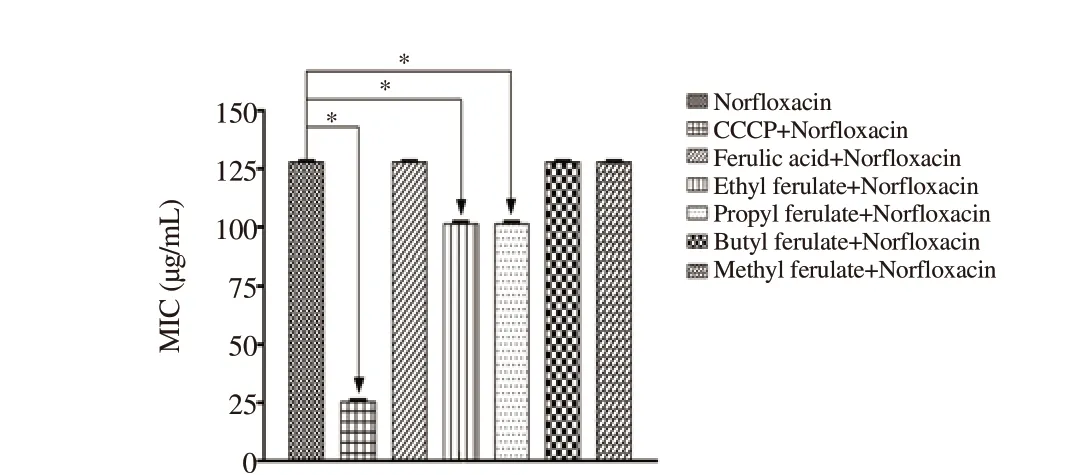
Figure 3. MIC of norfloxacin alone or in the presence of ferulic acid and its esterified compounds against Staphylococcus aureus strains. Statistical significance was determined by one-way ANOVA and Tukey’s post hoc test. *- indicates statistically significant differences between groups with P<0.05.
3.4. Docking results and ADMET pharmacokinetic properties predictions
Results from the in silico study of the physicochemical properties of ferulic acid derivatives are shown in Table 1. Based on Table 1,the log value of the octanol/water partition coefficient (CLog Po/w)ranged from 1.36 to 2.80 (<5), the number of hydrogen bond donnor ranged from 1 to 2 (≤ 5), and the number of hydrogen bond acceptor was 4 for all derivatives (<10). Thus, all derivatives meet Lipinski’s Rules of Five[28].
The docking results suggested different forces of interaction type including hydrogen bonds, van der Waals (VW), Pi-sigma, Pi-Alkyl are necessary to the NorA-ferulate complex formation. These fingerprints present in the active site demonstrated that amino acid residues TYR57, TYR58, and LEU255 were related with the interaction of these derivates with the struture of the efflux pump,mainly due to the effect of hydrophobic interactions and hydrogen bonds.
Based on the principal component analysis using physicochemical property, as shown in Table 1, it can be observed that some physicochemical properties are directly related with the antimicrobial activity. In PC1 axis, positive values indicated the main molecular characteristics of the compounds with action against the NorA efflux pump, as the number of atom donnors of hydrogen bonds and the molar refractivity (Figure 4). In PC2 axis, we observed that MIC activities of ferulate derivatives were influenced by lipophilicity, the rotations bond and the possible hydrogen bonds. Topological polarsurface area and accessible surface area were not correlated to NorA inhibition. Therefore, the model showed that not only lipophilia, but also the rotations bond and H-binding pattern are more relevant than efflux size and molecular shape (Figure 4).
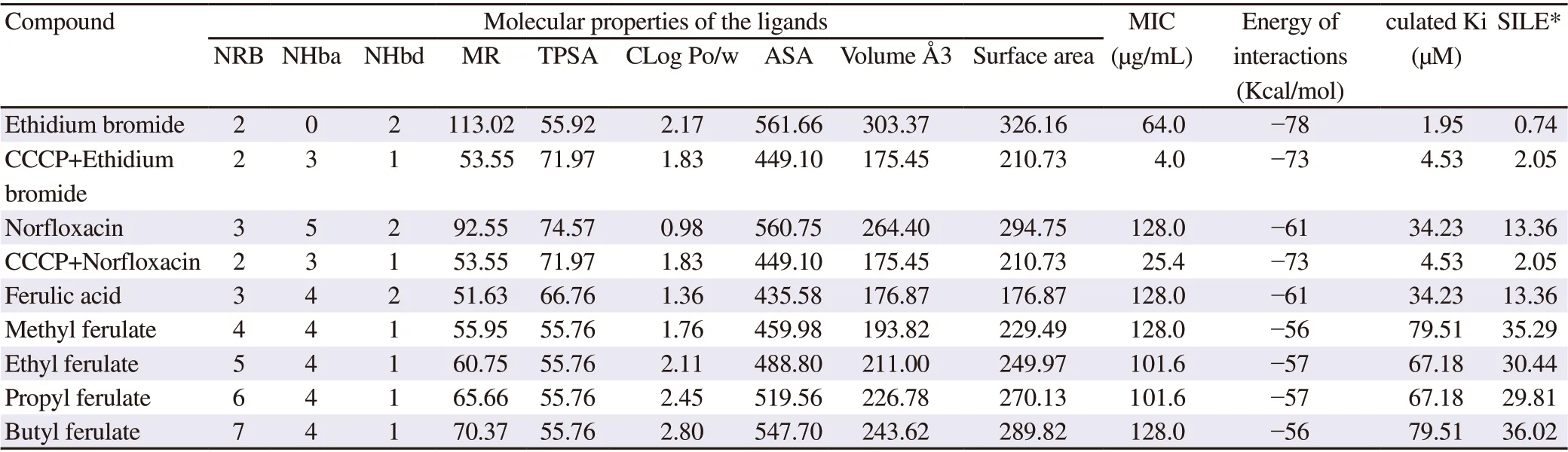
Table 1. Physicochemical predictions of ferulic acid derivatives from Swiss ADME and molecular docking results in NorA efflux pump.
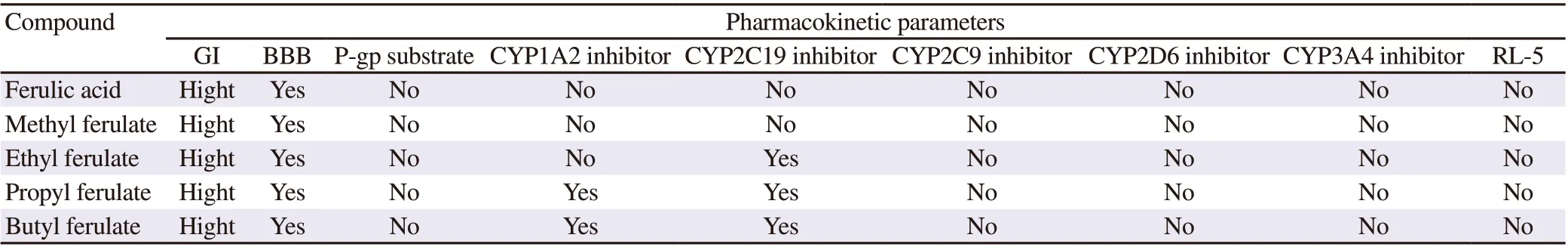
Table 2. Pharmacokinetic predictions by Swiss ADME.
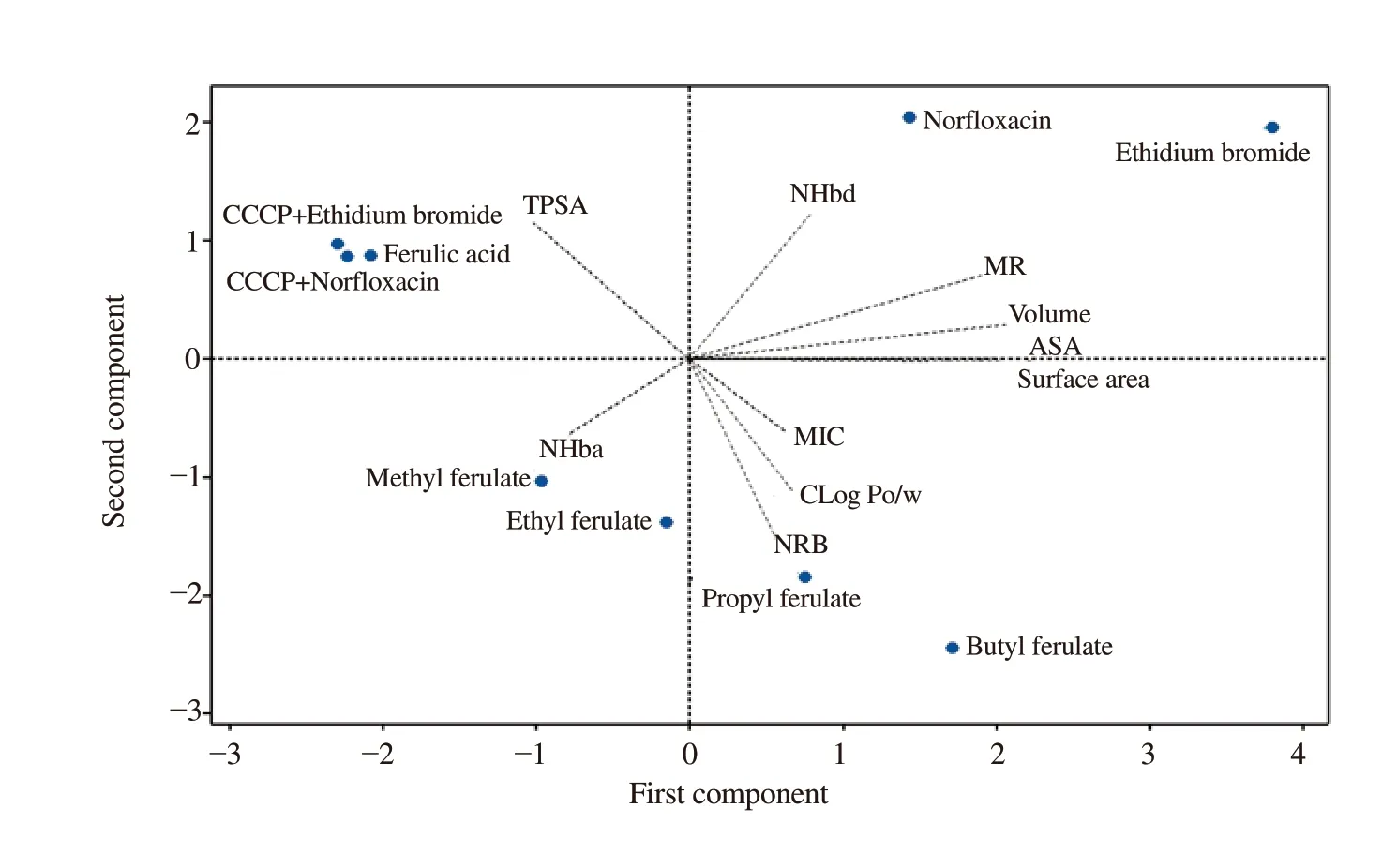
Figure 4. Chemometric multivariate analysis based on physicochemical parameters and antibacterial activity (MIC) using a principal component analysis correlation biplot (loading and score). NHbd: number of hydrogen bond donnor, NHba: number of hydrogen bond acceptor, ASA: water accessible surface area, MR: molar refractivity, MIC: minimum inhibitory concentration, NRB: number of rotatable bonds, CLog Po/w: octanol-water partition coefficient, TPSA: topological polar surface area.
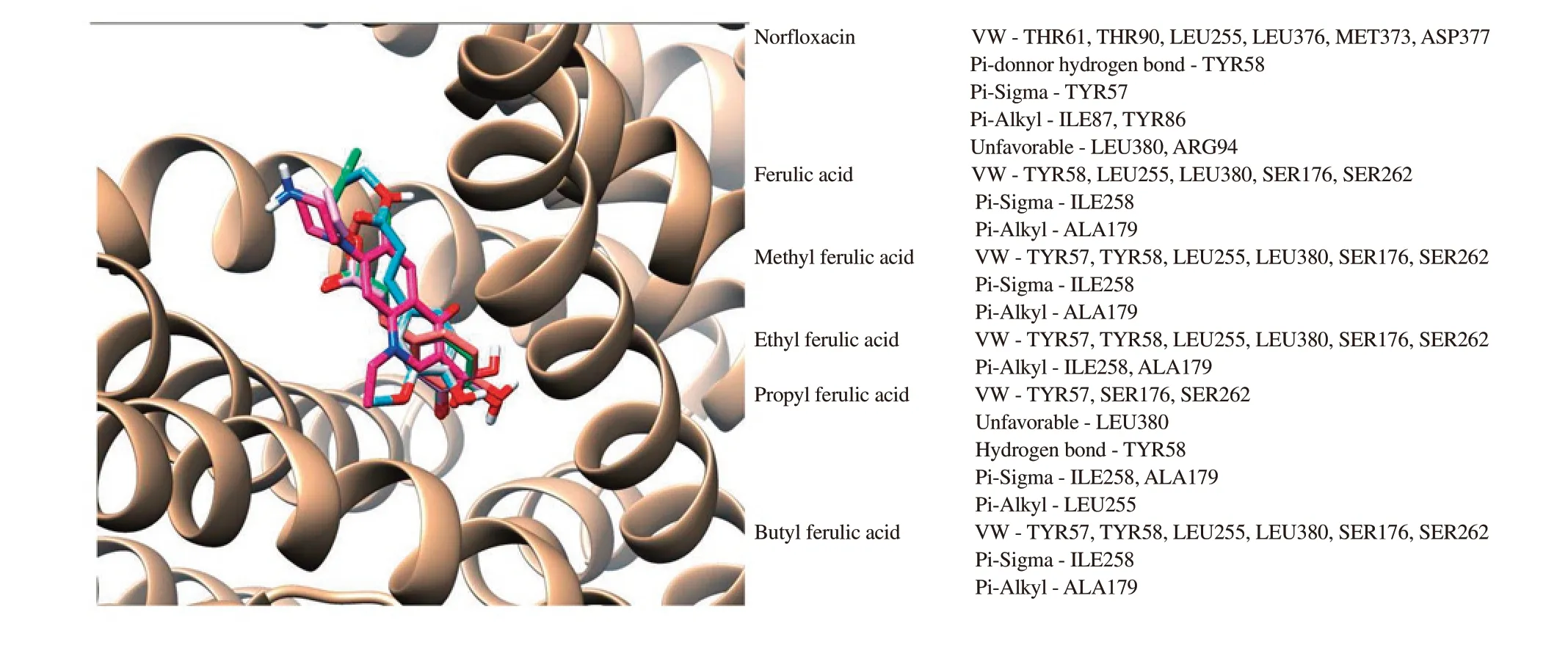
Figure 5. Binding pose with the best binding and stability for norfloxacin (red-violet), ferulic acid (white), methyl ferulic acid (pink), ethyl ferulic acid (blue),propyl ferulic acid (coral) and butyl ferulic acid (green) in the NorA efflux pump binding site and the interaction types between the residues.
In Table 2, it can be seen that almost all ferulic acid derivatives have physico-chemical characteristics that demonstrate high permeability and consequently good absorption capacity without affecting or inhibiting the cytochrome P450 enzyme complex. However, propyl and butyl derivatives can affect or inhibit CYP1A2 enzyme while ethyl, propyl, and butyl derivatives can affect or inhibit CYP2C19 enzyme.
4. Discussion
The ferulic acid esterification reaction with methanol, ethanol,propanol, and butanol produced the esters methyl ferulate, ethyl ferulate, propyl ferulate, and butyl ferulate, respectively, whose structural modifications, with respect to the substrate, occurred in the C-9 carboxylic carbon. The esterified compounds provided moderate to good yields (47%-73%), which were sufficient to carry out the tests, after purification by column chromatography.The synthesized products were characterized byH andC NMR spectra interpretation, where ferulic acid derivatives were obtained and confirmed by the presence of a pair of doublets assigned atδ
H 7.49 (J=16.0 Hz; 1H) andδ
H 6.93 (J=16.0 Hz; 1H), typical of the trans-olefinic system associated with C6-C3 benzene derivatives[29].The other chemical shifts, associated with the signals corresponding to the benzene ring, showed small differences in the series of esters, according to the insertion of the C-Calkyl groups. Taking into account small variations between ferulic acid and the four derivatives, the signals atδ
H 7.06 (d, J=1.70 Hz),δ
H 6.93 (dd,J=1.70 and 8.10 Hz) andδ
H 6.71 (d, J=8.10 Hz), observed in theH NMR spectrum, were associated with the H-2, H-6, and H-5 hydrogens, respectively.This information suggests the presence of a 1,3,4-trisubstituted benzene ring, thus confirming the basic structure of ferulic acid and its derivatives[29]. TheC NMR spectrum of ferulic acid showed a similar profile to those observed in the four reaction products, except for the presence of alkyl groups, a consequence of the structural modification caused by the esterification reaction. The hydrogenation pattern of the carbons in theC NMR was determined by the DEPT 135° technique, in combination with the variation of the signal multiplicities in theH NMR spectrum. The methanol derivative showed a signal atδ
C 52.14 (OCH), confirmed by the appearance of the singlet atδ
H 3.75 (3H). The product derived from the reaction with ethanol showed twoδ
H signals [1.30 (t) and 4.21 (q)] combined with twoδ
C signals [14.78 (CH) and 61.56 (OCH)]. Theδ
H signals [0.98 (t), 1.70 (m) and 4.12 (t)], in combination with the correspondingδ
C [10.90 (CH), 23.30 (CH) and 67.20 (OCH)]confirm the reaction with propanol. An n-butyl group, resulting from the reaction with butanol, was evidenced by the appearance ofδ
H signals [0.95 (t), 1.42 (m), 1.65 (m) and 4.15 (t)], associated withδ
C [14.22 (CH), 20.35 (CH), 32.06 (CH) and 56.41 (OCH)]. All the carbons inserted in the ferulic acid structure were justified by monitoring the signals present in the DEPT 135° spectrum.The literature reports compounds such as tannic acid, a phenolic compound capable of inhibiting the efflux pump effect and reducing bacterial resistance mediated by this mechanism, potentiate the activity of antibiotics against S. aureus[30]. Other study showed that ferulic acid esterification resulted in increased lipophilia when compared to ferulic acid[31]. The 24 ferulic acid-related compounds showed experimental partition coefficients ranging from 0.95 to 0.98 in the n-octanol/PBS assay, while ferulic acid obtained a value of 0.13. No significant lipophilicity difference was observed between ferulate derivatives, obtaining similar binding interaction values with the NorA efflux pump[32].
This activity may be associated with lipophilicity, assuming that the phenolic acids cross the cell membrane by passive diffusion in their undissociated compound form, or perhaps through binding of the pump substrates, causing a decrease in the drug inhibitory mechanism[33]. However, other efflux pump inhibitory methods,such as pump gene expression effects cannot be ruled out[33].
Ethyl ferulate is a compound whose lipid soluble nature can alter the fluidity of the bacterial membrane, making it more susceptible to antibiotic penetration[33], which demonstrated that ferulic acid has antimicrobial activity against Cronobacter sakazakii, affecting the integrity of the bacterial membrane and the synthesis of adenosine triphosphate, reducing the intracelular pH[34,35].
Other effects of the ferulic acid on the cell membrane can be a rupture of the lipid bilayer, causing extrusion of internal cell compounds[36]. Another study demonstrated that derivates of ferulic acid reduced the biofilm formation[37] and potentiated the antibiotic activity against S. aureus[38].
The compound methyl ferulate, similar to ferulic acid, did not show any activity due to its small side chain[39]. Another finding showed that an increase in the number of hydroxyl groups in the hydroxybenzoic acid side chain reduced the MIC and that the substitution of the hydroxyl groups with methoxy groups increased the activity of hydroxybenzoic acids, but not hydroxycinnamic acids.
However, the propyl ferulate and butyl ferulate compounds showed a larger side-chain, which does not leave them with unfavorable dynamic binding energy. Lipophilic substances cause disturbances in the bacterial membrane, resulting in damage to the fundamental elements necessary for membrane integrity, such as reduced membrane potential and loss of ions, cytochrome C, proteins, and radicals, followed by the collapse of the proton pump and adenosine triphosphate depletion[38,40].
Lipophilicity is a common characteristic of several compounds referred to as efflux pump inhibitors, as pointed out by Kikuzaki et al[31]. The ferulic acid related compounds show partition coefficients in n-octanol/PBD assay ranging between 0.95 to 0.98.No significant difference of lipophilicity was observed between ferulate derivatives, resulting in similar values of bindig interactions in NorA efflux pumb. This effect may, because efflux pumps constitute transmembrane proteins, have functions associated with cell membrane structure and fluidity.
The prediction of ADME property is utilized to reduce the fail in identifying candidate molecules in the drug discovery phase. Hence,these ferulic acid derivative compounds can be predicted to be easily absorbed with a low capacity for side effects or toxicity. The pattern fingerprint-based using correlations between molecular descriptors and ADME properties were particularly useful to increase the success rate to identify models from a medicinal chemistry perspective with better therapeutic potential[41].
The principal component analysis demonstrates using those physicochemical properties as hydrophobic properties, hydrogen bonds, and molecular dimensions are directly correlated with compound activity. Other studies suggest that lipophilia and rotating bonds and H-binding patterns may be more relevant than efflux size and molecular shape as the most probable pharmacophore-based model correlated with NorA inhibition. These results corroborate another study that performed a pharmacophore-based model for the NorA biological results and showed that a hydrogen-bond acceptor positive charge and aromatic rings led to the identification of potentially potent NorA inhibitors[42].
A study demonstrated that the antibacterial activity of ferulic acid affected membrane integrity, causing cell membrane hyperpolarization and intracellular pH reduction that could be correlated with a possible antibacterial mechanism[34]. Another study also demonstrated that significant alterations in membrane properties that correlate with hydrophobicity led to local rupture or pore formation in cell membranes. These results corroborate the hypothesis that lipophilic character is more relevant than efflux size and molecular shape[34,37].
Molecular docking was used to explain their inhibitory activities and the results obtained indicated that hydrogen bond interactions played a very important role in the combination that results in the energy required to form a bond between the ligand and the receptor.In Figure 5, the chemical conformation with the lowest binding energy is represented as the most stable interaction observed in the docking procedure, which shows correlations with the activity.This figure also describes the amino acid residues responsible for stabilizing the ferulate complex derived from NorA through the predominant molecular interactions of hydrogen bonds, van der Waals, Pi-sigma, and Pi-Alkyl interactions. The docking results revealed hydrogen bonds (H-bonds) and hydrophobic interactions that validate the described principal component analysis, through superimposition of the docking poses of all the ferulic acid derivatives and norfloxacin.
The association of the compound ethyl ferulate with norfloxacin and ethidium bromide showed a significant reduction in its MIC,which can be attributed to the inhibition of the NorA efflux pump.A correlation was drawn, through molecular docking, between the interaction of the compounds and the NorA efflux pump, which demonstrated good affinity. Although other derivatives did not modify the activity of the NorA pump, all compounds showed significant ADME parameters that demonstrate the potential as druglike. Nevertheless, further studies should be carried out to elucidate the complete mechanism of interaction between ethyl ferulate and the 1199B strain.
Conflict of interest statement
The authors declare no conflict of interest.
Authors
’contributions
PGP performed the formal analysis and investigation, the writing and the original draft preparation, reviewed, read and agreed to the published version of the manuscript. HDMC performed the supervision. JGMC performed the supervision and the project coordination. GMPS performed the formal analysis of the text.FEFS performed the formal analysis of the text. IRAM performed the docking assays. ACJA and SRT performed the microbiological assays. CRTO performed the chemical synthesis of the ferulic acid derivates. PRF performed the microbiological assays. JER performed the statistical analysis. JBAN performed the chemical synthesis.MMCS performed the chemical synthesis.
 Asian Pacific Journal of Tropical Biomedicine2021年9期
Asian Pacific Journal of Tropical Biomedicine2021年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Improved performance of naringenin herbosomes over naringenin in streptozotocininduced diabetic rats: In vitro and in vivo evaluation
- Tylophora hirsuta L. leaf extract attenuates alloxan-induced diabetes in mice by suppressing oxidative stress and α-amylase
- In vitro antimicrobial and synergistic effect of essential oil from the red macroalgae Centroceras clavulatum (C. Agardh) Montagne with conventional antibiotics
- Biopeptides of Pyropia yezoensis and their potential health benefits: A review
