Deterioration of Avellino corneal dystrophy in a Chinese family after LASlK
Xue Jiang, Hong Zhang
1Eye Hospital, the First Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
2Key Laboratory of Basic and Clinical Research of Heilongjiang Province, Harbin 150001, Heilongjiang Province,China
Abstract
● KEYWORDS: Avellino corneal dystrophy; granular corneal dystrophy type 2; LASIK; exacerbation
INTRODUCTION
G ranular corneal dystrophy (GCD) is a type of stromal malnutrition, which can be divided into two types according to clinical manifestations (GCD I and GCD II)[1‐2]. GCD II [Avellino corneal dystrophy (ACD), MIM No.601692] is an autosomal dominant genetic disease, characterized by granular and lattice dystrophy[3‐7]. ACD is strongly associated with the R124H mutation of theTGFBI(MIM No.601692) gene[2,6,8‐9],which was first reported in Italian population[1,3,6‐7,10‐11], but nowadays it has been reported all around the world[4,10‐12].The influence of homozygous R124H mutation on cornea is more seriously affected than heterozygous mutation[4,6,9‐10,13].Several literature have been reported that corneal deposits in the interface of laser‐assistedin situkeratomileusis(LASIK) flap increased postoperatively in the patients with heterozygous ACD initiating 2‐5y post‐LASIK[3‐5,10‐11,14‐20],which may cause serious vision loss. Until now, the only method to treat transforming growth factor beta‐induced protein(TGFBIp) corneal malnutrition has been a corneal transplant or keratectomy, but the recurrence of protein deposition is unavoidable[9,20]. Here, we report the results of both ophthalmologic examination andTGFBIgene analysis in one patient affected by ACD worsening after LASIK and his family from Heilongjiang province, China.
SUBJECTS AND METHODS
Ethical ApprovalThe study was authorized by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University and following the tenets of the Declaration of Helsinki, and all subjects signed an informed consent.

Figure 1 The pedigree of the proband’s family The arrow shows the proband (II‐11).

Table 1 Ophthalmic examination of family members harboring a heterozygous R124H mutation
Ophthalmic examination in family members with a heterozygous R124H mutation were showed in Table 1, and the family tree was displayed in Figure 1. The proband (II‐11), Chinese male,37 years old, was operated with bilateral LASIK for myopia in 2002. The vision in each eye was 1.0 after treatment. In the fourth year, he gradually felt that right eyesight had declined and underwent surgery to scrape away the opacities from the interface in another hospital. Right eyesight recovered to 0.3 postoperatively. He was then referred to the First Affiliated Eye Hospital of Harbin Medical University in 2019,complaining of the severe decline of right eye vision again.He was found to have haze in both corneas, and his vision of the right eye was hand motion and vision of the left eye was 0.3. Slit‐lamp photography showed that a large amount of dense and confluent granular opaque were observed in the center of the interfaces of the flap and remnant stroma bed in the right eye and light degree in the left eye(Figure 2A, 2B).In vitoconfocal microscope (IVCM) and optical coherence tomography (OCT) examination demonstrated numerous dense, confluent granular deposits of variable shape and size, which were extracellular structures of highly reflection,primarily lied at the interface between the flap and the stromal bed (Figure 2C‐2F). Sanger sequencing showed that there was a 371G>A mutation (CGC>CAC) in exon 4, which indicated that he was carrying a heterozygote R124H mutation, identifying the diagnosis of ACD (Figure 2G). However, the corneas of proband’s son were healthy, and had noTGFBImutation inherited.
Ophthalmic ExaminationAll subjects in this family accepted general ophthalmology examination, including vision acuity, intraocular pressure, slit‐lamp photograph, fundus examination, OCT of cornea (Heidelberg Engineering GmbH,Heidelberg, Germany), and IVCM (Heidelberg, Germany).
Genetic AnalysisGenomic DNA was prepared from peripheral blood (approximately 3 mL) of all 33 members in the family using QIAamp DNA Blood Mini Kit (Qiagen,Santa Clara, CA, USA) in accordance with the protocol of the manufacturer. All exons ofTGFBIgene were amplified by polymerase chain reaction and sequenced automatically using primer sequences and conditions described previously[21]. The nucleotide sequences were in comparison with the sequences of publishedTGFBIcDNA.
RESULTS
The proband’s third sister (II‐10) was a 56‐year‐old female,who had no history of ocular surgery or blurred vision. Slit‐lamp photography of the proband’s third sister demonstrated the classical superficial round granular deposits (Figure 3A,3B). OCT and IVCM showed opacities in the anterior stroma(Figure 3C‐3F), the same conditions with her two daughters(III‐11, a 28‐year‐old female; III‐12, a 25‐year‐old female). All three of them were tested positive. Genetic analysis manifested that they harbored a heterozygote R124H mutation. However,her eldest daughter (III‐10) was not inherited the mutation from her, with no opacities on both corneas.
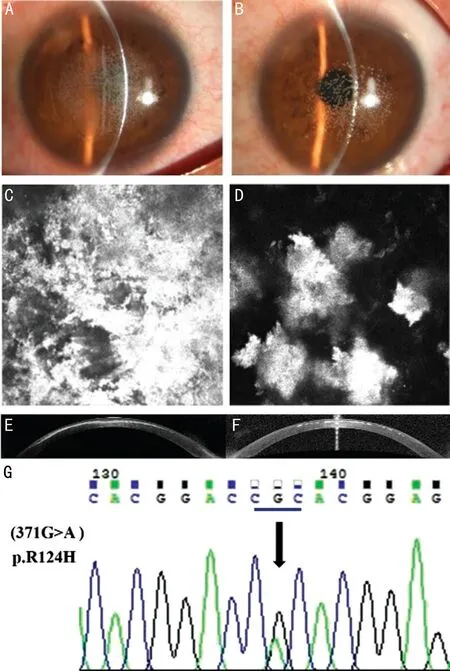
Figure 2 Both corneas of the proband in 2019, after interface deposits were removed from the right eye 4y after LASIK A:The slit‐lamp photograph showed that the numerous dense, confluent granular opaque deposits re‐accumulated in the center of the interface of the right eye flap and the remnant stroma bed. B: Recurrence of opacities due to ACD after LASIK showed in the left eye. The degree of opacities was lighter than that of the right eye. C, D: IVCM images of both corneal anterior stroma with highly reflective extracellular deposits.E, F: OCT images of the right eye and left eye of the proband were shown. Numerous dense, confluent granular opaque deposits were positioned on the interface between the Bowman layer and corneal stroma, especially in the right eye. G: Sequence chromatogram of mutation in TGFBI were shown. Mutation of 371G>A (CGC>CAC)was found in exon 4, indicating the presence of heterozygote R124H mutation, which comfirms the diagnosis of ACD.
The proband’s eldest sister (II‐2) was a 67‐year‐old female,who had a history of glaucoma surgery in both eyes six years ago. DNA screening revealed that heterozygous results of R124H mutation inTGFBI. Her daughter (III‐2, a 39‐year‐old female) and grandson (IV‐1, a 5‐year‐old male) were also tested positive with a heterozygote R124H mutation, but without any history of ocular surgery and no symptoms. It is worth mentioning that the grandson’s corneas were transparent,while grandma’s and mother’s corneas had opaque deposits.Other subjects displayed normal ophthalmic examination and no positive result of gene screening. It remains unclear whether the mutation was inherited from the proband’s mother or father. But the proband’s mother had poor eyesight during her life according to his family members, I‐1 is likely to be suspected of ACD.

Figure 3 Slit lamp images of II-10 (the proband’s third sister) in the right (A) and left (B) eye. IVCM images of II-10 in the right(C) and left (D) eye. OCT images of II-10 in the right (E) and left(F) eye. Opacities were located at corneal anterior stroma.
DISCUSSION
We reported a case of deterioration of corneal opacity in an ACD patient undergoing LASIK for myopia, and performed genetic screening in his family. In addition to the proband,six members in the family were detected the identical heterozygous R124H mutation inTGFBIas the proband,confirming the diagnosis of ACD. The images of slit‐lamp,OCT and IVCM images also exhibited irregular opacity of the anterior stroma. Homozygous ACD patients have a large amount of protein deposits in the early stages of life, while heterozygous ACD patients are impacted at a much later stage and present a generally less serious prognosis[20,22].Nevertheless, when patients with heterozygous ACD have the LASIK surgery, the accumulation of protein is expedited and can form a large protein accumulation in the years following surgery[20], leading to a significant decrease in vision[18].
The proband had no symptom of vision loss before LASIK,and passed the preoperative examination of LASIK operation.However, the opacities deposition was found on the interfaces of the flap and surrounding stroma bed postoperatively,which indicated that there might be mild opacities deposition in corneal stroma before operation, butTGFBIgene screening was not carried out. The patient reported that he did not underwent phototherapeutic keratectomy (PTK) or photorefractive keratectomy (PRK) to remove the opacities in 2006, but only mechanically removed granules of the interface in another hospital and later developed a severe relapse.Besides, theTGFBIgene test of the other 6 members were also positive, which proved the diagnosis of ACD. Since ACD will recur and exacerbate after LASIK and it is contraindication of LASIK, we might ask and remind the patients to consider the possibility ofTGFBIgene mutations inducing postoperative deterioration of ACD if they have a family history of corneal disease, or granular opacities in corneal stroma.
LASIK is an operation to correct myopia, hyperopia, and astigmatism. The flap of corneal epithelium and anterior stroma was incised and collapsed, and the laser was used to remodel the exposed stroma layer. PRK and PTK influence the vision correction or treat a variaty of eye diseases through the removal of the surface turbidity as well as surface irregularity[23].Numerous reports have indicated the deterioration of ACD following therapy with PRK, PTK, LASIK, and laser assisted subepithelial keratomileusis (LASEK)[3‐5,10‐18,20,23‐27].
LASIK may aggravate stromal dystrophies by various mechanisms. The formation of flap can release pro‐inflammatory cytokines, including TGF‐A, through the injury of epithelial cells and stromal cells. In addition, the trauma of flap lifting and mechanical removal of opaque further enhanced the cytokines production, resulting in more opacities[4‐5,11]but ultimately leads to a broader deposition of stroma material that requires penetrating keratoplasty[11]. The interface of flap and stroma may also serve as a potential accumulation space to enhance protein deposition[3‐4,9,15]. Because of the space that may be created after LASIK, or because this is the area where corneal cells stimulate the most, deposits may preferentially accumulate in the center of the cornea after LASIK[10],which was corresponding to the slit‐lamp photography of the proband.
According to Junet al[4], bilateral asymmetry of corneas and recurrent opacity were observed in a series of patients 1‐5y after bilateral LASIK, which was consistent with the proband in our case, who recurred in the 4y after LASIK with worse visual acuity in the right eye. This feature can be seen in both clinical manifestations and the time interval between excimer laser treatment and disease progression[11]. They reported a case in which the opacities scraped from the interface after the operation was more severe at a shorter interval than after the first operation[4]. Some opaque deposits are outside the ablative zone. The removed sediment turns out to be hyaline,which is part of the ACD granules[5,17,19], which was identical to the proband, although much of the interface was scraped and visual acuity improved to 0.3 in right eyesight , the deposits re‐accumulated and visual acuity reduced to pre‐debridement level. This may be due to the fact that there may be a potential space under the flap after LASIK, while there is no potential space after PTK[4,14]. Continuous deposition of corneal opacification after LASIK cannot be effectively prevented by surgical resection alone[4].
Recurrence of ACD after LASIK can result in multiple corneal transplants. After lamellar keratoplasty of any form in such patients, surgeons ought to pay attention to the possibility for rapid deposition of stroma interface, and limit the depth of lamellar incision in case of repeated operations[14]. For this proband, a deep anterior lamellar keratoplasty would be needed. However, he gave up the surgery treatment as the risk of recurrence of postoperative opacities was relatively high. Refractive surgery is prohibited for corneal dystrophy due to the possibility of recurrence and deterioration[9,16].Because the history of family and clinical workup alone might make it difficult to identify ACD diagnosis, preoperative gene‐screening forTGFBImutations should be advised for candidates of refractive surgery if possible.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.U20A20363; No.81970776); the Natural Science Foundation of Heilongjiang Province, China(No.LH2020H039).
Conflicts of Interest: Jiang X,None;Zhang H,None.
 International Journal of Ophthalmology2021年6期
International Journal of Ophthalmology2021年6期
- International Journal of Ophthalmology的其它文章
- A decrease in macular microvascular perfusion after retinal detachment repair with silicone oil
- Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography
- Dr. Father Wacław Szuniewicz, a forgotten pioneer in refractive surgery and his work in China
- A mutated CRYGD associated with congenital coralliform cataracts in two Chinese pedigrees
- Autophagy dysregulation mediates the damage of high glucose to retinal pigment epithelium cells
- Reliability of Chinese web-based ocular surface disease index questionnaire in dry eye patients: a randomized,crossover study
