Future of targeted therapy for gastrointestinal cancer:Claudin 18.2
Qian Niu,Jiamin Liu (Co-first author),Xiaoxiao Luo,Beibei Su,Xianglin Yuan (✉)
Department of Oncology,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
Abstract The treatment of gastrointestinal cancer has always been a crucial research area,and targeted therapy has been receiving increasing attention.At present,the effect of targeted therapy is unsatisfactory for gastric cancer.Thus,the discovery of new targets is crucial.Claudin 18.2 (CLDN18.2),a member of the claudin family,belongs to the tight junction protein family that controls the flow of molecules between cell layers.CLDN18.2 expression has been discussed in many studies.In recent years,there have been many studies on targeted therapy with CLDN18.2-ideal monoclonal antibody 362.Furthermore,CLDN18.2-specific chimeric antigen receptor T therapy has been used for CLDN18.2-positive tumors,such as gastric and pancreatic cancers.Considerable research has been focused on CLDN18.2.CLDN18.2,a newly discovered marker for precise targeted therapy of gastric cancer,could offer new hope for the treatment of gastric cancer.
Key words: gastrointestinal cancer;claudin 18.2 (CLDN18.2);targeted therapy;ideal monoclonal antibody 362 (IMAB362);chimeric antigen receptor (CAR) T cells treatment
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide,with almost one million new cases diagnosed every year[1].The early symptoms of gastric cancer are not easily observed,and hence,it is usually diagnosed at an advanced stage.Surgery and chemotherapy,which are the main treatments for advanced gastric cancer,cannot provide striking survival benefits[2].
To improve the treatment of gastric cancer,an increasing number of studies are being conducted on targeted therapy.The monoclonal antibody,trastuzumab,which targets anti-human epidermal growth factor receptor 2 (HER2),is listed as a targeted drug for gastric cancer by the National Comprehensive Cancer Network[3].Other drugs,such as pertuzumab,an anti-HER2 monoclonal antibody[4],ramucirumab,an antivascular endothelial growth factor receptor 2 monoclonal antibody[5],and pembrolizumab,an anti-procedural cell death protein 1 monoclonal antibody[6],also showed satisfactory results in clinical trials.For targeted therapy,claudin 18.2 (CLDN18.2),a newly discovered target,is expected to become a breakthrough in the treatment of gastric cancer.
Claudin protein belongs to the family of tight junction proteins that can control the flow of molecules between cell layers.Claudin 18 (CLDN18) is a protein encoded by the CLDN18 gene in humans,with two splicing variants,claudin 18.1 (CLDN18.1) and CLDN18.2.CLDN18.2 is primarily expressed in differentiated gastric parietal cells[7].
Ideal monoclonal antibody (IMAB) 362 (claudiximab/zolbetuximab) is a therapeutic monoclonal antibody against CLDN18.2[8].The safety and efficacy of IMAB362 have been demonstrated in completed phase I and II clinical trials[9-10],indicating that CLDN18.2 is a very valuable target.Moreover,a large number of phase III studies have been conducted.Additional treatments associated with CLDN18.2 are also being developed.In this review,we focus on the progress of CLDN18.2.
Characteristics of the claudin protein
In 1998,Furuseet al[11]first discovered the claudin protein in chicken liver.Claudins are the major transmembrane protein components of tight junctions in human endothelia and epithelia[12].The functions of claudins include adherence to cells,maintenance of cell polarity,regulation of cell permeability,and participation in the regulation of cell proliferation and differentiation[13].To date,27 members of the claudin protein family have been discovered in mammals,with expression in different tissues.For example,claudin 1 is highly expressed in the liver,lungs,and kidneys,and although,claudin 3 is present in the lung and liver,it is rarely expressed in the kidney.In tumor cells,related studies have shown that the occurrence of colon cancer is related to claudin 1,3,4[14].Claudin 7 is expressed in gastric[15],renal[16]and ovarian cancers[17].The decreased expression of claudin 10 benefits the prognosis of hepatocellular carcinoma[18].In addition,the distribution of CLDN18.1 and CLDN18.2 expression is different because of the differences in the eight amino acids of the two sequences.CLDN18.1,which is selectively expressed in normal lung cells,and CLDN18.2 are expressed in differentiated gastric parietal cells,but not in stem cells[7].In addition,CLDN18.2 is retained during the malignant transformation of gastric cancer.Moreover,the ectopic activation of CLDN18.2 has been observed in gastric cancer and its metastatic foci[19].CLDN18.2 can also be expressed in pancreatic,esophageal,ovarian,and lung cancers.
The claud in protein consists of four transmembrane regions,including two extracellular rings.The N-terminal and C-terminal regions of the protein are located in the cytoplasm[20].The two extracellular rings make it a potential antibody target.As an ideal therapeutic target,a monoclonal antibody,IMAB362,has been developed against CLDN18.2.
CLDN18.2 expression in gastric cancer patients
Clinical and therapeutic features of CLDN18.2 in a gastric cancer population have been studied.
Dottermuschet alconducted a cohort study on Caucasians[21].They investigated the association between CLDN18.2 expression and clinicopathological features including survival time in gastric cancer patients.CLDN18.2 expression was observed in 42.2% of the patients (n=203/481),and 14.8% (n=71/481) showed weak immunostaining (ICH 1+).The study also pointed out that CLDN18.2 expression is associated with mucin phenotype,EBV status,integrin αvβ5,EpCAM extracellular domain EPEX,and lysozyme.
Gastric cancer is more common in the Asian population.A recent study in Japan[22]compared CLDN18.2 expression in primary gastric cancer patients with that in patients with lymph node metastasis.In the study,52% of primary tumors (n=135/262) and 45% of cancers with LN metastases (n=61/135) had moderate to strong CLDN18.2 membrane staining (≥ 40% tumor cells with CLDN18.2 expression ≥ 2+).They also found a higher expression of CLDN18.2 in diffuse gastric cancer and high-grade (G3) tumors,which was not consistent with the results of Dottermuschet al.In addition,the positive rate of CLDN18.2 in gastric cancer patients in Japan (87%,n=228/262) was higher than that in Europe(77%,n=51/66)[7].
Based on the aforementioned study,Baeket alexplored the expression of CLDN18.2 in gastric cancer in Korea[23].The study included 367 gastric cancer patients(pathological stage II to III) who had undergone radical surgery.The authors carried out immunohistochemical staining for CLDN18.2,followed by a semi-quantitative analysis based on the intensity and percentage of staining.They reported that CLDN18.2 expression was observed in 237 patients,with 108 patients (29.4%) categorized as CLDN18.2-positive cases,based on staining scores of 51%-100% and moderate to strong staining intensity.The results demonstrated that there was a higher expression of CLDN18.2 in diffuse and HER2-positive (HER2 2+or 3+)gastric cancer.In addition,they reported that CLDN18.2 expression was independent of survival outcome,age,sex,primary tumor location,or TNM stage.
A significant difference in the expression rate of CLDN18.2 in different studies may be related to ethnic characteristics.Different testing methods and contemporary standards for determining CLDN18.2 expression have also produced varied results (Table 1).High expression of CLDN18.2 in gastric cancer patients in Asia indicates that it might be a potential and exclusive therapeutic target for gastric cancer.
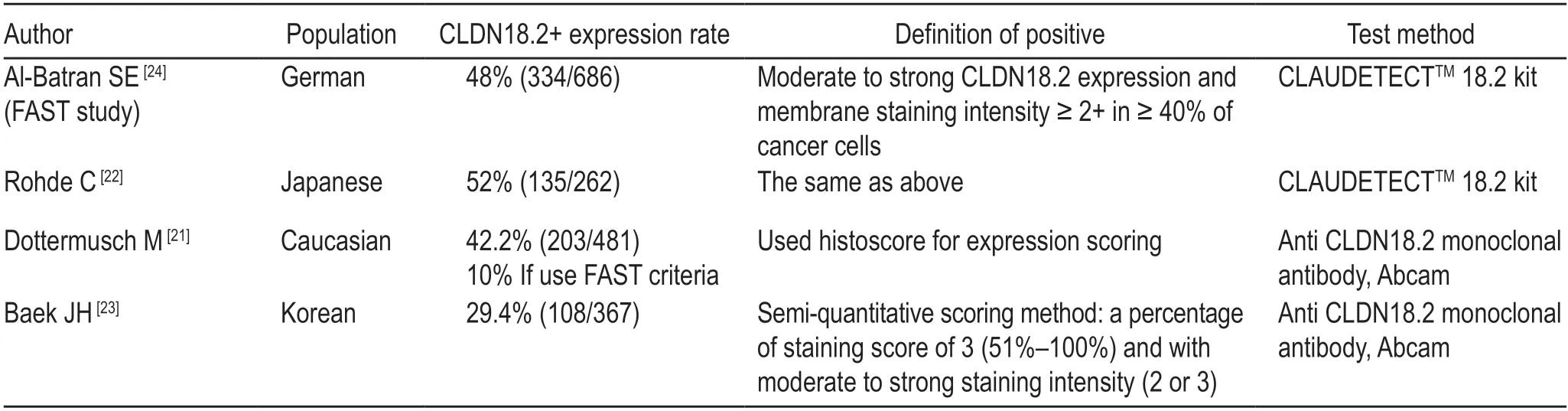
Table 1 The expression of CLND18.2 in different studies
Therapy
Mechanism of targeted therapy
IMAB362 (zolbetuximab/claudiximab) is a new IgG-1 antibody that is highly specific to CLDN18.2[25].Studies have shown that IMAB362 mediates cell killing through a series of immune mechanisms[20],which stimulates the activation of antibody-dependent cytotoxicity and complement-dependent cytotoxicity-induced cellular and soluble immune effects[26].In addition,it could interfere with the biological function of CLDN18.2 in cancer cells,resulting in anti-proliferation and pro-apoptosis effects[27].However,further exploration of the mechanism is still necessary.Furthermore,IMAB362 combined with chemotherapy plays a role in the recruitment of T cells,alteration of the tumor microenvironment,and prolongation of the overall survival time (OS) and progression-free survival time (PFS)[9].
Application of IMAB362/claudiximab/zolbetuximab
Many studies have investigated the effect of IMAB362 in various types of tumors,including advanced gastric[28],pancreatic[29],non-small-cell lung[30],and esophagogastric cancers[31].Clinical studies on IMAB362 have also yielded encouraging results.
Clinical trials on IMAB362/claudiximab/zolbetuximab
In a phase I clinical study (PILOT,NCT01671774)[32],the safety and efficacy of claudiximab in combination with zoledronic acid (ZA) and interleukin-2 in the treatment of esophageal and gastric adenocarcinoma were investigated.In the study,28 patients were included,and in 11 of the 20 evaluable patients,disease control was achieved.One patient showed partial response and the other 10 patients stable disease.The OS and PFS were 40 weeks and 12.7 weeks,respectively,which suggests that claudiximab,as a single therapy,has anti-tumor effect and can be used in combination with ZA for the treatment of tumors.
In a phase II clinical study (MONO,NCT01197885)[10],the safety and efficacy of zolbetuximab in the treatment of metastatic,refractory,and recurrent adenocarcinoma of the stomach or the lower esophagus were investigated.Patients with CLDN18.2 expression in more than 50% of tumor cells were intravenously injected with zolbetuximab five times every 2 weeks (two dose levels:four received 300 mg/m2in cohort 1 and 50 received 600 mg/m2in cohort 2/3).A total of 54 patients were evaluated.The antitumor activity data of 43 patients were finally obtained;the objective response rate,partial remission rate,and clinical benefit rate were 9%,9%,and 23%,respectively.The effect was more significant in the subgroup;out of 29 patients with CLDN18.2 expression in ≥ 70% of tumor cells,14% (four of 29) achieved partial response and 17% (five of 29) had stable disease.This study confirmed the efficacy of zolbetuximab monotherapy.
In a phase IIb clinical study (FAST,NCT01630083)[24],the researchers evaluated IMAB362 as a first-line treatment for advanced,recurrent gastric cancer and gastroesophageal junction adenocarcinoma.Patients with CLDN18.2 expression (with immunohistochemical score≥ 2+) in ≥ 40% tumor cells were included and randomly assigned to the first-line standard EOX (epi-doxorubicin,oxaliplatin,and capecitabine) group;chemotherapy was administered once every three weeks,with or without IMAB362 (the loading dose was 800 mg/m2,600 mg/m2on the first day).
There was also an exploratory third arm in which a high-dose monoclonal antibody (1000 mg/m2) plus EOX was administered.IMAB362 combined with EOX significantly improved the PFS of patients compared with EOX alone (PFS:7.9 monthsvs.4.8 months;HR 0.47,P=0.0001 and OS:13.2 monthsvs.8.4 months;HR 0.51,P=0.0001).Patients with high CLDN18.2 expression (≥ 70%tumor cells with CLDN18.2 expression ≥ 2+) had a better prognosis (PFS:7.2 monthsvs.5.6 months;HR 0.36,P< 0.0005;OS:16.6 monthsvs.9.3 months;HR 0.45,P<0.0005).PFS was significantly improved in the IMAB362 high-dose group compared with the chemotherapy group(PFS:7.1 monthsvs.4.8 months;HR 0.59,P=0.0026).The study demonstrated that IMAB362 combined with EOX is a safe and effective therapeutic strategy.The FAST study also provided a solid foundation for largescale phase III clinical studies.
A phase III clinical study on the first-line treatment of CLDN18.2-positive,HER2-negative,locally progressive,metastatic gastric cancer or gastroesophageal junction adenocarcinoma with mFOLFOX in combination with zolbetuximab or placebo (NCT03504397) is ongoing.A clinical study on CAPOX plus zolbetuximab(NCT03653507) has also been conducted.
All clinical studies showed good prospects for IMAB326,and the results of the phase III clinical study are anticipated.Previous studies are limited to Western countries;however,the high expression of CLDN18.2 in gastric cancer patients in Asia has encouraged the exploration of the effect of zolbetuximab in Asian populations.
CLDN18.2-specific chimeric antigen receptor(CAR) T cell therapy
Dr.Li Zonghai's team explored the use of CLDN18.2-specific CAR T cells for gastric cancer treatment,and they published their findings in the Journal of the National Cancer Institute in 2018[33].In their study,they developed specific humanized antibodies 8E5 (hu8E5)scFv,using the hybridoma and humanization techniques,and prepared CLDN18.2-specific CAR T cells -CAR(hu8E5-28Z,hu8E5-BBZ,and hu8E5-2I-28Z) T,using the lentiviral vector transduction method.Cytotoxicity test and enzyme-linked immunosorbent assay were then performed to detect the antitumor activity and cytokineproducing ability of CAR T cells against gastric cancer cellsin vitro.Thein vivoantitumor activity of CAR T cells was evaluated using a mouse gastric cancer cell line and a human transplanted tumor (PDX) model.CAR T cells could lyse CLDN18.2-positive cellsin vitro,but not CLDN18.2-negative cells.In the PDX model of gastric cancer (GA0006),the hu8E5-2I-28Z group was compared with the untransduced T (UTD) group.The tumor size(hu8E5-2I-28Z groupvs.UTD group:252.6 (158.6) mm3vs.1029.1 (852.7) mm3,P< 0.001) and weight (P< 0.007)were significantly lower in the hu8E5-2I-28Z group than in the UTD group.All the results showed that CAR T cell therapy is an effective way to treat CLDN18.2-positive gastric cancer.Furthermore,after treatment with hu8E5-2I T cells,no obvious damage was observed in the stomach tissue or other organs of the mice.
Similarly,some researchers conducted a clinical study(NCT03159819) in which CAR T cells targeted CLDN18.2 in gastric and pancreatic cancers.According to their mid-term data,12 patients with advanced gastric and pancreatic cancers were enrolled in the study.Most of the patients had multiple organ metastases and eight patients had different degrees of tumor regression.In particular,in a modified treatment subgroup,according to RECIST 1.1,five of the six patients achieved objective remission,including one complete remission.
Targeting CLDN18.2 using antibody drug conjugates (ADCs) or CD3-bispecifc modality
Zhuet aldemonstrated that targeting CLDN18.2 using ADCs and CD3-bispecifc modality may be an effective treatment strategy for gastric and pancreatic cancers[34].The anti-CLDN18.2 ADC and CD3-bispecifc molecules inhibited tumor cell growthin vitro.In addition,the efficacy and safety of the strategy have been demonstratedin vivoin PDX animal models of gastric and pancreatic cancers.
Conclusion and prospect
The treatment of advanced gastric cancer remains challenging.To date,chemotherapy has been the mains treamtreatment for advanced tumors[35].With the innovation of surgical methods and the emergence of new chemotherapy regimens,the 5-year survival rate of patients with advanced gastric cancer has improved to some extent,but it is still low,approximately 5%-20%,and the median OS is approximately 10 months[36].Targeted therapy is an effective treatment and has become the most promising therapy in oncology.Especially in gastric cancer,the variety of targeted drugs has become increasingly abundant[37].Currently,some progress has been made in the use of trastuzumab for the treatment of HER2-positive tumor cells[3,38].We expect that CLDN18.2 will be explored in the development of a targeted therapy for gastric cancer and other CLDN18.2-positive tumors.
Existing studies have pointed out the different expression characteristics of CLDN18.2.Some studies have shown that the downregulation of CLDN18.2 is a characteristic of gastric cancer[21,39].This is mainly due to the destruction of tight junctions,which leads to the disruption of epithelial cell cohesion[40]and promotes the invasive potential and metastasis of tumor cells[41-42].However,in the study by Rohdeet al,CLDN18.2 expression remained the same with the presence of lymph node metastasis[22],which is consistent with the results of the FAST study.Junet alfound that reduced expression of CLDN18.2 was associated with peripheral invasion and poor OS[42].However,Baeket alshowed that CLDN18.2 expression in gastric cancer patients was independent of survival outcome[23].Thus,more studies are required to elucidate the role of CLDN18.2 in gastric cancer metastasis.
Moreover,CLDN18.2 and HER2 co-expression has been mentioned in some studies[23-24].The dual-target therapy (monoclonal antibody of anti-HER2 and anti-CLDN18.2) creates a combination treatment mode for gastric cancer patients,which could provide additional survival benefits to specific patients.
Presently,IMAB is a better choice for the treatment of solid tumors and hematological tumors,as IMAB binds to the selective target of the tumor[43].It is mainly expressed in tumor cells and rarely expressed in healthy tissues.The limited expression of IMAB in normal tissues can reduce adverse events and improve curative effect[29].
CLDN18.2-specific CAR T cells also provide a new strategy for the treatment of gastric cancer and other CLDN18.2-positive tumors in clinical practice.This therapeutic approach should provide great benefit to cancer patients,such as those with pancreatic or gastric cancer[44].
At present,treatments for pancreatic and gastric cancers are not effective.CLDN18.2 expression in these cancers brings more options for treatment.However,there is still a need to further explore how much survival benefits CLDN18.2 could provide for patients with pancreatic or gastric cancer.In addition,the role of CLDN18.2 in tumor progression is also worth investigating.Furthermore,the combination of targeted therapy and radiotherapy has not been studied in depth.We hope to find more clinical applications of this strategy for the treatment of tumors.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年3期
Oncology and Translational Medicine2021年3期
- Oncology and Translational Medicine的其它文章
- Construction and validation of an immune-related lncRNA prognostic model for rectal adenocarcinomas*
- Antitumor and vascular effects of apatinib combined with chemotherapy in mice with non-small-cell lung cancer
- A study of the potential adverse effects of electrosurgical smoke on medical staff during malignant tumor surgery*
- Diagnostic value of lncRNAs as potential biomarkers for oral squamous cell carcinoma diagnosis:a meta-analysis*
- Recombinant human vascular endostatin injection to synchronize craniospinal radiotherapy for the treatment of recurrent medulloblastoma in children:A retrospective clinical study*
- Seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with cancer and the impact of anti-tumor treatment on antibodies*
