Recombinant human vascular endostatin injection to synchronize craniospinal radiotherapy for the treatment of recurrent medulloblastoma in children:A retrospective clinical study*
Yang Song,He Xiao,Chuan Chen,Ping Liang,Wenyuan Ji,Mingying Geng (✉)
1 Cancer Center,Institute of Surgery Research,Daping Hospital,Army Medical University (Third Military Medical
2 Department ofNeurosurgery,Children's Hospital Affiliated to Chongqing Medical University,University),Chongqing400042,China Chongqing 400014,China
Abstract Objective Medulloblastoma (MB) is the most common primary central nervous system malignancy in children.Nonetheless,there is no standard treatment for recurrent MB.The purpose of this study was to investigate the clinical value and toxicity of recombinant human endostatin injection (Endostar®) combined with craniospinal radiotherapy for the treatment of recurrent MB in children.Methods This study retrospectively analyzed 13 patients with recurrent MB aged 5-18 years.Endostar®7.5 mg/m2/d was synchronized during craniospinal radiotherapy for 7 children with a portable micro uniform speed infusion pump.Endostar® was applied 3 days prior to the initiation of radiotherapy.The drug was in continuous use for 7 days.Similarly,the withdrawal of the drug took place over 7 days.This represented a cycle.During radiotherapy,the application was repeated until the end of radiotherapy (experimental group).In the other 6 cases,only craniospinal radiotherapy was used (control group).Results The complete remission rate was 71.4% in the experimental group and 16.7% in the control group.The median progression-free survival (PFS) was 14 months (95% CI:0.0-29.60) and 19 months(95% CI:0.0-39.53) in the experimental and control groups,respectively.The median overall survival (OS)was 19 months (95% CI:0.0-38.20) and 23 months (95% CI:2.47-43.53) in the experimental and control groups,respectively.The most common adverse events included grade 1 thrombocytopenia (7.7%),grade 3 neutropenia (38.5%),and grade 1 anemia (30.8%).Conclusion Endostar® synchronizing craniospinal radiotherapy significantly improved the complete response rate of children with recurrent MB.It did not increase the side effects of radiation therapy.However,it did not improve the PFS or OS.
Key words: recombinant human vascular endostatin;craniospinal radiotherapy;medulloblastoma
Medulloblastoma (MB) is the most common primary neurological malignancy in children.It mainly occurs in the vermis of the cerebellum and accounts for approximately 20% of intracranial tumors in children[1].Prior to the combined treatment with surgical radiotherapy and chemotherapy,there was less than a 20%event-free survival for 3-5 years[2].With improvements in surgical and radiotherapy techniques the survival rate of MB in children has significantly improved[3].Children aged 5-14 years have a relatively good prognosis,with a 5-year OS rate of approximately 67%[4].However,the prognosis is poor for patients who relapse after treatment.The median survival time is only approximately 1 year after the secondary surgery,secondary radiotherapy,or high-dose chemotherapy supplemented with stem cells.This results in a 2-year OS rate of 25%[1,5-6].Consequently,it is essential to develop a new comprehensive treatment method for recurrent MB.
Recently,molecular targeting therapy has become a novel way to treat tumors.Molecular targeting plays an anti-tumor role by inhibiting key molecules in the tumor signal transduction pathway.In 1971,Folkman first proposed the hypothesis that tumor growth and infiltration depend on tumor angiogenesis[7].Subsequently,anti-angiogenesis therapy targeting tumor angiogenesis has become one of the most important anti-tumor strategies.Recombinant human endostatin injection (Endostar®) is one of the most effective angiogenesis inhibitors.Its' pan-target anti-angiogenesis effect reduces abnormal angiogenesis and remodeling by affecting the dynamic balance of angiogenesis in the tumor microenvironment.This promotes the normalization of the tumor microenvironment,reducing tumor hypoxia and improving drug delivery.Thus,it plays a sensitization role in radiotherapy and chemotherapy[8-14].Vascular endothelial growth factor receptor 2(VEGFR-2) is a kinase insert domain-containing receptor(KDR).Studies have confirmed that Endostar®can be combined with radiotherapy for the simultaneous treatment of brain metastatic tumors resulting from lung cancer.This has a significant effect in patients with a high expression of VEGFR2 protein or increased copy numbers of the KDR gene[15-16].The VEGFR gene not only has expression in the MB vascular endothelial cells,but it also has high expression in tumor cells.In patients with a poor prognosis,the KDR gene expression level is higher.Endostar®combined with radiotherapy and chemotherapy for the treatment of metastatic intracranial tumor glioma and other tumors has achieved good efficacy with a satisfactory safety profile[15-16].This brings the hope of an effective treatment for central nervous system tumors with endostatin.Combining Endostar®'s antiangiogenesis characteristics with classical radiotherapy synchronous treatment has important clinical value.This combination will pave the way for the exploration of new strategies for the treatment of recurrent MB in children.
Materials and methods
Patients
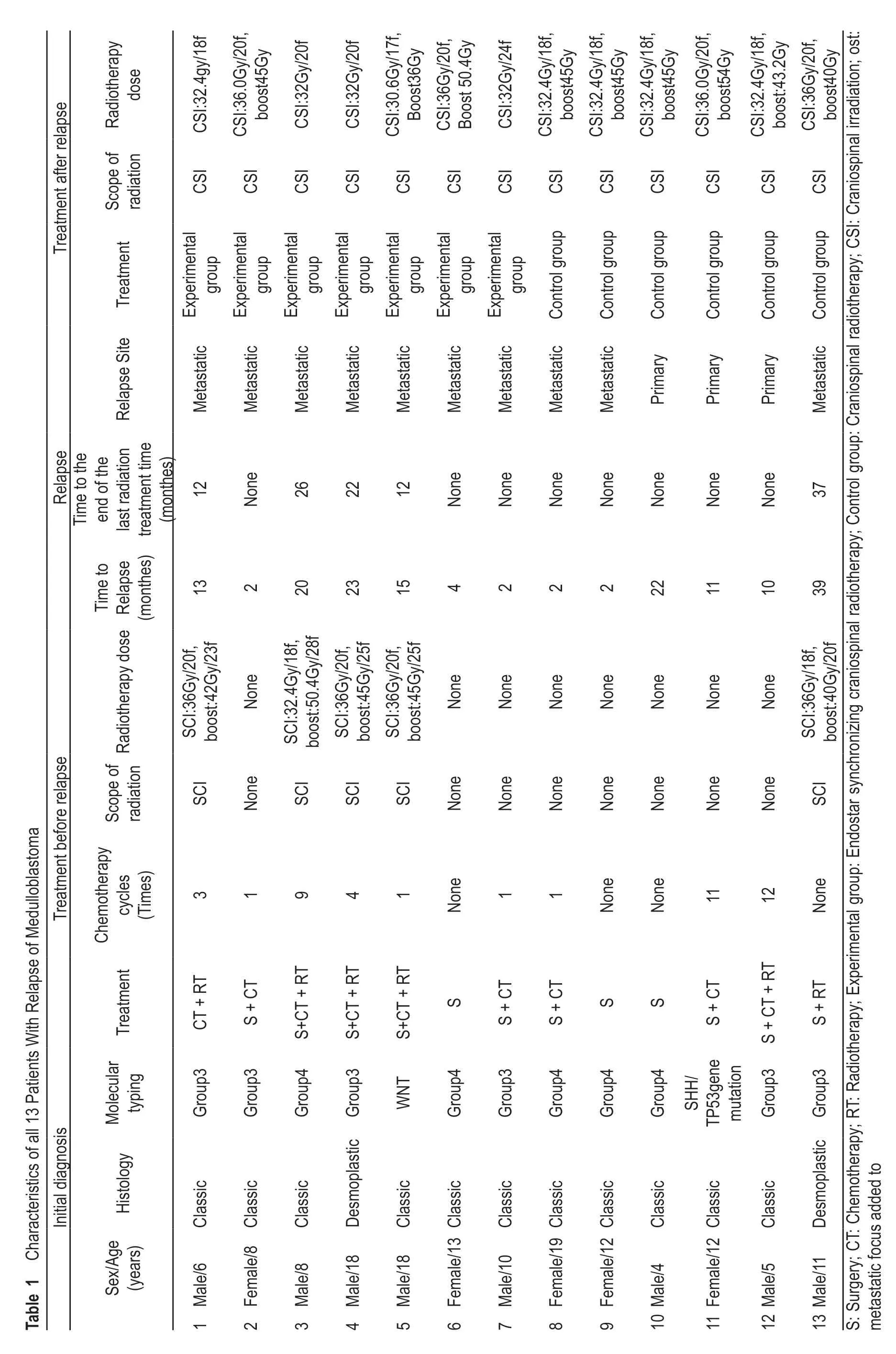
From February 2018 to July 2019,a total of 13 recurrent MB children were treated in the cancer center of the Daping Hospital of the Army,Military Medical University.These children were diagnosed with MB by pathology after tumor resection by a neurosurgeon.The median age at diagnosis for the 13 patients who relapsed was 10 years(range,5-18 years).Eight of 13 (61.5%) were male and 2 (15.4%) had the desmoplastic subtype.No secondary surgery was performed in the recurrent children (Table 1).After recurrence,M stage (Chang stage)[17]was observed in 10 M3 cases (76.9%),in 2 M2 cases (15.4%),and in 1 M0 case (7.7%) in the total group of children.According to the molecular subtype results,6 cases were classified into Group 3 (46.2%),5 cases were classified into Group 4 (38.5%),1 was WNT (7.7%),and 1 was a SHH type associated with TP53 gene mutations (7.7%).According to the patients' physical condition or personal wishes of the patients' family,3 patients (23.1%) received only surgery,4 patients (30.8%) underwent surgery and chemotherapy,1 patient (7.7%) underwent surgery and radiotherapy,and 5 patients (38.5%) underwent surgery followed by radiotherapy and chemotherapy for the initial treatment (Tables 1,2).Initial chemotherapy regimens included nitrosourea compounds,vincristine,methotrexate,etoposide,cyclophosphamide,or platinum derivatives (carboplatin cisplatin).
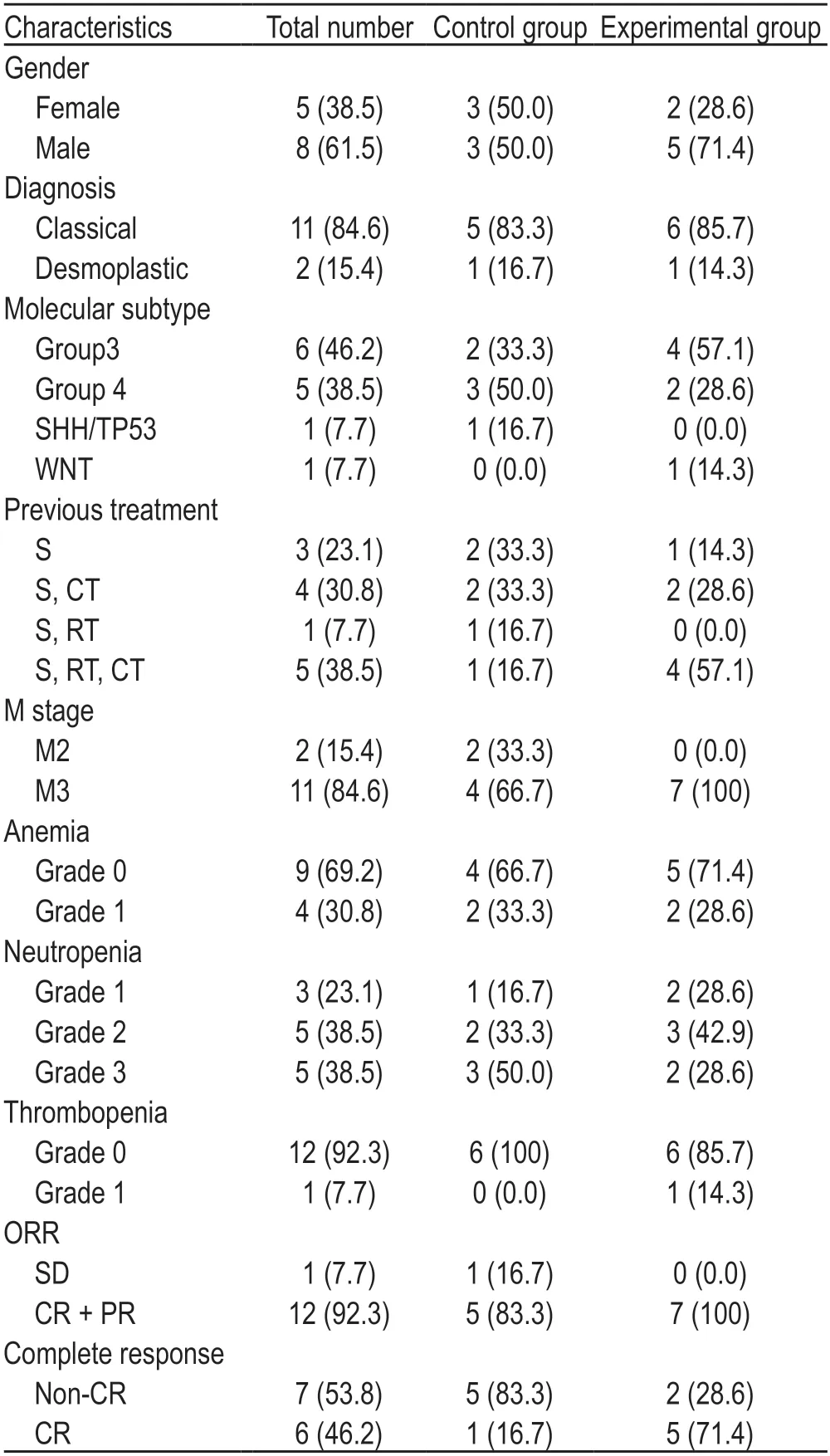
Table 2 Baseline characteristics of control group and experimental group [n (%)]
Inclusion criteria
Patients received an enhanced MRI scan of the craniospinal area and were confirmed to have myeloblastoma recurrence or tumor spread.This was confirmed prior to the initiation of treatment in our department.These patients also had measurable tumor lesions.There were no hematology contraindications prior to the initiation of radiotherapy treatment.Informed consent was obtained from the patient and legal guardian.
• Experimental group:Endostar® synchronizing craniospinal radiotherapy.
• Control group:Craniospinal radiotherapy.
The experimental group received continuous intravenous Endostar®via an infusion pump during craniospinal radiotherapy:7.5 mg/m2/d.A portable micro constant speed infusion pump was used for continuous intravenous infusion.Drug administration was initiated 3 days prior to the beginning of radiotherapy.This was followed by continuous infusion for 7 d.Drug withdrawal over the next 7 days completed the cycle.This cyclic application was conducted during radiotherapy until the end of radiotherapy.
Radiotherapy:All the children received irradiation,8MV X-ray,3 fields,conventional segmentation (1.6-2.0 Gy) using three-dimensional conformal radiotherapy technology.The radiotherapy dose was adjusted according to the total dose of the first radiotherapy,tumor size,neurological symptoms,tumor site,the child's age,and a limited dose considering the major organs.
Main purpose:To evaluate the objective response rate(ORR) and complete remission rate (CRR) of Endostar®combined with craniospinal radiotherapy for recurrent MB in children.Secondary purpose:the progression-free survival (PFS),OS,and side effects of the treatment were also analyzed.
Therapeutic evaluation
The baseline assessment included a complete history of the child's general condition,a neurological examination,and blood work.Routine blood work was performed at least once a week.A physical and nervous system examination was also performed at least once a week.Every 2-3 weeks a blood,liver,and kidney function biochemistry was performed.Toxicity ratings were based on the third edition of the National Cancer Institute's conventional toxicity criteria (NCICTCAE,version 3.0).
An enhanced MRI scan of the whole brain and whole spine was completed within 2 weeks prior to the initiation of treatment.An additional MRI was conducted 1 week after the termination of radiotherapy.If signs or symptoms of suspected MB clinical progression occurred during treatment,an MRI scan was required.
Efficacy was evaluated according to RANO criteria.
Statistical method
Statistical analysis was performed using SPSS 20.0.Kaplan-Meier curves were used to evaluate PFS rates,overall survival (OS) rates,median PFS,OS,and corresponding 95% confidential intervals between the experimental and control groups.
Results
Overall,13 children with MB were treated with a secondary radiotherapy after MB recurrence.This included 7 in an experimental group (53.8%) and 6 in a control group (46.2%).The craniospinal radiotherapy was 30.6-36.0 Gy (experimental group,30.6-36.0 Gy;control group,32.0-36.0 Gy) plus metastatic focus added to 32.0-54.0 Gy (experimental group 32.0-50.4 Gy,40.0-54.0 Gy)(Table 1).Subsequent chemotherapy was administered post-radiotherapy.
The CRR of the experimental and control groups was 71.4% and 16.7% and ORR was 100% and 83.3%,respectively.In the entire population,the median PFS was 19.0 months (95% CI:8.431-29.569) and median OS was 23 months (95% CI:11.257-34.743).The median PFS was 14 months (95% CI:0.0-29.60) and 19 months (95%CI:0.0-39.53) in the experimental and control groups,respectively.The median OS was 19 months (95% CI:0.0-38.20) and 23 months (95% CI:2.47-43.53) in the experimental and control groups,respectively.The PFS rates at 6,12,and 18 months in the experimental and control groups were 71.4%,55.4%,44.6%,and 83.3%,54.8%,and 38.9%,respectively.The OS rates at 6,12,and 18 months in the experimental and control groups were 85.7%,71.4%,51.8%,and 66.7%,61.7%,and 51.7%,respectively.After radiotherapy,the efficacy of one child was evaluated as the SD.The histology test of one case indicated desmoplastic MB.The disease progressed,and the child died after 9 months.(Table 2;Fig.1)
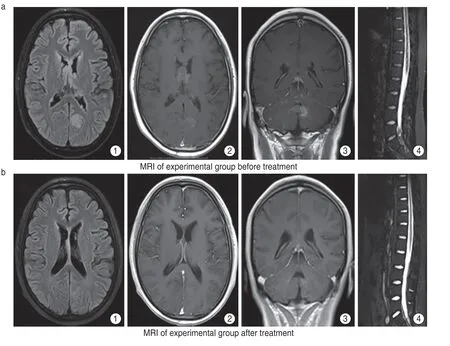
Fig.1 (a) MRI of experimental group before treatment,multiple nodular or long T1 long T2 signals were seen in the cerebellum and left occipital lobe of bilateral lateral ventricles.(a1) FLAIR showed hyperintensity and (a2) obvious nodular abnormal enhancement,(a3) the largest was about 1.5 × 1.2 cm located in the left cerebellopontine foot,(a4) also multiple abnormal signals were seen in the medulla of cervical,thoracic and lumbar segments;(b) MRI of experimental group after treatment,intracranial lesions and spinal cord lesions were significantly reduced and some nodules disappeared
Toxic manifestations and side effects:None of the patients had grade 3-4 hematologic extrinsic toxicity or life-threatening events.Bone marrow suppression usually occurs 1 week after radiotherapy.The most common adverse events included grade 3 tropenia (two in the experimental group and three in the control group),grade 1 thrombocytopenia (one in the experimental group) and grade 1 anemia (two in the experimental group and two in the control group) (Table 2).
Discussion
Treatment options for children with recurrent MB are limited.Although all patients with postoperative recurrence or implantable dissemination receive active surgery and chemotherapy,the prognosis remains poor.Few children can receive radical treatment after recurrence.Unfortunately,there is a lack of standard treatment for recurrent MB.
In this study,13 children with recurrent MB were enrolled.Of these,5 patients underwent surgery followed by radiotherapy and chemotherapy during the initial treatment.Four children received surgery and chemotherapy.One child received surgery and radiotherapy,whereas three patients received only surgery.In 2016,MB was divided into four molecular subtypes,including Wnt,SHH (TP53 wild type and TP53 mutant type),G3,and G4 by WHO.This was based on molecular biology and histomathology[18].Patients with G3,MYC amplification,or TP53 mutations have a poor overall prognosis[19].Group3 was the most common subtype in this study.One child had SHH with a TP53 mutation.One child with WNT underwent only surgery and radiotherapy without chemotherapy.Among the five G4 patients,only three children underwent surgical treatment without radiotherapy or chemotherapy.
Secondary radiotherapy,targeted therapy,and other comprehensive therapies are being investigated for the treatment of recurrent MB.Zhaoet al[20]used bevacizumab combined with stereotactic radiotherapy to treat CR in children with relapsed MB.Raoet al[21]performed secondary radiotherapy on 67 children with recurrent brain tumors (including 20 MB cases).The average OS was 12.8 months in the entire cohort,while the median OS was 8.4 months in MB.Guptaet al[22]treated 28 patients with recurrent MB with secondary radiotherapy in combination with platinum-based chemotherapy.This yielded a 46% 2-year PFS rate and 51% OS rate.In recent years,targeted therapy has become a promising therapeutic approach for the treatment of recurrent MB.
Endostar®is one of the most effective angiogenesis inhibitors discovered thus far.Its' mechanism may be related to the following factors.The mechanism by which Endostar® achieves radiosensitization may be:(1) Normalization of tumor blood vessels[12,23-25];(2)blockade of the cell cycle at the radiotherapy sensitive stage[26-30];(3) Induction of apoptosis of endothelial cells and tumor cells[31],and (4) Improvement of the tumor microenvironment (TME)[8-11].In addition,the molecular mechanism of Endostar®anti-angiogenesis may be related to the inhibition of tyrosine phosphorylation of KDR/ flk-1 (vegfr-2) and the activation of ERK,p38 MAPK,and AKT[32].In addition,clinical studies have shown that Endostar®combined with radiotherapy in the simultaneous treatment of brain metastatic tumors from lung cancer has a significant effect in patients with high expression of VEGFR2 protein (or increased copy number of the KDR gene)[15-16].VEGFR is not only expressed in MB vascular endothelial cells but it is also highly expressed in tumor cells.The worse the prognosis,the higher the expression level of the KDR gene.Targeted VEGF signaling may be a new treatment option for MB[33].Taken together,the combination of radiotherapy and Endostar® may have a synergistic effect.
The blood brain barrier (BBB) regulates homeostasis of the central nervous system by forming a tightly regulated neurovascular unit[34-35].However,these same features also hinder the delivery of systemic therapies to brain tumors.The BBB is disrupted during tumor progression and is referred to as the blood tumor barrier (BTB).Although the BTB is more permeable than the BBB,its' heterogeneous permeability to small and large molecules as well as heterogeneous perfusion contribute to suboptimal drug accumulation in brain tumors[36-39].As such,the BBB is one of the rate-limiting factors in clinically effective therapies.Radiotherapy can cause damage to the BBB[40].This may increase the concentration of the drug within the tumor tissue and improve the effectiveness of treatment.In this study,the CRR and ORR of the experimental group were better than those of the control group.
Secondary radiotherapy for recurrent MB remains controversial due to its' potential for toxicity and the uncertainty of improving overall viability[41-43].MB spreads easily within the cerebrospinal fluid.Total central or subtotal central radiotherapy remains an important guarantee to reduce the recurrence of irradiation in this area[44].The survival rate of children who received radiotherapy was significantly higher than that of those who did not[45].Radiobiology data showed that age,chemotherapy use,radiated volume,total dose of radiotherapy,and the time interval between the first and second radiotherapy were important factors in determining survival.This data was also important in determining the recovery of the central nervous system from radiation injury.Although there are several case reports of severe brain damage caused by conventional radiotherapy doses[46],Lawrenceet al[47]discovered that for patients receiving brain radiation therapy,the biologically effective dose of 150 Gy (BED) was 10%.Patients with symptomatic radioactive necrosis accumulated BED doses of 204 Gy and were born with irreversible complications[48].For the second radiotherapy of the spinal cord,if the BED dose of each radiotherapy is 98 Gy and the interval between the two radiotherapies is no less than 6 months,the accumulated BED dose can reach 120 Gy without causing spinal cord injury[49-51].In our study,the cumulative dose of craniospinal radiotherapy was less than 72.0 Gy.None of the patients had grade 3-4 hematological extrinsic toxicities or lifethreatening events.Bone marrow suppression often occurs 1 week after radiotherapy.The most common adverse events include thrombocytopenia,neutropenia,and anemia.Compared with the control group,the experimental group did not show acute toxicity or side effects,including hematological toxicity.Hematological toxicity was more closely related to early chemotherapy and total central radiotherapy.The critical functional areas of the brain are not suitable for excessive irradiation.Therefore the radiation dose is limited.Certain scholars believe that reducing the radiation dose,combined with chemotherapy,can reduce the neurotoxicity caused by high-dose radiotherapy.This is accomplished without reducing the curative effect.It also further improves the curative effect compared to chemotherapy alone[4,52].In this study,PFS and OS of patients in the experimental group did not benefit from the increase in ORR and CRR.We speculated that subsequent treatment of recurrent MB may play an crucial role in influencing PFS and OS.In addition,statistical bias was inevitable due to the small sample size in this study.
In conclusion,Endostar®combined craniospinal radiotherapy may be an effective treatment for the management of recurrent MB in children.Considering that this therapy has a high clinical remission rate and acceptable tolerance,all patients in the control group were radiotherapy-naive prior to recurrence.Compared to the treatment group,there was a selective statistical bias.Therefore,in a future study we will expand the sample size and multi-center enrollment to confirm the efficacy and safety of Endostar®.We will include this in a future study as well as a discussion of a maintenance program for recurrent MB in children.
 Oncology and Translational Medicine2021年3期
Oncology and Translational Medicine2021年3期
- Oncology and Translational Medicine的其它文章
- Construction and validation of an immune-related lncRNA prognostic model for rectal adenocarcinomas*
- Antitumor and vascular effects of apatinib combined with chemotherapy in mice with non-small-cell lung cancer
- A study of the potential adverse effects of electrosurgical smoke on medical staff during malignant tumor surgery*
- Diagnostic value of lncRNAs as potential biomarkers for oral squamous cell carcinoma diagnosis:a meta-analysis*
- Seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with cancer and the impact of anti-tumor treatment on antibodies*
- Future of targeted therapy for gastrointestinal cancer:Claudin 18.2
