A mutated CRYGD associated with congenital coralliform cataracts in two Chinese pedigrees
Su-Ping Cai, Lan Lu, Xi-Zhen Wang, Yun Wang, Fen He, Ning Fan, Jing-Ning Weng,Jun-Hua Zhang, Xu-Yang Liu
1Shenzhen Key Laboratory of Ophthalmology, Shenzhen Eye Hospital, Jinan University, Shenzhen 518000, Guangdong Province, China
2Department of Ophthalmology, Fujian Medical University Union Hospital, Fujian Medical University College of Medical Technology and Engineering, Fuzhou 350001, Fujian Province,China
3Xiamen Eye Center, Xiamen University, Xiamen 361100,Fujian Province, China
4Department of Ophthalmology, Shenzhen People’s Hospital,the 2nd Clinical Medical College, Jinan University, Shenzhen 518020, Guangdong Province, China
Abstract
● KEYWORDS: congenital cataract; mutation; CRYGD gene; autosomal dominant
INTRODUCTION
Congenital cataract is defined as opacity of the crystalline lens that usually presents at birth or short thereafter.It remains the most common cause of lifelong visual impairment in childhood worldwide[1]. As a phenotypically and genotypically heterogeneous disease, up to date, approximately 22.3% of childhood cataracts are inherited[2], with more than 100 genes and about 200 locus (http://cat‐map.wustl.edu/)being identified[2‐3].
Autosomal dominant is the most prevalent inherited manner in congenital cataract[4], and there are at least 34 genes linked to non‐syndromic congenital cataracts[2], including genes encoding crystalline proteins (CRYAA,CRYAB,CRYBB,CRYGC,CRYGD), cytoskeletal proteins, membrane proteins,gap junction proteins, and others[4‐9].
Crystallins are water‐soluble lens crystalline proteins and play an essential role in maintaining lens transparency,90% of the total lens proteins are crystallin[10‐11]. Mutations in crystalline encoding genes has been reported to induce congenital cataract with a wide variety of phenotypes[11]. In this study, a wide spectrum of cataract gene panel was screened in two unrelated Chinese pedigrees with inherited autosomal dominant congenital cataract, and mutation inCRYGDgene was identified as causal gene of cataract in these two families.The molecular genetic mechanism and clinical characteristics of the two families were evaluated.
SUBJECTS AND METHODS
Ethical ApprovalThis study was approved by the Medical Ethics Committee of the Shenzhen Eye Hospital, Jinan University and Fujian Union Hospital, Fujian Medical University. Informed consent was obtained from all participants.
PatientsTwo unrelated pedigrees with congenital cataract were enrolled in the study, with 42 members (20 patients and 22 normal individuals) in pedigree I (Figure 1, from Xiapu,Fujian Province, China) and 36 individuals (15 patients and 21 normal individuals) in pedigree II (from Shenzhen, China).
Clinical ExaminationFamily histories were obtained from these two pedigrees, and detailed ocular examination was performed for each member. Diagnosis was made by experienced ophthalmologists and the type of cataract was classified according to lens characteristics by slit lamp examination or the images which were recorded before the cataract extraction surgeries. Age at disease onset was defined as the age at which the visual symptom was first noted by patient or his/her family members. When the information regarding the age of disease onset was not available, the age at diagnosis was recorded. Visual acuity (VA) was classified into the following criteria as defined by the World Health Organization. Ten‐year follow up has been made for these two families and no other ocular or systemic disorders other than congenital cataract were noticed.
DNA ExtractionPeripheral venous blood was obtained from all members of these two families included in the study. Genomic DNA was isolated and purified from 200 μL venous blood using QIAamp DNA Blood Mini Kit (Qiagen,Hilden, Germany), according to a standard procedure of the manufacturer. The integrity of the DNA samples was evaluated by electrophoresis on 1% agarose gel.
Mutation Screening and Sequence AnalysisTargeted next generation sequencing (NGS) panel containing a large spectrum of 134 cataract‐associated genes was used in the research. The 1 to 5 μg genomic DNA from the proband was used for Target‐Capture sequencing according to manufacturer protocol.

Figure 1 Pedigree I: a congenital cataract pedigree inherited in an autosomal dominant pattern The square indicates male, the circle indicates female; The filled shape indicates the affected patients with congenital cataract; The arrow indicates the proband.
Sanger Sequencing ValidationThe identified mutation was screenedviadirect automated sequencing to ascertain whether the indicated mutation was co‐segregated with the disease phenotype in each of the pedigrees. Based on the Primer Premier 5 software, polymerase chain reaction (PCR) primers were obtained and synthesized by BGI (BGI‐Shenzhen,Guangdong, China). PCR amplification was then performed in other family members of this pedigree. The 30 μL PCR components included 15 μL 2×Taq PCR Master Mix (SinoBio,Shanghai, China), 1.4 μL DNA (30 ng), 0.8 μL of both forward and reverse primers (1.0 μmol/L), and 12 μL ddH2O. The PCR products were first incubated at 95℃ for 3min, followed by 35 cycles of 95℃ for 30s, then annealing at 55℃ for 30s,and extension at 72℃ for 1min, with final extension at 72℃for 5min. After purification, PCR amplified reactions were sequencedviaan ABI 377XL automated DNA sequencer(Applied Biosystems, Foster City, CA, USA). The re‐assembly DNA sequences were analyzed by the DNA Star software and compared pairwise with reference sequence on Human Genome databases. All identified mutations and its variants were interpreted and classified according to the nomenclature recommended by the Human Genomic Variation Society(HGVS).
RESULTS
Clinical EvaluationThe four‐year‐old female proband (IV:5)presented with diminished vision in her both eyes since age two and subsequently experienced progressive vision loss. On her last visit, the VA was counting fingers (CF)/30 cm in her right eye and CF/80 cm in her left eye, no improvement with refractive correction. The intraocular pressures were normal bilaterally. Patient had bilateral coralliform shape opacification characterized by the white opaque involving the whole lens except for capsule, with appearance resembling the coralliform shape (Figure 2). Bilateral dilated fundoscopy showed no detectable retinal abnormality. The child was generally healthy without notable systemic abnormalities.
Almost the same type of lens opacification as that in pedigree I was noted in pedigree II. The seven‐year‐old male proband(Figure 3, patient IV:1) manifest with reduced vision in his both eyes since birth and experienced subsequent progressive vision loss. No history of physical and other ocular disease was found. On his last visit, his VA was CF/50 cm in his both eyes, no improvement with refractive correction. The bilateral intraocular pressures were normal. Axial length was 23.63 mm in his right eye and 23.40 mm in his left eye. Bilateral dilated fundoscopy showed no notable abnormality.
Genetic EvaluationAfter sequencing the 134 genes of the cataract gene panel for these two probands, we identified a heterozygous missense mutation c.70C>A (p. P24T) in exon 2 ofCRYGDgene (Figure 4). The automated Sanger sequencing was used to verify the indicated mutation ofCRYGDwithin the family members of two pedigrees. The mutation inCRYGDwas co‐segregated with the diseases in these two families.
DISCUSSION
Around between 8.3% and 25% of congenital cataracts are inherited and nearly half of inherited cataract are induced by crystallin genes mutation. Crystallin genes encode a wide spectrum of soluble structural proteins in the lens. There are three major types of crystallin genes in human lens including α‐, β‐, and γ‐crystallin[12]. Candidate genes of crystallin for congenital cataract include αA‐crystallin (CRYAA), αB‐crystallin (CRYAB), βA‐crystallin (CRYBA), βB‐crystallin(CRYBB), γC‐crystallin (CRYGC), and γD‐crystallin (CRYGD)genes, one‐third of congenital cataract were associated with γD‐crystallin, which amounts for 25% of the total crystallin in the human lens. Functionally, αA‐crystallin and αB‐crystallin have roles in maintaining the solubility of the other lens proteins like β‐ and γ‐crystallin. β‐crystallin remains the most elusive in their structural significance due to their greater number of subunits and possible oligomer formations.γ‐crystallin proteins folded tightly in two domains, each domain consists of two Greek‐key motifs, and a folded hairpin act to maintain the stability between two beta‐sheets. The highly expression of γC‐crystallin and γD‐crystallin are highly expressed in the fiber cells results in embryonic lens nucleus formation. The lens nucleus will remain its transparency owing to the regular micro‐architecture and the stability in nucleic fiber, the solubility of lens protein is also essential in maintaining the lens transparency, mutation inCRYGDgene might involve the solubility and stability of the crystallin proteins, subsequently reduce lens transparency causing congenital cataract[13‐16]. The crystal opacity display irregularly along the peripheral cortical region reveals the restructure of anatomic lens fibers, which influences the light scattering and lens nucleus transparency[17].
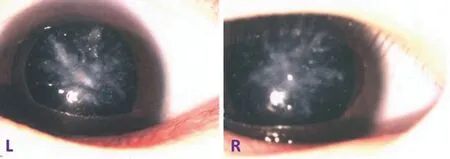
Figure 2 Slit lamp photograph showing representative phenotype of coralliform cataract (IV:5).

Figure 3 Pedigree II: a congenital cataract pedigree inherited in autosomal dominant pattern The square indicates male, the circle indicates female; The filled shape indicates the affected patient; The arrow indicates the proband.
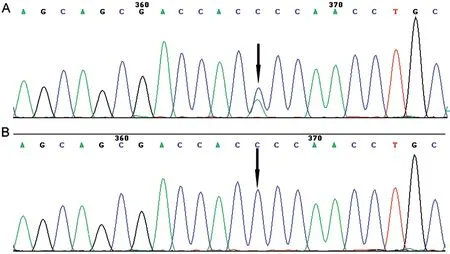
Figure 4 The mutation in CRYGD was verified using Sanger sequencing in affected patients and unaffected individuals Black arrow: C→A transversion induced a threonine substitution for proline at amino acid sequence. A: Patients; B: Normal individuals.
In current study, a wide spectrum cataract gene panel screening was performed in two probands from two separate Chinese congenital cataract pedigrees with autosomal dominant inheritance, a heterozygous variant ofCRYGDgene in exon 2 (70C>A, P24T) was identified as a disease‐causing gene as this mutation was co‐segregated with the disease within two families. Previous studies reported that ten mutated variants inCRYGDgene, including P24T, and others such as R15S,R15C, P24S, R37S, R59H, G61C, and Y134X[18‐20], manifested with vast phenotypic variations as a result of genotypic heterogeneity[18,21‐29]. As examples, Y56X, Y134C, and R58H were reported to be related with nuclear cataract, lamellar cataract and aculeiform/coral‐like cataract, respectively[22,30‐31].In current study, P24T mutation was associated with coralliform cataract, with most of family members in two Chinese families manifesting with significant coralliform cataract which resulting in severe visual impairment after birth. However,other than coralliform cataract, P24T was also known to be responsible for several different phenotypes of congenital cataract,e.g., cerulean cataract, lamellar cataract, and the fasciculi form cataract[15,26,28‐29,32], but with coralliform cataract being the most common phenotype from P24T variant in previous reports[17].Congenital coralliform cataract is closely related toCRYGDmutation, several different mutated variants inCRYGDgenes including R15S, R15C, G61C, and P24T mutations cause coralliform cataract. Clinically vast coralliform variations from diverse variants fromCRYGDgenes[15,26,28,30,33]. Guet al[34]found a six‐generation Chinese pedigree with R14C inCRYGDpresenting with the phenotype of either coralliform or nuclear cataract. In current study variant P24T occurred in two Chines pedigrees presenting with coralliform phenotype as previous demonstration, all affected patients presented with typical symmetric coralliform cataract in their both eyes,with clumps of thick coralliform opacification in the central portion of the lens cortex, across in a radiating direction from the cortical region towards the peripheral capsule, resulting in severe visual impairment after birth, patients had their cataract removal in their young ages. It was different from the effects of R15S mutation ofCRYGDgene in previously report, which resulted in bilateral coralliform cataract, but did not have detectable lens opaque until nine years of age. The molecular basis for lens opacification induced by P24T inCRYGDgene remained unclear[18]. Biophysical analysis showed that the solubility of P24T mutant protein is significantly lower than wild‐type human γD‐ crystalline. The understanding of phenotypic characteristic from P24T mutation may suggest the occurrence of a bilateral symmetric severe coralliform cataract at early age after birth may drive an evaluation of the P24T mutation inCRYGDgene[35‐36]. TheCRYGDp.P24T has been detected previously in families with congenital cataract in East Asia and functional analysis showed that the P24T mutatedCRYGDplayed a biological role in cataract formation.Analysis with a 3D structure prediction software indicated that the p.P24T mutant ofCRYGDchanged a pyrrole ring structure into a hydrophilic structure[37], therefore affecting γD‐crystallin’s solubility and leading to cataract formation.
In conclusion, a reported P24T mutation ofCRYGDgene,occurred in two Chinese pedigrees with bilateral symmetric coralliform cataract, causing severe visual impairment after birth. In current study,CRYGDmutations persistently caused congenital coralliform cataracts, indicating that the coralliform phenotype and theCRYGDgene are closely related.
ACKNOWLEDGEMENTS
The authors thank the two families for granting permission to share their information.
Foundations:Supported by the National Natural Science Foundation of China (No.81770924; No.82070963); Fujian Health and Family Planning Research Talent Training Project(No.2017‐CX‐18).
Conflicts of Interest: Cai SP, None;Lu L, None;Wang XZ,None;Wang Y, None;He F, None;Fan N, None;Weng JN,None;Zhang JH, None;Liu XY, None.
 International Journal of Ophthalmology2021年6期
International Journal of Ophthalmology2021年6期
- International Journal of Ophthalmology的其它文章
- A decrease in macular microvascular perfusion after retinal detachment repair with silicone oil
- Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography
- Dr. Father Wacław Szuniewicz, a forgotten pioneer in refractive surgery and his work in China
- Deterioration of Avellino corneal dystrophy in a Chinese family after LASlK
- Autophagy dysregulation mediates the damage of high glucose to retinal pigment epithelium cells
- Reliability of Chinese web-based ocular surface disease index questionnaire in dry eye patients: a randomized,crossover study
