Seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with cancer and the impact of anti-tumor treatment on antibodies*
Bili Wu,Bo Liu,Xueyan Jiang,Ye Yuan,Wan Qin,Kai Qin,Qi Mei,Li Zhang,Huilan Zhang,Guangyuan Hu (✉),Xianglin Yuan (✉)
1 Department of Oncology,Tongji Hospital,Tongji Medical College,University of Science and Technology,Wuhan 430030,China
2 Department of Gastroenterology,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
3 Department of Respiratory and Critical Care Medicine,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
Abstract Objective The aim of this study was to examine the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among patients with cancer and followed up changes in SARS-CoV-2-specific antibodies to explore the impact of anti-tumor treatment in patients.Methods Patients with cancer who visited the Outpatient Clinic of Oncology,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan,China between March 9 and April 30,2020 were enrolled in this retrospective cohort study.SARS-CoV-2 immunoglobulin (Ig) G,IgM,and viral load at various time points during the disease course were determined.Results We examined the serological results of 779 patients with cancer.The overall seroprevalence(IgG-positive or IgM-positive) rate of SARS-CoV-2 was 3.4%.The probability of seropositivity was significantly higher in patients with gastric cancer than in those without gastric cancer (odds ratio:6.349,95% confidence interval:2.191-18.396).Follow-up data showed that SARS-CoV-2 IgM and IgG levels decreased and the polymerase chain reaction test result remained negative in seropositive patients with cancer.Conclusion This study investigated the seroprevalence of SARS-CoV-2 in coronavirus disease (COVID-19)-positive patients with cancer in Wuhan,China.The seropositivity in patients with cancer was lower than or similar to that in the general population.Irrespective of anti-tumor therapy,the levels of SARS-CoV-2 antibodies decreased in these patients.More studies are needed to better understand the impact of antitumor therapy on change in the levels of SARS-CoV-2 antibodies.
Key words: coronavirus disease (COVID-19);cancer;immunoglobulin (Ig) G;IgM
Patients with cancer,as a special population,are prone to viral or bacterial infections due to immune suppression[3-4].Previous studies on COVID-19 have revealed that patients with cancer have a poor prognosis,which is treatment related;however,recent studies do not support this notion[5-10].
Currently,several studies have investigated the seroprevalence of SARS-CoV-2 in the general population.However,limited studies have been conducted in patients with cancer.It is unclear whether COVID-19-positive patients can be administered anti-tumor treatment[11].Therefore,in this study,we examined the seroprevalence of SARS-CoV-2 among patients with cancer and followed up changes in SARS-CoV-2-specific antibodies to explore the impact of anti-tumor treatment in patients.
Patients and methods
Study design and participants
This retrospective study was conducted in Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan,China from March 9 to April 30,2020 and included 852 patients who presented to our outpatient oncology clinic.Detailed data were obtained from the electronic medical records.Tumor diagnosis was established based on clinical manifestation and laboratory examinations.After excluding 73 patients who lacked data on the results of the throat swab or serological tests,779 patients were included in the study.We investigated the seroprevalence of SARS-CoV-2 in the population.Twenty-five patients were positive for SARS-CoV-2-specific antibodies (Fig.1).We dynamically observed the change in the antibodies during anti-tumor treatment,with the cutoff date set on September 9,2020.
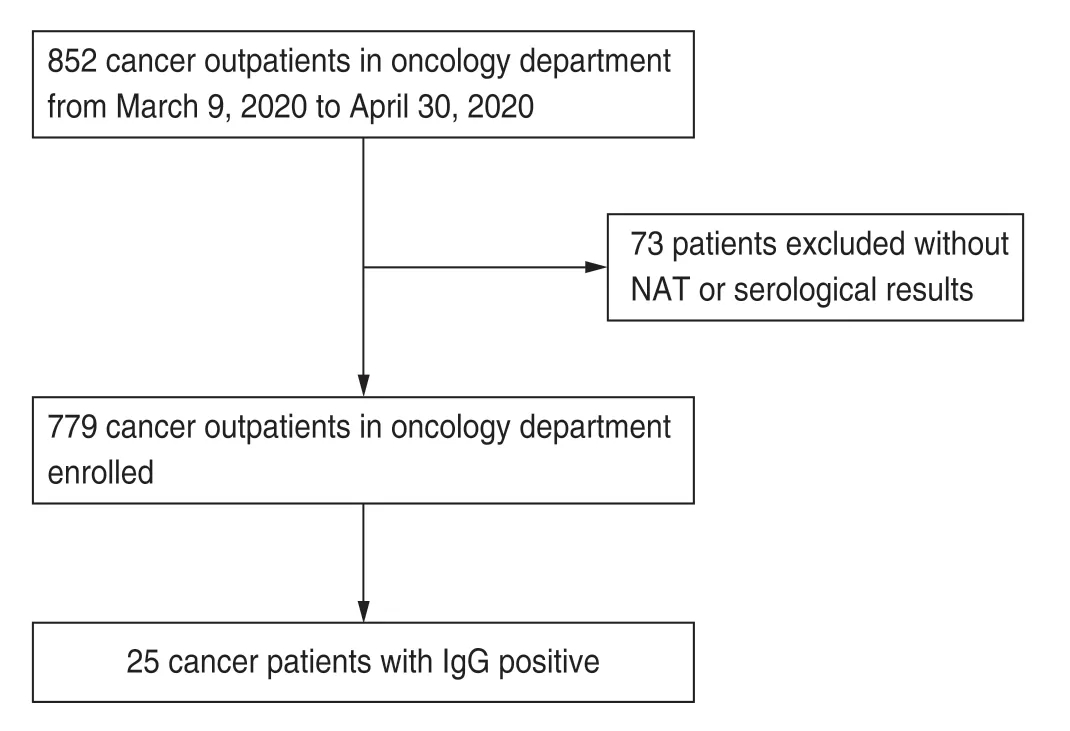
Fig.1 Patient flow diagram.NAT:nucleic acid tests
This study was approved by the Ethics Committee of Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan,China,which waived the requirement of informed consent.
Observation indicators and data collection
Epidemiological data,such as data on sex,age,and tumor type,were obtained from the electronic medical records and through patient interviews.Data on radiological findings and treatment information of the patients positive for SARS-CoV-2 antibodies were also included.All data were separately reviewed and verified by two physicians.Any missing or uncertain data were retrieved by contacting the physicians or the patients themselves.Data on laboratory examinations,including chest computed tomography (CT),were obtained at the first outpatient visit.No patients were lost-to-follow-up.
Cancer stage was categorized according to the tumornode-metastasis staging system[12].Using quantitative real-time (RT) polymerase chain reaction (PCR) and nextgeneration gene sequencing,SARS-CoV-2 can be detected using nasopharyngeal swab samples[13].Wantai kits were used for antibody detection[14].The serum antibody level was standardized against the ratio of the signal value to the cut-off point (S/CO).The ratio was measured using the optical density (OD) value generated by the sample relative to the cut-off point OD value.When the ratio was > 1,the sample was considered positive for SARSCoV-2.
Statistical analysis
Quantitative variables are expressed as medians[interquartile range (IQR)],and qualitative variables are presented as frequencies.The Mann-WhitneyU-test,the chi-square test,and Fisher's exact test were used to compare seropositive and seronegative patients in terms of demographic statistics where appropriate.Univariable and multivariable Logistic regression models were used to screen or identify the risk factors of seropositivity.To follow the 10 events per variable (EPV) principle to avoid overfitting,we eventually entered three factors in the multivariate model.The normality of data was tested using the Shapiro-Wilk test.The Wilcoxon signed-rank(nonparametric) test was used for the comparison of paired data.All statistical tests were two-sided andP≤0.05 was considered statistically significant.SPSS version 22.0 statistics software package (IBM Corp.,Armonk,NY,USA) was used for statistical analysis.
Results
Clinical features of patients with cancer and influencing factors of positive SARS-CoV-2 antibodies
Among the 779 patients with tumors,384 (49.3%)were men,and the median patient age was 54.0 (45.0-62.0) years.The most common tumor types were lung cancer (n=201),breast cancer (n=132),cervical cancer(n=65),and nasopharyngeal carcinoma (n=48;Table 1).
Now when the boy had come to his full strength the King of that country fell in love with his mother, and wanted to marry her, but he knew that she would never part from her boy
Of the 779 patients,25 (3.2%) were positive for SARSCoV-2 IgG antibody and four (0.5%) were positive for SARS-CoV-2 IgM antibody,with an overall seropositive rate of 3.2% (Table 2).
Univariable analysis showed that the rate of seropositivity was higher in patients with gastric cancer than in those without gastric cancer;however,it was not significantly different among other tumor types,sexes,and age groups.Univariable logistics analysis revealed that gastric cancer was associated with seropositivity.Considering the seropositive patients (n=25),we included three variables in the multivariable logistic regression model to avoid over-fitting the model.The results showed that gastric cancer was associated with a higher rate of seropositivity than the other cancer types(Tables 3 and 4).
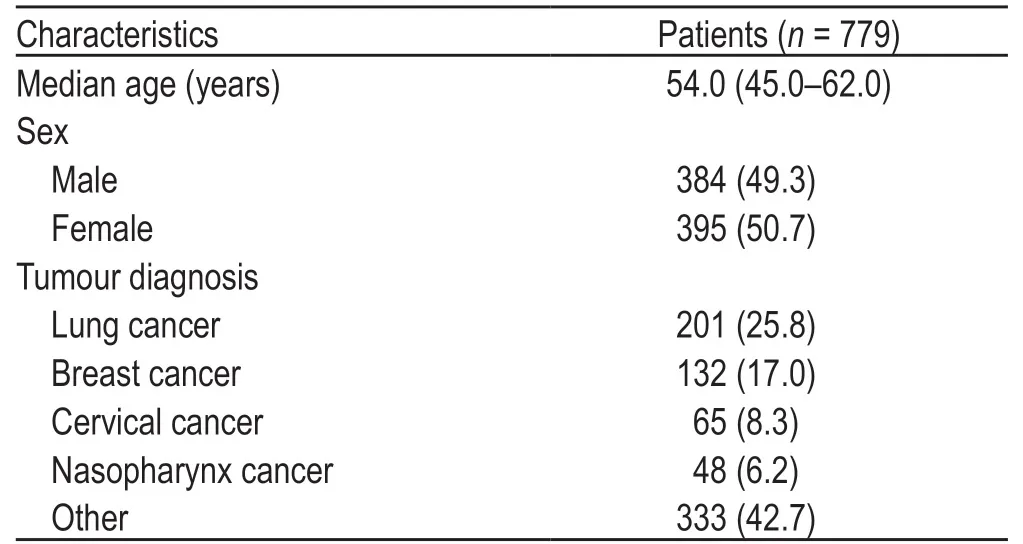
Table 1 Demographic and baseline clinical characteristics of cancer patients [n (%)]
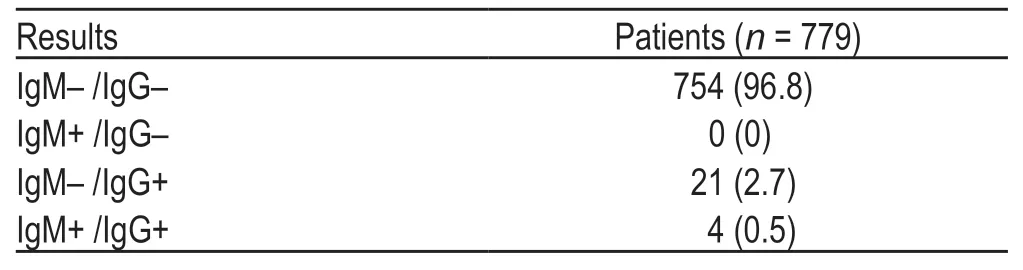
Table 2 Rates of the serological test results [n (%)]
None of the patients presented with typical COVID-19 symptoms according to the Chinese Clinical Guidelines for COVID-19 Pneumonia Diagnosis and Treatment(8th edition).PCR test results of all patients remained negative.
Clinical characteristics and dynamic changes in SARS-CoV-2 antibodies in seropositive patients
Table 5 presents the clinical characteristics of 25 seropositive patients.Among them,14 (56%) patients were men,and the median patient age was 56.0 (48.0-61.0)years.The most common tumor types were lung cancer(n=5),gastric cancer (n=5),nasopharyngeal cancer (n=3),and cervical cancer (n=3).Thirteen patients were diagnosed with stage IV cancer.Two patients had been surgically treated,12 patients had received chemotherapy,seven patients had undergone radiotherapy,five patients were receiving target therapy,and two patients received immunotherapy.At their first visit to the hospital,radiological examinations showed that 22 patients had nodules,10 patients had pleural thickening,nine patients had fibrous stripes,four patients had patchy shadows,and one patient had ground-glass opacity.
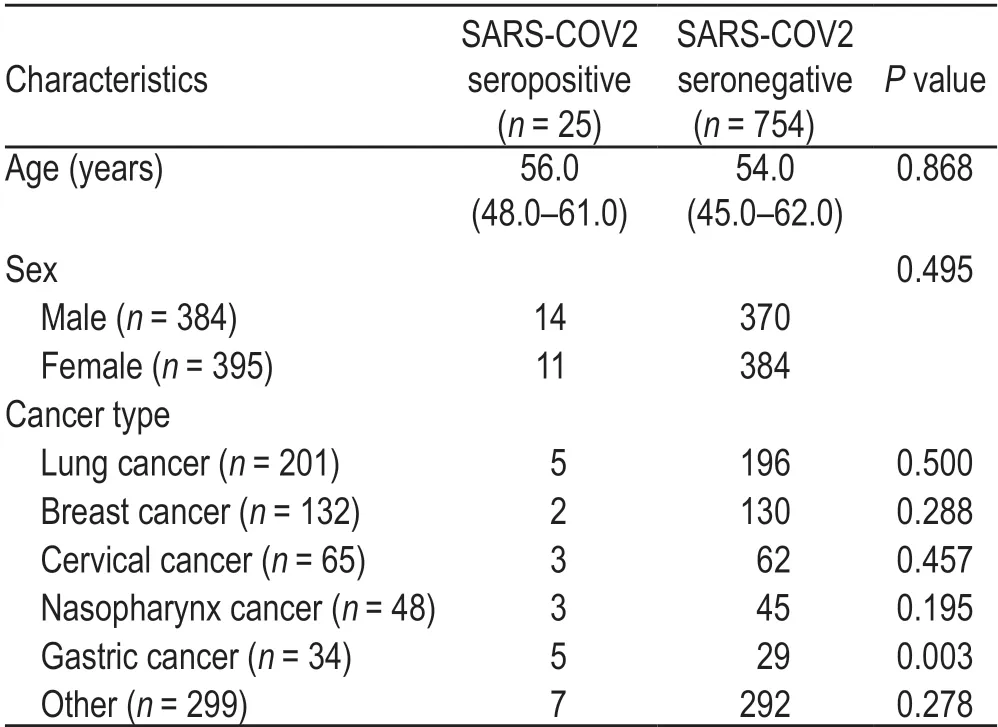
Table 3 Demographic characteristics of the patients based on seroprevalence
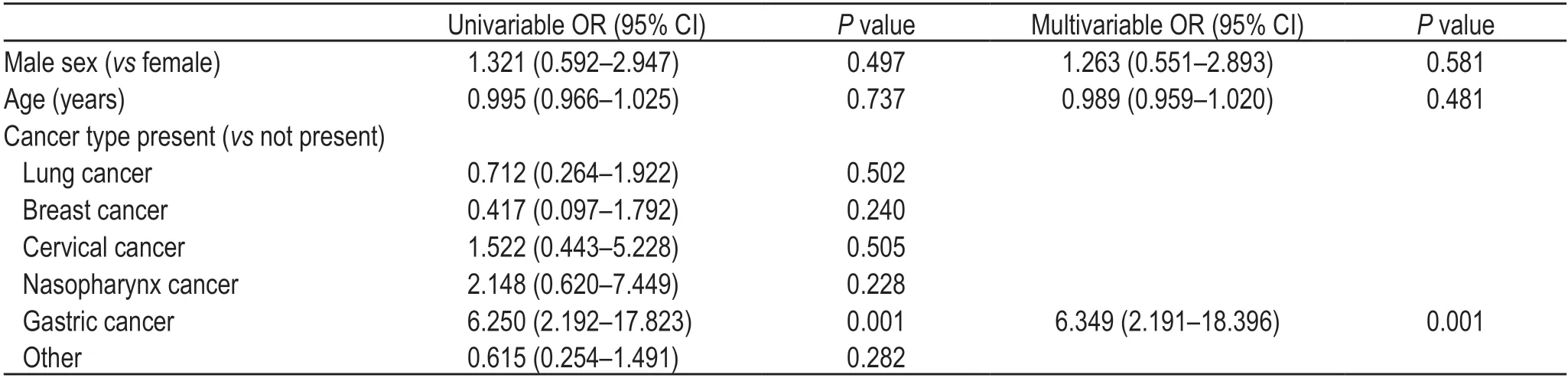
Table 4 Risk factors associated with seropositivity

Table 5 Demographic and baseline clinical characteristics of cancer patients with SARS-COV-2 IgG positive
We followed up 25 patients seropositive for SARSCoV-2 for a median period of 60 (16-141) days.All patients were tested for SARS-CoV-2 antibodies and SARS-CoV-2 RNA using nasopharyngeal swab samples.Their RT-PCR test yielded negative results.The IgM levels in four SARS-CoV-2 IgM-positive patients showed a downward trend,and in three of them,the level shifted from above to below the cut-off point.Twenty-five SARSCoV-2 IgG-positive patients also showed a downward trend in IgG levels during the follow-up period (Fig.2).However,the results of five patients eventually turned negative.One patient received radiotherapy,one cycle of tegaful,and one cycle of capecitabine;one patient was administered osimertinib;one patient received three cycles of tegaful,and one patient was initiated on one cycle of PP (pemetrexed and cisplatin) protocol;and one patient was treated with one cycle of XELOX (oxaliplatin and cisplatin) regime in combination with bevacizumab,one cycle of capecitabine plus bevacizumab,one cycle of concurrent capecitabine and radiotherapy,and three cycles of capecitabine and bevacizumab therapy.Fig.3 shows the SARS-CoV-2 IgG and IgM antibody levels at the first and last tests.The antibody levels conspicuously reduced at the last test and the difference in IgG level wasstatistically significant.
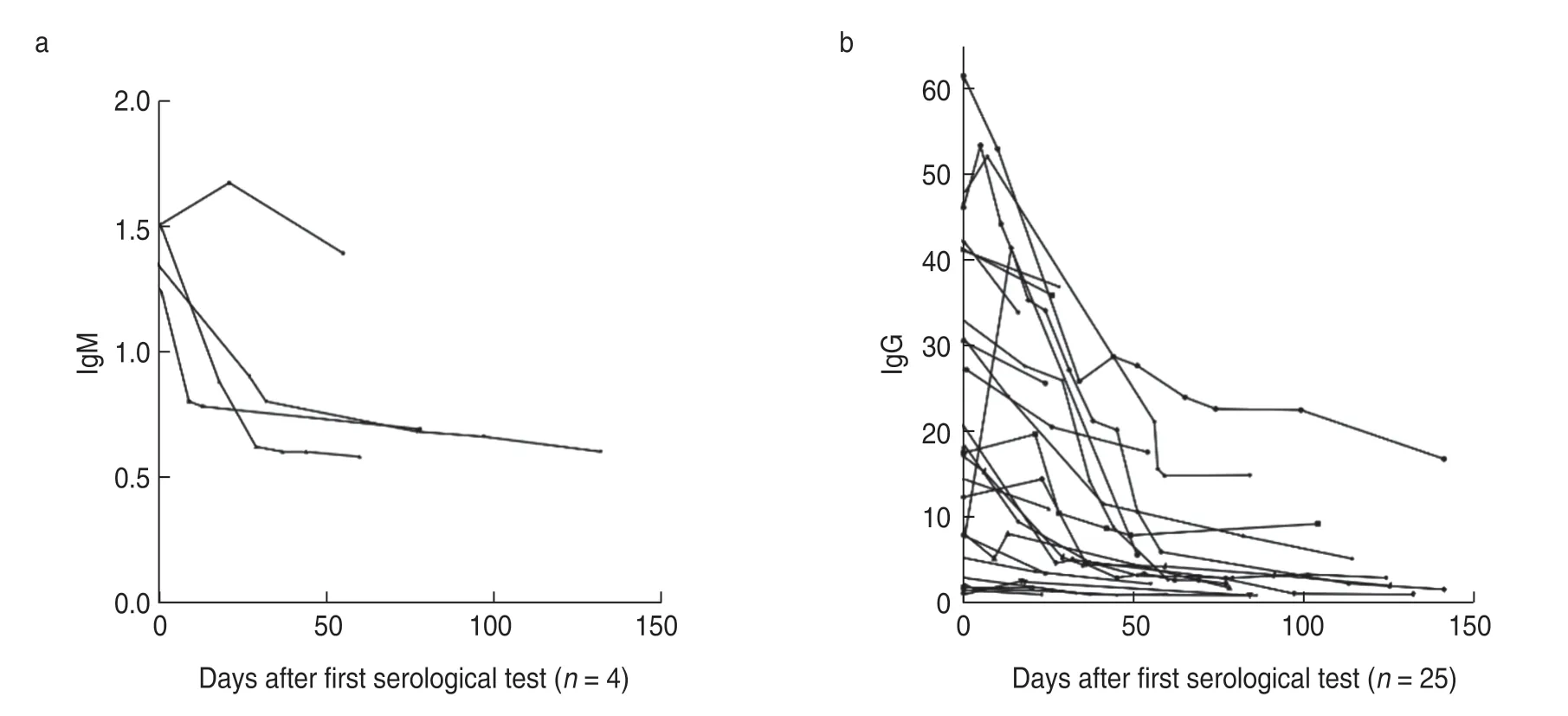
Fig.2 Serological courses and decline in (a) IgM and (b) IgG levels in patients with cancer who were initially seropositive
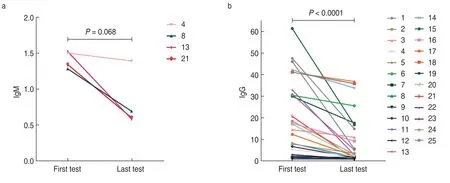
Fig.3 Changes in SARS-CoV-2 serum IgM and IgG levels in patients with cancer who were seropositive at the first and last test.The P value for the difference between first and last test is 0.068 and < 0.0001 for IgM and IgG,respectively
Discussion
Patients with cancer,as a special population,are vulnerable to COVID-19.The effect of anti-tumor treatment on this population was still unclear.To provide evidence regarding the prognosis and treatment of patients with cancer infected with SARS-CoV-2,we investigated the clinical characteristics,treatment,and serological results of 779 patients with cancer between March 9 and April 30,2020 at our oncology clinic at Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan,China.We found that 25 asymptomatic individuals were positive for SARS-CoV-2 IgG antibodies.An association was found between the seropositivity of the SARS-CoV-2 antibodies and gastric cancer.Serological follow-up revealed a declining trend in both IgG and IgM levels.
Among the 779 patients with cancer enrolled in this study,25 were positive for SARS-CoV-2 IgG and four were positive for IgM.The overall seropositivity rate was 3.2%.In a study involving medical workers and their close contacts in Wuhan from March to April 2020,the seropositivity was approximately 3.2%-3.8%[15].Another study examined Wuhan citizens during the same period and found a positivity rate of 3.57%[16].The seropositivity in patients with cancer is lower than or similar to that in the general population.In Madrid,Spain,the positive rate of SARS-CoV-2 antibodies in outpatients of a cancer center was 31.4%,whereas the IgG positive rate in the general population in the same area was 20.18%[17].Compared to the seroprevalence of SARS-CoV-2 in other regions,that in Wuhan was lower.This might be contributed to the different infection rates of SARSCoV-2 in different regions.The immunity of patients with cancer is impaired after receiving chemotherapy,immunotherapy,or other anti-tumor treatment.They have to frequently visit the hospital,and such visits make them more susceptible to COVID-19,as demonstrated by previous studies[4,18].In a previous study,the SARSCoV-2 positivity rate was higher in patients with cancer than in the general population[17];however,this was not the case in China.Such discrepancy might be attributed to the difference in the study populations.Early studies on COVID-19 mainly focused on symptomatic patients.A study conducted in Spain also included patients with pneumonia.However,in our study,all SARS-CoV-2-infected patients were asymptomatic.Moreover,each patient was followed up for >14 days and they could be regarded as“truly asymptomatic”patients.Therefore,the possibility of patients being symptomatic in the incubation period was excluded.
In addition,we showed that gastric cancer was associated with a higher positivity rate of SARS-CoV-2 antibodies.The invasion of SARS-CoV-2 into host cells relies on the combination of spike protein and angiotensinconverting enzyme 2 (ACE-2).Transmembrane protease serine 2,cathepsin L,and cathepsin B (CTSL/B) assist in the binding,tailoring,and activation of the spike protein.Both ACE-2 and CTSL/B are highly expressed in stomach adenocarcinoma[19-20].These might facilitate the entry of SARS-CoV-2 into the host cells,thereby causing viral infection in patients with stomach adenocarcinoma.
Further,we conducted a follow-up study on patients with cancer positive for SARS-CoV-2 antibodies.Their antibody levels showed a declining pattern.An asymptomatic patient was defined as a patient who tested positive on the nucleic acid test but did not show clinical symptoms throughout the infection.At present,the serological changes in these patients are not clear.Patients with COVID-19 were mainly screened by nucleic acid testing and antibody testing.Nucleic acid tests are most commonly used for detection of the SARSCoV-2 RNA in the nasopharynx and oropharynx by RTPCR and can yield positive results before and within a few weeks of symptom onset.Three types of antibodies(IgA,IgM,and IgG) are produced by the human body in response to SARS-CoV-2 infection.The first two tend to appear within 1 week of symptom onset and their levels gradually decrease after reaching a plateau.IgG is produced within 2 weeks of symptom onset and persists in the serum for some time,serving as an indication of host immunity to SARS-CoV-2[21-22].The nucleic acid test results of the patients in our study were negative,but the receptor-binding domain (RBD) in the spike proteins IgG and IgM showed a downward trend.The current longterm follow-up study on antibodies against the spike protein or RBD of SARS-CoV-2 shows that IgG level stay positive for a long time or decrease gradually.The IgM level stays negative for a shorter time than the IgG level[23-25].Flehmig and Crawford examined the ability of antibodies to neutralize the virus and found that 3-4 months after symptom onset,the neutralizing ability of antibodies diminished[26-28].These findings are consistent with the antibody results in our study,suggesting that human immunity against SARS-CoV-2 dropped gradually over time.
Many studies have demonstrated that patients with cancer are more likely to have adverse events or a higher mortality rate after being infected with SARS-CoV-2.The risk factors include sex,age,complications,tumor types,and disease stage[29-30].Thus far,researchers have failed to agree on the impact of anti-tumor therapy on the prognosis of the disease.Some researchers believe that receiving anti-tumor treatments such as recent chemotherapy are unfavorable factors for the prognosis of patients,while other researchers do not believe in this phenomenon[6,9].The inconsistencies might be attributed to the differences in patient selection.Few studies investigated the impact of anti-tumor therapy on SARS-CoV-2 infection.Our study investigated changes in antibody levels in cancer patients with asymptomatic infection.Although we could not definitively determine the exact time point of the viral infection,based on a sufficiently long follow-up,we could assume that most people were in the convalescent period of the infection.During follow-up,none of the patients died or experienced any adverse event.Some patients received chemotherapy,radiotherapy,targeted therapy,immunotherapy,and other treatments,while others did not receive any anti-tumor therapy.We observed that SARS-CoV-2 antibodies of both groups presented a downward trend.In some patients,the IgG antibody level was transiently elevated.Patients 1,16,17,and 25 did not receive anti-tumor therapy during the high antibody level period,while patient 8 was administered icotinib orally and patient 9 was administered radiotherapy,patient 15 was treated with capecitabine plus radiotherapy,patient 21 was administered oral tegaful;patient 24 was initiated on the EP protocol (etoposide+cisplatin).The reason for the elevated antibody level might have been the IgG spiked in response to a recent infection.Antibody levels in patients 8,15,21,and 24 increased after decreasing for some time,which might be related to the anti-tumor treatment the patient received.Recent studies have demonstrated that patients with cancer have a lower positive rate of serum IgG than patients without cancer.Immunosuppressive therapy has been shown to be related to a lower rate of seropositivity in patients with a history of COVID-19[31].Further studies are needed to evaluate whether anti-tumor therapy can cause transient elevations in antibody levels.
Further,there is another question,can anti-tumor treatment cause the recurrence of SARS-CoV-2 infection? The PCR test performed using nasopharyngeal swab samples from the patients in our study all yielded negative results.No close contacts of these patients developed SARS-CoV-2 infection.At present,articles on SARS-CoV-2 recurrence are mostly case reports.In study,asymptomatic patients with COVID-19 developed symptoms after treatment with high-dose glucocorticoids[32].In another study,COVID-19 symptoms appeared after immunosuppressive treatment in patients with acute B-lymphocytic leukemia[33].Therefore,for patients on anti-tumor treatment,a longer period of follow-up is needed.
This study has several limitations.First,this study was a single-center retrospective nonrandomized study and only included 25 SARS-CoV-2 seropositive patients.The patients were heterogeneous in terms of factors such as tumor type,age,and treatment.Second,because most of the patients were outpatients,data on anti-tumor treatment received by seronegative patients,tumor stages,and other information were lacking,and the time and frequency of serological testing varied among patients during the follow-up.Finally,the method used in our study could only detect antibody quantity,not titers,and could not reflect the virus-neutralizing ability of the antibodies.
Conclusion
At present,the precise mechanism underlying the role of SARS-CoV-2 antibodies in COVID-19 infection is poorly understood.This study explored the seroprevalence of asymptomatic patients with cancer infected with SARS-CoV-2 in the Wuhan area and tracked antibody changes in seropositive patients.More studies are needed to better understand the impact of anti-tumor therapy on antibody changes.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年3期
Oncology and Translational Medicine2021年3期
- Oncology and Translational Medicine的其它文章
- In memory of Academician Mengchao Wu,the pioneer of Chinese hepatobiliary surgery
- Future of targeted therapy for gastrointestinal cancer:Claudin 18.2
- Recombinant human vascular endostatin injection to synchronize craniospinal radiotherapy for the treatment of recurrent medulloblastoma in children:A retrospective clinical study*
- Diagnostic value of lncRNAs as potential biomarkers for oral squamous cell carcinoma diagnosis:a meta-analysis*
- A study of the potential adverse effects of electrosurgical smoke on medical staff during malignant tumor surgery*
- Antitumor and vascular effects of apatinib combined with chemotherapy in mice with non-small-cell lung cancer
