Association of MTHFR C677T polymorphism with primary open angle glaucoma: a Meta-analysis based on 18 casecontrol studies
Yu-Mei Yang, Yu-Ping Liu,2, Dong-Yu Li,2, Man Yu, Bo Gong, Lin Wang, Ping Shuai,2
1Health Management Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu 610072, Sichuan Province, China
2School of Medicine, University of Electronic Science and Technology of China, Chengdu 610054, Sichuan Province,China
3Department of Ophthalmology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu 611731, Sichuan Province, China
4Sichuan Provincial Key Laboratory for Human Disease Gene Study, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu 611731, Sichuan Province, China
Abstract
● KEYWORDS: methylenete trahydrofolate reductase;polymorphism; primary open angle glaucoma; Meta-analysis
INTRODUCTION
Glaucoma, defined as an acquired degeneration of retinal ganglion cells and a progressive neurodegeneration of the optic nerve, is the second most common cause of irreversible vision loss worldwide[1]. According to the epidemiological studies, Glaucoma affects approximately 64.3 million people worldwide, and will increase to 111.8 million in 2040[2‐3]. Among them, 74% were diagnosed as primary open angle glaucoma (POAG)[4‐5]and half (47%) of them were from Asia[6]. The methylenetetrahydrofolate reductase (MTHFR)enzyme is responsible for the conversion the reduction of 5,10‐methylenetetrahydrofolate into 5‐methyltetrahydrofolate,is plays an important role in the homeostasis and normal metabolism of folic acid. C677T single‐nucleotide polymorphism (SNP) is one of the most extensively studied genetic polymorphism of MTHFR gene, which can cause a missense mutation leading to a valine to alanine exchange at position 222 of the enzyme, causing the synthesis of a thermolabile enzyme with a decrease in activity[7‐10]. Previous researches have demonstrated that MTHFR deficiency could lead to hyperhomocysteinemia and give rise to the development of vascular injury or direct toxicity to retinal ganglion cell (RGC)[7,11]. MTHFR C677T polymorphism were found to be association with POAG in many studies, but the results are inconsistent. Therefore, we conducted a Meta‐analysis of all available studies to get a more comprehensive evaluation of the relationship between MTHFR C677T polymorphism and POAG risk.
MATERIALS AND METHODS
Searching StrategyAccording to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA)criteria[12], we performed a comprehensive literature. To identify all relevant available studies published in Google Scholar, PubMed, Science Citation Index (SCI), Foreign Medical Literature Retrieval Service, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases up to August 2019. The search strategy identified as the eligible studies using the following search term/phase,‘methylenetetrahydrofolate reductase or MTHFR’ AND‘mutation or variant or polymorphism or genotype’ AND‘glaucoma’. The articles only selected those studies in humans and no population, time period or sample size restriction.
Inclusion and Exclusion CriteriaStudies included in this Meta‐analysis had to meet the following criteria: 1) evaluated the MTHFR C677T polymorphism and POAG risk; 2) case‐control studies ; 3) provide sufficient data for calculating odds ratios (ORs) with 95% confidence intervals (CIs); 4)publications in English or Chinese. The studies were excluded those not relevant to C677T polymorphisms and glaucoma,only abstracts, case reports, reviews, animal studies, letter to editors. For duplicate reports, only the largest sample size was selected for analyses. Moreover, studies lack of definite information of genotypes and other essential data were also excluded.
Data Extraction and Quality AssessmentDate for analysis were extracted independently by two investigators (Yang YM and Shuai P) according to the inclusion and exclusion criteria listed above. The following variables were collected from each study if available: name of the first author; year of publication;ethnicity; country; study design; diagnosis criteria; genotyping method; total numbers of cases and controls; source of control(hospital‐based or community‐based); the frequencies of each genotype; evidence of Hardy‐Weinberg equilibrium (HWE) in normal controls (P<0.05 of HWE was considered significant).Disagreements were resolved by discussion until a consensus was achieved. Otherwise, a third investigator (Liu YP) was consulted to resolve the dispute. The Newcastle‐Ottawa scale(NOS) was performed to assess the quality of eligible studies[13]. If the scores >7 were considered to be of high quality studies.
Statistical AnalysisSummary ORs and corresponding 95%CIs were estimated for various genotypic models,including TvsC (allelic mode), TTvsCC (additive model),TT+TCvsCC (dominant model) and TTvsTC+CC (recessive model). The pooled ORs were performed through a fixed‐effect model (Mantel and Haenszel method) if there was no heterogeneity. Otherwise, the random‐effect model(DerSimonian and Laird method) was used. The Cochran’sQtest and the inconsistency index (I2) were used to assess the level of heterogeneity (P<0.05 orI2>50% was considered the existence of significant heterogeneity). The source of heterogeneity can detect by Meta‐regression. Additionally,we performed stratified analysis with respect to ethnicity(Caucasians and Asians). Sensitivity analysis was carried out by excluding one study at a time to explore whether the results were significantly influenced by a specific study. Funnel plots and Begg’s test were performed to evaluate for the presence of publication bias. The HWE for SNP polymorphism was tested by theχ2test. The threshold for significance was set asP<0.05(two‐sided). All statistical analysis was carried out by using the STATA 12.0 (STATA Corp, College Station, TX, USA).
RESULTS
Literature Search and CharacteristicsThe literature search generated 2054 results initially. Totally 1586 irrelevant articles and 328 duplicate ones were excluded after careful review of abstracts and/or full‐text, with 140 articles being retrieved for further evaluation. Among the remaining studies, 122 publications were excluded for they were review articles,letters to editors, case reports, articles not relevant to MTHFR C677T or evaluation of other diseases instead of glaucoma.Seventeen studies evaluated the association between risk of POAG and MTHFR C677T polymorphism. The additional study, which did not provide the MTHFR C677T genotype frequency both in cases and controls, was obtained with the detailed information by contacting the original author through email[14]. As the inclusion criteria of POAG was defined as intraocular pressure greater than 21 mm Hg, Fanet al’s[15]study, which aimed normal tension glaucoma, was not included. Total of eighteen studies[14‐31]were found suitable for the inclusion in the present Meta‐analysis, which comprises 2156 cases and 2201 controls (Figure 1).
The detailed characteristics of all the included studies were listed in Table 1. The score of NOS ranged from 6 to 9(average 7.6), hinting a low‐level of bias. In terms of ethnicity,nine studies were performed in Caucasian population and the same numbers were conducted in Asian group. There were four studies based on community population, while fourteen were hospital‐based. One study’s NOS score was less than 6 and two studies did not follow the HWE.

Figure 1 Flow diagram of the study selection process.

Table 1 Characteristics of 18 studies included for the Meta-analysis
Meta-analysis ResultsThis Meta‐analysis included 18 publications comprising 2156 cases and 2201 controls. The whole population was divided into the Caucasian and the Asian group, according to the studies’ different geographic regions. Heterogeneity in Caucasian, Asian and pooled populations was assessed respectively. Results showed that,for the overall populations and the Caucasian group, obvious heterogeneities were detected in the allelic model (TvsC) and dominant model (TC+TTvsCC). While for the Asian group,no significant heterogeneity was found in all models (Table 2).Fixed‐effect model would be used in the Asian group and revealed that MTHFR C677T polymorphism was significantly increased the risk of POAG in allelic model (TvsC: OR=1.34,95%CI: 1.12 to 1.59,P=0.001) and dominant model (TT+TCvsCC: OR=1.41, 95%CI: 1.14 to 1.76,P=0.002). While in the Caucasian group, no visible association was observed under the random‐effect model (Pvalues were all over 0.05). Overall,a mild relationship of MTHFR C677T polymorphism with POAG was seen for the pooled population in the allelic model and dominant model (bothP=0.02; Table 2, Figure 2).
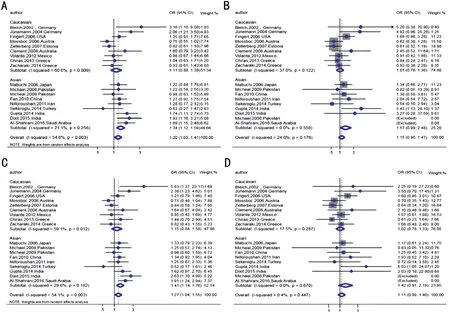
Figure 2 Forest plots of POAG risk association with MTHFR C677T polymorphism in Caucasian, Asian and overall populations A:Allelic model (T vs C); B: Additive model (TT vs CC); C: Dominant model (CT+TT vs CC); D: Recessive model (TT vs CT+CC). Fixed effects models were used for all models of Asian studies as no significant heterogeneity was observed (I2=21.1%, 0, 29.6%, 0, respectively).Random effects models were performed for allelic model and dominant model as heterogeneity was seen in Caucasian studies (I2=60.6% and 59.1%) and overall studies (I2=54.6% and 54.1%). The summary pooled ORs and 95%CIs are displayed with the blue diamond. No significant association was observed between all models and the risk for POAG in Caucasian subgroup (for T vs C OR=1.11, 95%CI: 0.88 to 1.39; for TT vs CC OR=1.01, 95%CI: 0.76 to 1.36; for TT+TC vs CC OR=1.15, 95%CI: 0.84 to 1.58 and for TT vs TC+CC OR=1.02, 95%CI: 0.78 to 1.33) and overall studies (for TT vs CC OR=1.15, 95%CI: 0.90 to 1.47; for TT vs TC+CC OR=1.11, 95%CI: 0.89 to 1.40). However, significant association were seen in two genetic models for Asian subgroup (for T vs C OR=1.34, 95%CI: 1.12 to 1.59; for TT+TC vs CC OR=1.41, 95%CI:1.14 to 1.76). The overall studies (for T vs C OR=1.20, 95%CI: 1.03 to 1.41; for TT+TC vs CC OR=1.27, 95%CI: 1.04 to 1.55).
Heterogeneity Test and Sensitivity AnalysisIn order to evaluate the source of heterogeneity, a Meta‐regression was conducted considering the possible factors such as ethnicity(CaucasianvsAsian), genotyping methods (sequencing/PCRvsothers), NOS (≥7vs<7), HEW (P≥0.05vsP<0.05 ), population source of control (hospital‐basedvscommunity‐based). Results demonstrated that the ethnicity contributed 33.3% and the genotyping methods contributed 22.7% to the heterogeneity in the allelic model, respectively. After pooling the two factors together, 77.6% of the heterogeneity sources was explained.The Meta‐regression found that the ethnicity and genotyping methods might be the major sources of between‐study heterogeneity (similar results were got in the other models,data not shown). Moreover, sensitivity analysis was conducted to examine the influence of each independent study on the pooled ORs by the sequential removal of each individual study.After each study was excluded, the corresponding pooled ORs were not materially changed, indicating that the result of this Meta‐analysis was reasonably robust and reliable.
Publication BiasPublication biases in this work wereevaluated by funnel plot and Begg’s test. Visual inspection of the funnel diagram revealed no asymmetry (Figure 3).

Table 2 Meta-analysis of MTHFR C667T polymorphism with POAG in 18 studies subgroup by different population

Figure 3 Funnel plots showed symmetric distribution logOR is plotted against the SE of logOR for studies on MTHFR C677T. The dots show specific studies for the indicated association.
The Begg’s test was then performed to provide quantitatively statistical evidence. No significant publication biases were revealed for the four genetic models (the allelic model,P=0.121; the addictive model,P=0.137; the recessive model,P=0.224; the dominant model,P=0.449).
DISCUSSION
The pathogenesis of POAG has not been studied clearly.Reports supported that genetic factors might play a significant role in POAG. Many epidemiological studies have assessed the roles of MTHFR C677T polymorphism and POAG risk in different populations, nevertheless, the results remain inconsistent. In this study, we conducted a comprehensive and detailed Meta‐analysis derived from 18 case‐control studies in 11 publications containing 2156 cases and 2201 controls.Our results revealed that there were significant associations between MTHFR C677T polymorphism and POAG for Asian population, indicating the T allele or TT+TC genotype might increase the risk of POAG.
So far, there are five studies showing that the T allele and TC genotypes of MTHFR C677T polymorphism may increase the POAG risk. Bleichet al[16]provided the first evidence of the association and discovered a significantly higher prevalence of the MTHFR C677T polymorphism in patients with POAG when compared with the controls (P=0.022, OR=5.34,95%CI: 1.14 to 29.56). Dixitet al[17]also found a very obvious association of MTHFR C677T with POAG (χ2=9.05,P=0.01)in north Indian population. Similarly, Al‐Shahraniet al[18]reported the T allele and TC genotype were related with POAG as a risk factor. However, many other reports revealed no relationship of MTHFR C677T with POAG, such as those studies in American, Chinese, Australian, Japanese, Pakistani,Mexican populations. Huoet al[32]carried out a Meta‐analysis including ten studies with 1224 cases and 1105 controls in 2012 and reported that for the overall population, no obvious association was found. However, when stratifying the source of control, significant correlation was shown in the allelic and additive genetic model for the population‐based subgroup(OR=1.39, 95%CI: 1.05 to 1.83 and OR=1.88, 95%CI: 1.04 to 3.43 respectively), indicating that the T allele or TT genotype might increase the risk of POAG.
We found some problems when reviewing previous literatures.For example, Gohariet al[33], based on 33 studies with 3504 glaucoma patients and 2525 controls, offered different opinion on the MTHFR C677T susceptibility with glaucoma(containing POAG patients). We found they might miscalculate the frequency of TC genotype and misclassified the source of control from hospital‐based group into the community‐based ones[15]. A study from India miscounted the gene frequency of the patients as those in controls[17]. One previous Meta‐analysis which considered MTHFR C677T as genetic biomarkers of POAG in West Asians, might wrongly grouped the normal tension glaucoma patients to the POAG subgroup[34‐35].
A potential issue is Heterogeneity, which might affect the interpretation of the association. Many factors, such as inclusion criteria, ethnicity, source of controls, deviation from Hardy‐Weinberg equilibrium, and genotyping methods, could be the source of heterogeneity. In our study, we conducted an ethnicity subgroup analysis firstly, then, performed a Meta‐regression analysis to find the source of heterogeneity. The results revealed that the ethnicity and genotyping methods might be the main ones, which contributed 77.6% of the heterogeneity.One limitation of our Meta‐analysis is that the source and definition of the control group are not completely consistent in all the literatures. The degree of risk between the MTHFR C677T and POAG may be weakened by taking individuals with cataract, hypertension, coronary heart disease and other vascular diseases as the controls. Also, the publications were limited as English and Chinese language, and predominantly on Asian and Caucasian studies. This suggested that a possible partial result only relevant to the Asian and Caucasian populations. Another possible limitation was that for the Asian group in the additive model, the OR was 1.57 (95%CI: 0.99 to 2.48) with thePvalue was 0.053, which was just over 0.05 a little bit. It is hard to conclude that the no significant association can be revealed for the TT genotype, especially under the situation that increased risk of POAG was detected in the allelic and dominant model. More Asian studies and samples are needed to give a clear relationship for the MTHFR C677T polymorphisms and POAG. As a complex disease, POAG is the result under the effect of both genetic and environmental factors. Further research between MTHFR polymorphisms and glaucoma will be incentive and worthy to explore the multi‐factors’ effects, especially the potential gene‐gene and gene‐environment interactions. Our Meta‐analysis presented distinct advantages with largest sample size to date and a relatively reliable conclusion through the sensitivity analysis.
In summary, the current Meta‐analysis showed that MTHFR C677T polymorphisms were significantly associated with POAG in Asians, indicating that the T allele or TT+TC genotype might increase the risk of POAG in this group. No association was detected for the Caucasians. In the future,large well‐designed studies from different ethnicities should be encouraged to provide more comprehensive understanding of the association of MTHFR C677T polymorphism with risk of POAG.
ACKNOWLEDGEMENTS
Foundations:Supported by National Key Research and Development Plan (No.2017YFC0113901); the Department of Science and Technology of Sichuan Province, China(No.2017JZ0039); the Science Research Project for Cadres’ Health Care of Sichuan Province (No.2017‐205);Science and Technology Department of Sichuan Province(No.2019JDJQ0031; No.2020ZYD035).
Conflicts of Interest:Yang YM,None;Liu YP,None;Li DY,None;Yu M,None;Gong B,None;Wang L,None;Shuai P,None.
 International Journal of Ophthalmology2021年6期
International Journal of Ophthalmology2021年6期
- International Journal of Ophthalmology的其它文章
- A decrease in macular microvascular perfusion after retinal detachment repair with silicone oil
- Surgical outcomes in acute dacryocystitis patients undergoing endonasal endoscopic dacryocystorhinostomy with or without silicone tube intubation
- Dr. Father Wacław Szuniewicz, a forgotten pioneer in refractive surgery and his work in China
- Deterioration of Avellino corneal dystrophy in a Chinese family after LASlK
- A mutated CRYGD associated with congenital coralliform cataracts in two Chinese pedigrees
- Autophagy dysregulation mediates the damage of high glucose to retinal pigment epithelium cells
