利用适用于SAPO-34的有机结构导向剂合成SSZ-13分子筛
王 磊,孙毯毯,闫娜娜,马 超,4,刘晓娜,田 鹏,郭 鹏,刘中民
(1.郑州大学化学学院,绿色催化研究中心,郑州 450001;2.中国科学院大连化学物理研究所甲醇制烯烃国家工程实验室,洁净能源国家实验室,大连 116023;3.中国科学院大学,北京 100049;4.大连理工大学张大煜学院,大连 116023)
1 Introduction
The aluminosilicate zeolite SSZ-13 and zeolitic silicoaluminophosphate molecular sieve(SAPO MS)SAPO-34 with the identical CHA topology have been regarded as the important solid-acid catalysts[1],especially for the selective catalytic reduction(SCR)of diesel exhaust[2,3]and the methanol to olefins(MTO)reaction[4,5].Therefore,preparations of both catalysts are widely and systematically investigated.Both SSZ-13 and SAPO-34 are three-dimensional(3D)CHA framework containingchacages with small pore openings(delimited by eight tetrahedral atoms such as Si,P,or Al).It is of interest to note that SAPO-34 could be synthesized with tunable Si contents with the assistance of multifarious and economical organic structure-directing agents(OSDAs)[6-10],while appropriate OSDAs for preparing SSZ-13 are relatively limited.The initial OSDA for synthesizing SSZ-13 wasN,N,N-trimethyladamantammonium hydroxide(TMAdaOH)[11],which has displayed a strong directing capability even in different synthetic systems,such as the traditional hydrothermal method[12],inter-zeolite transformation[13],dry gel conversion[14],and steam-assisted conversion[15].However,the utilization of TMAdaOH will come with huge budgets and further impede large-scale industrial production.In this case,developing cost-effective and capable OSDAs to completely or partially replace TMAdaOH has a great practical significance.
A series of quaternary amines imitating the shape of TMAda+cation has been identified for completely replacing TMAda+to synthesize SSZ-13.For example,Itakuraet al.[16]used benzyltrimethylammonium hydroxide(BTMOH)as OSDA to synthesize SSZ-13,which would take a longer crystallization time(21 d).Other OSDAs with a similar structural feature were also discovered,namelyN,N,N-dimethylethylcyclohexylammonium hydroxide(DMECHAOH)and its bromide[17,18],but they are not commercially available and require additional chemical preparation.Based on this,a commercialized and security quaternary amine,choline chloride(CC),was selected and could largely reduce the cost[19,20].However,it is still challenging to synthesize SSZ-13 with high silicon-to-alumina ratio(SAR)by utilizing CC as the OSDA.Except for the use of traditional organic amines,Renet al.[21]developed the one-pot method to directly synthesize Cu-SSZ-13 by employing a metal complex Cu-tetraethylenepentamine(Cu-TEPA).This strategy can also relieve the economic burden but is difficult to acquire Cu-free SSZ-13 or SSZ-13 zeolites with tunable SARs.Moreover,it is worth mentioning that the OSDA-free method,which totally abandons the use of OSDAs,could also be an alternative approach for the preparation of SSZ-13[22,23].However,the products still held the low SAR under the hydrothermal condition.
Another route for preparing SSZ-13 is to partially replace TMAdaOH by other OSDAs with a reasonable price.For instance,Martínez-Francoet al.[24]found that the employment of Cu-TEPA and TMAdaOH enabled the synthesis of Cu-SSZ-13 catalysts with controlled SARs and the samples obtained by this modified one-pot method showed good NH3-SCR performance.Moreover,Guoet al.[25]also developed an economic approach to synthesize SSZ-13(SAR~10)by using tetramethylammonium hydroxide(TMAOH)and TMAdaOH.The addition of TMAOH not only decreased the consumption of TMAdaOH,but also facilitated crystal growth by bridging the neighboring grains.This signifies that OSDAs appropriate for other zeolites might be favorable to the synthesis of SSZ-13.
Since SAPO-34 and SSZ-13 are closely related from the structural point of view,it would be interesting to attempt to fully or partially utilize commercialized OSDAs used for SAPO-34 to synthesize SSZ-13.However,there are few literatures regarding the direct synthesis of SSZ-13 by using OSDAs of SAPO-34[26].
Herein,we proposed an economic method to synthesize SSZ-13 through the partial replacement of TMAda+by OSDAs directing the synthesis of SAPO-34.The as-made SSZ-13 samples showed an applicable SAR region(11-22)and the Cu2+exchanged SSZ-13 displayed excellent NH3-SCR performance.
2 Experimental
The synthesis of SSZ-13 was carried out by the traditional hydrothermal method and the results are listed in Table 1.The detailed synthesis process,catalytic performance tests,and characterization conditions are exhibited in the Supporting Information of this paper.

Table 1 Synthesis of SSZ-13 under different conditions*
3 Results and Discussion
In our previous work[27],we have synthesized SAPO-34 with different Si contents by employing various OSDAs,such as diisopropylamine(DIPA),tetraethylammonium hydroxide(TEAOH),n-butylamine(nBA),and morpholine(MOR).It is of significance to note that unprotonated DIPA could emerge in the pure-silicachacage of high-silica(22.1% in molar ratio)SAPO-34.This finding inspires us to explore OSDAs used for SAPO-34 to direct the synthesis of the aluminosilicate SSZ-13,replacing TMAdaOH fully or partially.
As shown in Table 1,a series of OSDAs,such as DIPA,dipropylamine(DPA)andnBA,were selected for the preparation of SSZ-13 based on the conventional recipe of Sample 1 in molar ratio 20NaOH∶20TMAdaOH∶2.5Al(OH)3∶100SiO2∶4400H2O(if needed,seeds would be introduced into the initial gel composition).The PXRD patterns and SEM images are displayed in Fig.S1 and Fig.S2(see the Supporting Information of this paper).In order to better illustrate the synthesis procedure in detail,one of the secondary amines,DIPA,was chosen.It is worth pointing out that the attempts(Sample 2 and Sample 3)to prepare SSZ-13 by employing DIPA solely failed as shown in Fig.S1 and Table 1,even though the addition of the DIPA and seeds were doubled.It indicates that the complete replacement of OSDAs by DIPA is not feasible.In this case,the strategy of partial replacement was carried out.Replacing three quarters of TMAda+by DIPA will lead to the generation of SSZ-13 with by-products of MOR-type zeolites and cristobalite(Samples 4-6).Pure SSZ-13 with tunable SAR(Samples 7-9)can be obtained with an equal ratio of TMAdaOH and DIPA in the initial gel as listed in Table 1 and Table 2.More importantly,pure SSZ-13 with SAR of 11(Sample 10)can be successfully prepared even in the absence of SSZ-13 seeds.
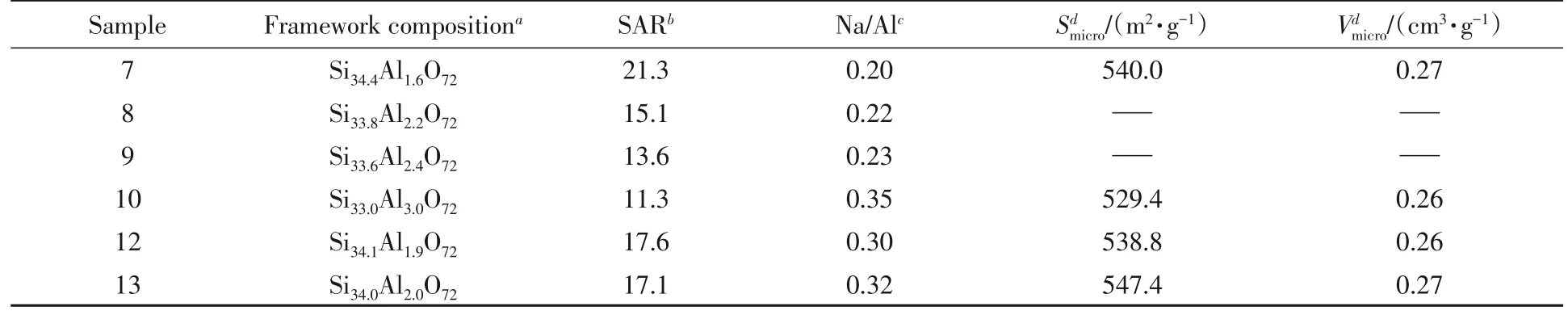
Table 2 Chemical compositions and textural properties of Samples 7―13
We further probe the crystallization mechanisms of SSZ-13(Sample 10)co-templated by TMAda+and DIPA.Besides,a control sample denoted as Sample 11 was also synthesized for the better comparison.The only difference of synthetic recipes between Sample 10 and Sample 11 is that the DIPA was not added into the gel composition of the latter one.As shown in Fig.1,at the very beginning(0-24 h),the crystallization of SSZ-13 in both Sample 10 and Sample 11 did not take place.The strong peak at 18.4° is attributed to the undissolved Al sources.The changes of this peak indicate that the Si sources dissolved firstly in this system and then Al sources began to dissolve after 12 h.This process could also be verified by SEM as shown in Fig.S3(see the Supporting Information of this paper).At the time of 24 h,weak PXRD peaks of SSZ-13 could be detected firstly in both two samples,which means the dissolved Si and Al have formed SSZ-13(Fig.S3).In addition,the most obvious difference between Sample 10 and Sample 11 is that when the crystallization time arrives at 50 h,the crystallinity of the former one is better,indicating that the introduction of DIPA significantly accelerates the crystallization process(Fig.1).Moreover,the absence of DIPA will lead to the undesirable amorphous phase(AP)in Sample 11 as displayed in Fig.S2.

Fig.1 PXRD patterns of Sample 10(A)and Sample 11(B)during 0―96 h
For further investigating the incorporation of TMAda+and DIPA,13C MAS NMR characterization of Sample 10 was carried out(Fig.2).Notably,the chemical shift atδ22 is attributed to the methyl of DIPA,which can be easily recognized in the whole spectra,while the peak atδ51 of DIPA is covered by the strong signal of methyl in TMAda+atδ48.Interestingly,the introduction of TMAda+firstly occurred at 3 h while DIPA did at 12 h.Besides,it is obvious that although DIPA is present in the as-synthesized SSZ-13,TMAda+isstill more abundant than DIPA.

Fig.2 13C MAS NMR spectra of Sample 10 at different time
In order to investigate the practicability of the as-synthesized SSZ-13 in catalytic fields,Sample 10 was exchanged with Cu(OAc)2for NH3-SCR tests.For a better comparison,the SSZ-13 seeds used in this work,which were prepared by the classical TMAdaOH,were chosen as a reference for evaluating the catalytic performance.Both SSZ-13 seeds and Sample 10 have a similar SAR of 11(Table S1,see the Supporting Information of this paper)with an ion-exchange level ofca.60% in terms of the currently commercialized recipe[28].As shown in Fig.3,compared with SSZ-13 seeds,copper exchanged Sample 10 displays no less activity and possesses a wide and applicable active window(200-400°C)for the diesel vehicle.
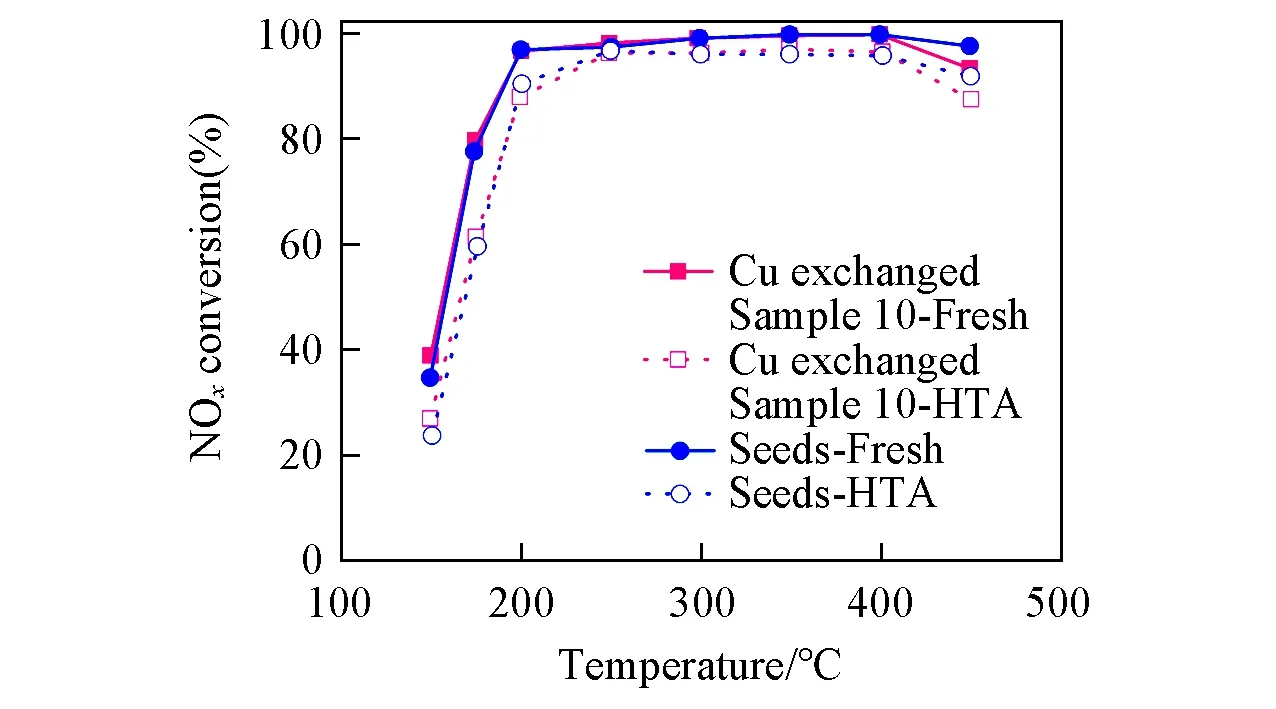
Fig.3 Results of NH3-SCR tests for fresh or HTA Sample 10 and seeds with the same SAR
In a short summary,by simply replacing part of TMAda+by DIPA in the initial gels,SSZ-13 with high crystallinities can be successfully synthesized.The addition of DIPA not only promotes the yield and crystallinity of SSZ-13,but also inhibits the undesirable impure phases.Moreover,the copper exchanged Sample 10 displays excellent NH3-SCR performance even after high temperature aging(HTA)at 750 °C for 16 h.
To understand whether effective OSDA replacement is influenced by SAR,three samples with different SARs(denoted as Sample 7,Sample 8,and Sample 9)were selected.The inorganic framework compositions of the three samples were calculated by XRF and are listed in Table 2,while the organic parts were investigated by13C MAS NMR.The clear trend shown in Fig.4 demonstrates that there are more DIPAs incorporated with the increase of SARs.
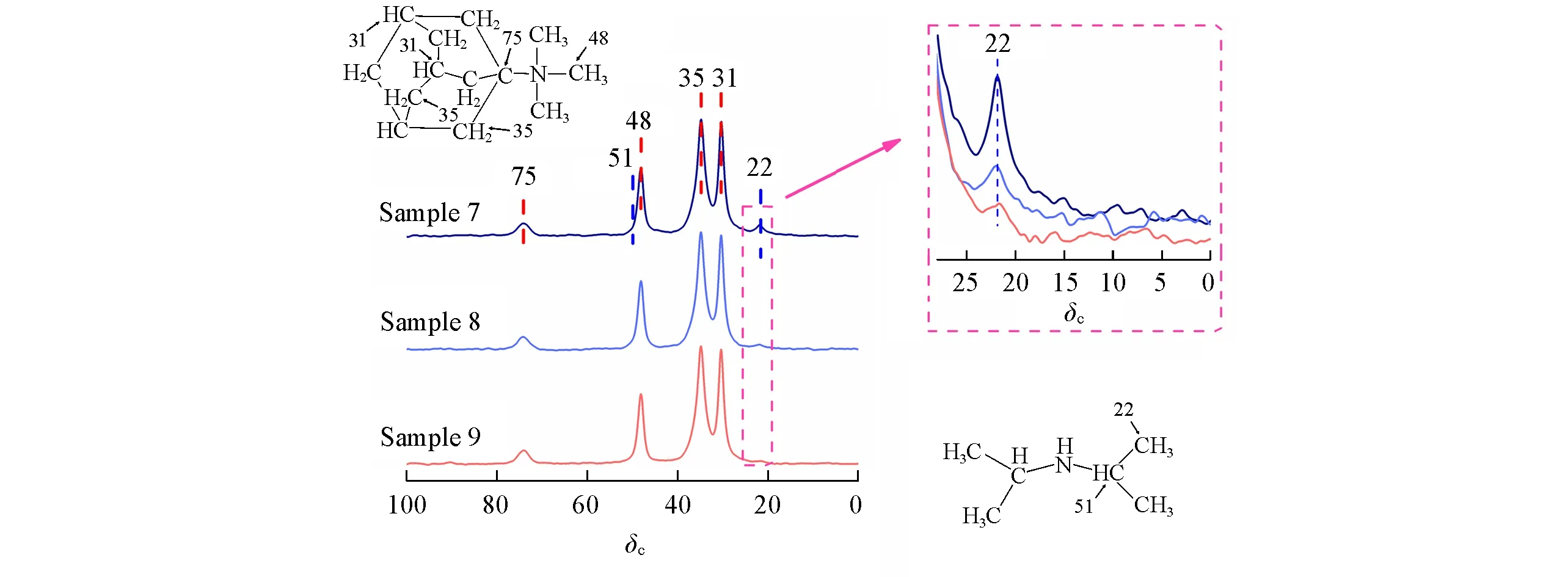
Fig.4 13C MAS NMR spectra of SSZ-13 with different SAR
Now that the partial replacement of TMAdaOH by DIPA is successful,other frequently-used OSDAs for SAPO-34 might be feasible.To verify this hypothesis,DPA andnBA were employed for the partial replacement of TMAdaOH to prepare SSZ-13,and it turns out that well-crystallized SSZ-13(Sample 12 and Sample 13)can be successfully obtained as shown in Table 1.There is no doubt that DPA andnBA have been introduced into SSZ-13 according to the results of13C MAS NMR spectra(Fig.5).Furthermore,the amounts ofnBA in the SSZ-13 cages are higher than DIPA as observed in the higher intensity ofδ22 fromnBA than the one from DIPA(inset of Fig.5).It is attributed to the fact that there are twonBAs locating in onechacage,while only one DIPA occupies onechacage[29].
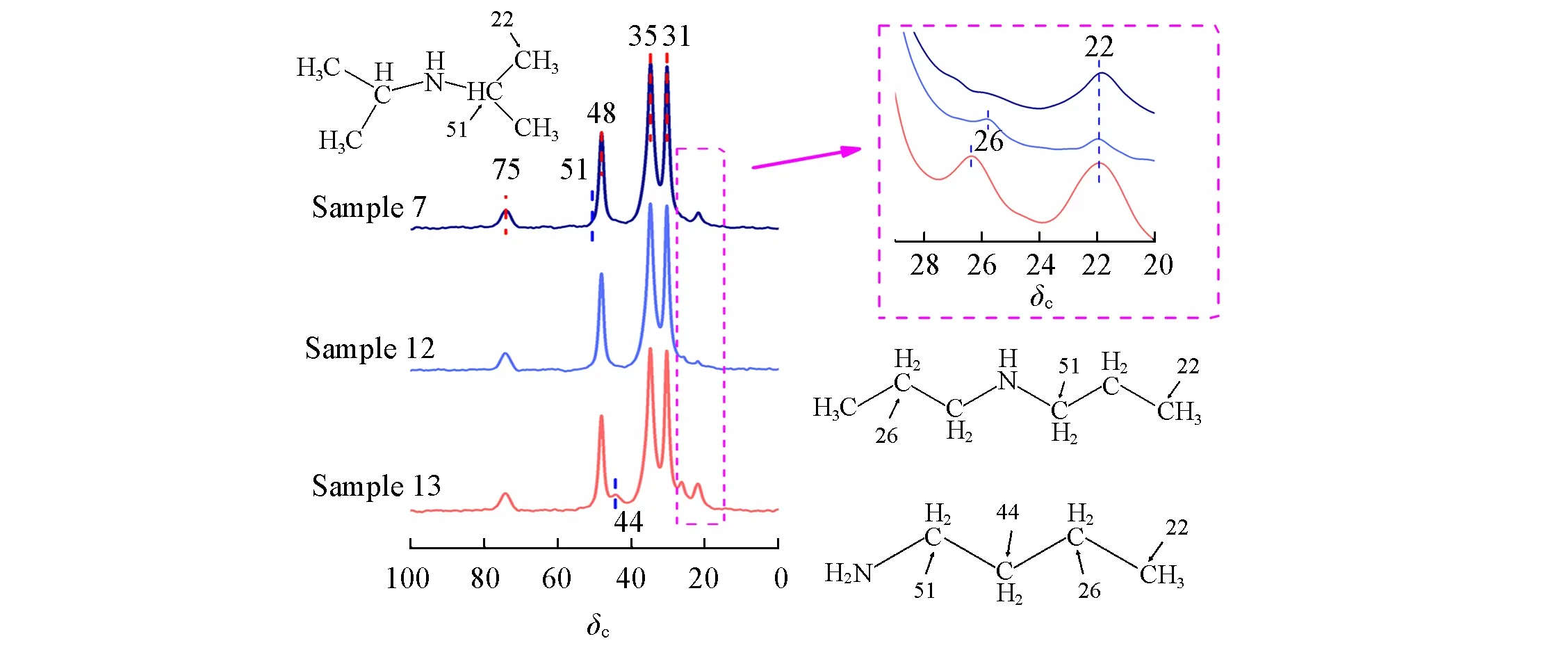
Fig.5 13C MAS NMR spectra of SSZ-13 prepared with different OSDAs
4 Conclusions
In this work,we have synthesized well-crystallized SSZ-13 by employing TMAdaOH and OSDAs used for SAPO-34(DIPA,DPA,andnBA).The studies on the crystallization mechanisms of SSZ-13 co-templated by TMAdaOH and DIPA were carried out.The results show that the partial replacement of expensive TMAdaOH by DIPA accelerates the crystallization process,inhibits the formation of amorphous phase,and increases the yields.The Cu-SSZ-13 shows excellent NH3-SCR reactivity even compared with recent state-of-art catalysts.
The supporting Information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20200855.
This paper is supported by the National Natural Science Foundation of China(Nos.21972136,21676262,21991091),the CAS Pioneer Hundred Talents Program(No.Y706071202),the Dalian National Laboratory for Clean Energy(DNL)Cooperation Fund,Chinese Academy of Sciences(No.DNL201908)and the Key Research Program of Frontier Sciences,Chinese Academy of Sciences(No.QYZDBSSW-JSC040).
——李振声

