Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography
Ze-Bing Li, Zhong-Jing Lin, Na Li, Huan Yu, Yan-Lin Wu, Xi Shen
Department of Ophthalmology, Ruijin Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
Abstract
● KEYWORDS: optical coherence tomography angiography; Alzheimer’s type dementia; Mini-Mental State Examination; Montreal Cognitive Assessment scale; RETeval system; flash electroretinogram
INTRODUCTION
Alzheimer’s type dementia (ATD) is the most common type of dementia and accounts for 60% to 70% of all causes of dementia. ATD is a progressive neurodegenerative disease that usually manifests as irreversible impairments in cognitive function and behaviour. Characteristic pathological changes include brain atrophy, tangles, loss of neurons in the brain tissue and deposition of extracellular amyloid protein[1].The increase in the ageing population in society is attributed to the continuous increase in the standard of living and development of medical technology; thus, the incidence of ATD inevitably shows an increasing trend. Therefore, basic and clinical studies of ATD have gradually begun to attract attention from researchers. Currently, the clinical diagnosis of ATD mainly relies on the patient’s complaint, Mini‐Mental State Examination (MMSE) score, Montreal Cognitive Assessment (MoCA) score, magnetic resonance imaging(MRI) and other imaging examinations designed to assess the intracranial atrophy of the brain tissue. In recent years,an increasing number of ophthalmologists have observed symptoms, such as decreased resolution and reduced contrast sensitivity in visual function in many patients with ATD[2‐4].Changes in the fundus of the retina in patients with ATD have become the focus of research in recent years. Many experts and scholars are eager to determine whether these studies will provide new insights and perspectives for the early clinical diagnosis and prevention of ATD.
The most common vessel problems in the brains of patients with ATD include impaired Aβ clearance, impairments in the blood‐brain barrier, decreased vessel density, decreased vessel diameter and decreased blood flow[5]. Additional studies are needed to determine whether similar changes will occur in the retina. In recent years, the introduction of optical coherence tomography angiography (OCTA) technology and its gradual promotion as a non‐invasive and rapid fundus retinal vessel examination technology has gradually been welcomed by clinicians. Our study aims to discover changes in retinal vessels and the function of the fundus in patients with ATD using OCTA and flash electroretinograms (ERG). These findings may contribute to the early diagnosis, prevention, and follow‐up of ATD.
SUBJECTS AND METHODS
Ethical ApprovalThis research received the ethical approval from Ruijin Hospital, affiliated to Shanghai Jiao Tong University School of Medicine. All procedures were in accordance with the Declaration of Helsinki. Each subject carefully read the informed consent form and signed it to indicate his/her willingness to participate in the study prior to recruitment.
Availability of Data and MaterialsAll data and materials are fully available in the paper without restriction.
Research SubjectsThis cross‐sectional study was conducted at Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. Patients with neurological memory disorders were recruited at the clinic from December 2017 to January 2019. Untreated patients with ATD were diagnosed by experienced senior neurologists, while local healthy residents were recruited as the normal control group.
The participants with ATD were diagnosed according to the criteria for dementia established in the 4thedition of the Diagnostic and Statistical Manual of Mental Disorders[6]. All participants were subjected to an analysis of complete routine blood biochemical parameters, including measures of thyroid function, vitamin B12 levels and folic acid levels; MMSE and MoCA scores; and MRI and electroencephalogram (EEG).Additionally, the spherical equivalent was between +3.0 D and‐6.0 D, and the cylinder was between ±3.0 D for all subjects at enrollment. The slit lamp examination indicated that the anterior segment of the eye was normal and the signal strength of the OCTA image must be greater than 6. The following exclusion criteria were used: severe cardiovascular disease,liver and kidney dysfunction, respiratory diseases, intracranial lesions and blood diseases, patients with anterior segment diseases (such as keratitis, anterior chamber opacity and hyphema, severe cataract), fundus diseases (such as fundus haemorrhaging, macular degeneration, diabetic retinopathy,uveitis), other eye diseases that affect vision (such as colour blindness, retinitis pigmentosa, optic neuritis), a history of eye surgery, history of trauma, and history of laser therapy.

Figure 1 Schematic of 1, 3, and 6 mm ETDRS of the left eye.
Ophthalmic ExaminationAll subjects underwent a series of detailed eye examinations, including the best corrected visual acuity measured using the new national standard visual acuity chart, the anterior segment examination using the slit lamp, the refractive error (RK‐F1 from Nidek, Japan), and the intraocular pressure (IOP; Goldmann applanation tonometer) before the OCTA test, central corneal thickness (CCT) and axial length(IOL‐Master of Zeiss, Germany), and chamber angle examined using a gonioscope. Fundus examination was performed using spectral‐domain OCT (Carl Zeiss Meditec, Germany).Both eyes of all subjects were examined, if both eyes met the enrollment conditions, one eye was randomly selected for inclusion in the study to avoid binocular interactions.
Optical Coherence Tomography AngiographyAll OCTA measurements were performed by the same physician skilled in the technique. Inspections were performed using a Zeiss light coherence tomography scanner (CIRRUSTMHD‐OCT Model 5000 instrument, AngioplexTMOCT, Carl Zeiss Meditec, Germany). Compound tropicamide eye drops were administered to both eyes to dilate the pupils. After at least 30min, the physician ensured that the pupil sphincter of the tested eye was relaxed and pupil dilation was complete.Angiography 6×6 mm2, angiography 3×3 mm2and enhanced depth imaging (EDI) modes were chosen.
The angiography 6×6 mm2model was chosen and centred on the macular area through the 1, 3, and 6 mm ETDRS(Figure 1) to partition the macular area foveolar subfield (F),inner temporal subfield (IT), inner inferior subfield (II), inner nasal subfield (IN), inner superior subfield (IS), outer temporal subfield (OT), outer inferior subfield (OI), outer nasal subfield(ON), and outer superior subfield (OS). Figure 2A shows the superficial retina from the internal limiting membrane (ILM)to the inner plexiform layer (IPL), which was examined to collect the superficial retina blood flow signals and analyse the overall vessel length density and vessel perfusion density. The vessel length density calculates the value of the vessel length density in the region by depicting the linear length of blood vessels to more accurately observe the degree of perfusion of microvessels. The vessel perfusion density reflects the diameter of blood vessels and the degree of perfusion of blood flow in blood vessels by depicting the diameter and width of blood vessels and calculating the density of blood vessel coverage in the region. The angiography 3×3 mm2model was used to measure the area of the foveal avascular zone (FAZ; Figure 2B‐2D).
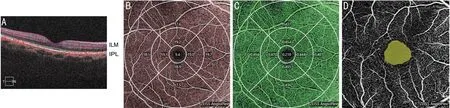
Figure 2 Schematic showing the measured parameters around the macular area using OCTA A: Image of the surface retina blood flow signals from the ILM to the IPL, which were used to analyse the overall vessel length density and vessel perfusion density; B: OCTA image overlaid on the schematic diagram showing the vessel length density image acquired in angiography 6×6 mm2 mode; C: Image of the vessel perfusion density acquired in angiography 6×6 mm2 mode; D: FAZ area obtained using angiography 3×3 mm2 mode.
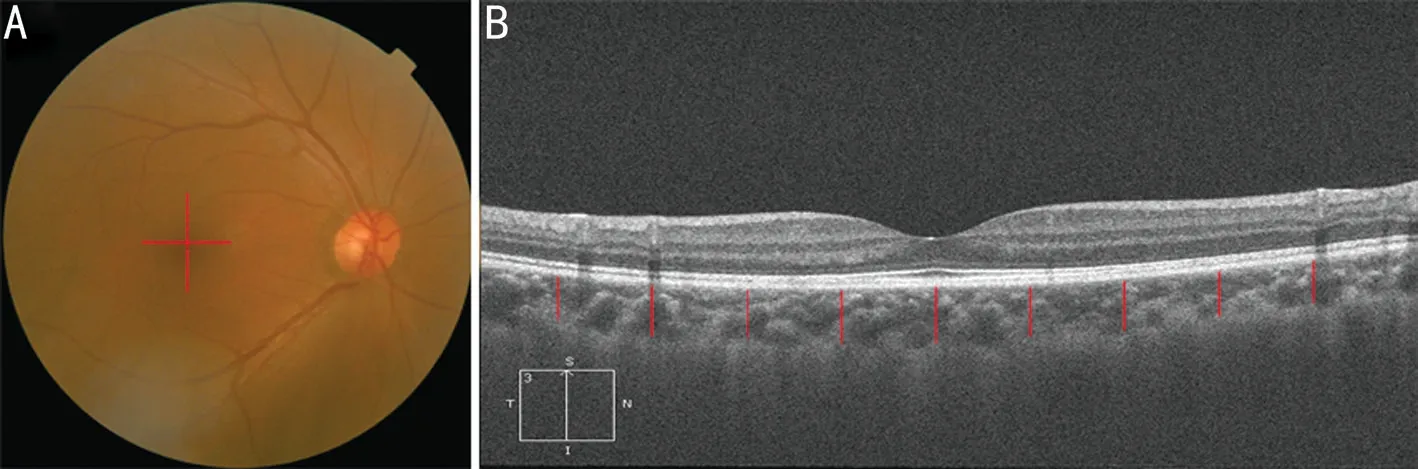
Figure 3 Schematic showing the measurement of the choroid thickness around the macular area using OCT A: The positions of the horizontal and vertical scan lines in the macular area; B: The location at which the choroidal thickness was measured in the macular area; each red line is separated by 500 μm.
In EDI mode, the scanning line of 6 mm was used to scan the 0° horizontal line and the 90° vertical line around the macular area. The site of the macular area that was measured is the central concave and the upper, lower, nasal, and temporal sides, and the distances from the fovea were 500 μm, 1000 μm,1500 μm, and 2000 μm, respectively (Figure 3). Self‐designed measurement software[6‐7]was used to measure the vertical thickness of the choroid at the corresponding position. The inner boundary of the choroid is the outer border of the retinal pigment epithelial layer, while the outer boundary of the chorioscleral border is the inner sclera[8], and the mean choroidal thickness was calculated.
RETeval System Records of Flash ElectroretinogramsThe new handheld RETeval recording ERG system[9]reduces the damage to the cornea and ocular surface caused by rigid corneal contact electrodes and improves the comfort level of subjects compared with traditional retinal galvanogram recording devices. The method could be used for the collection of retinal ERGs from subjects with partial poor corneal epithelial conditions and low degree of fit.
We chose the international general ISCEV five‐step protocol that starts with dark adaptation. Subjects first underwent dark adaptation in a dark environment for 30min. The second step was mainly used to collect the mixed reaction data from cone and rod cells [flash: 3.0 cd·s/m2, chromaticity (0.33, 0.33;0.1 Hz) backlight: 0 cd/m2]. Many waveforms are present in the response wave, the most important of which are the first two waveforms: a negative waveform (a‐wave) followed by a positive waveform (b‐wave). The amplitude of a‐wave is measured from the baseline down to the base of a‐wave,and the amplitude of b‐wave is measured from the base of a‐wave to the peak of b‐wave. A wave reflects the activity of photoreceptor cells, and its amplitude mainly reflects the function of rod cells. The b‐wave reflects the function of the inner layer of the retina (the bipolar cells of rods and cones and Müller cells). The amplitude (the distance from peak to trough)was used as the main reference index to evaluate the responses of cones, rods and Müller cells, and to explore the difference in ocular visual function between patients with ATD and the normal control group.
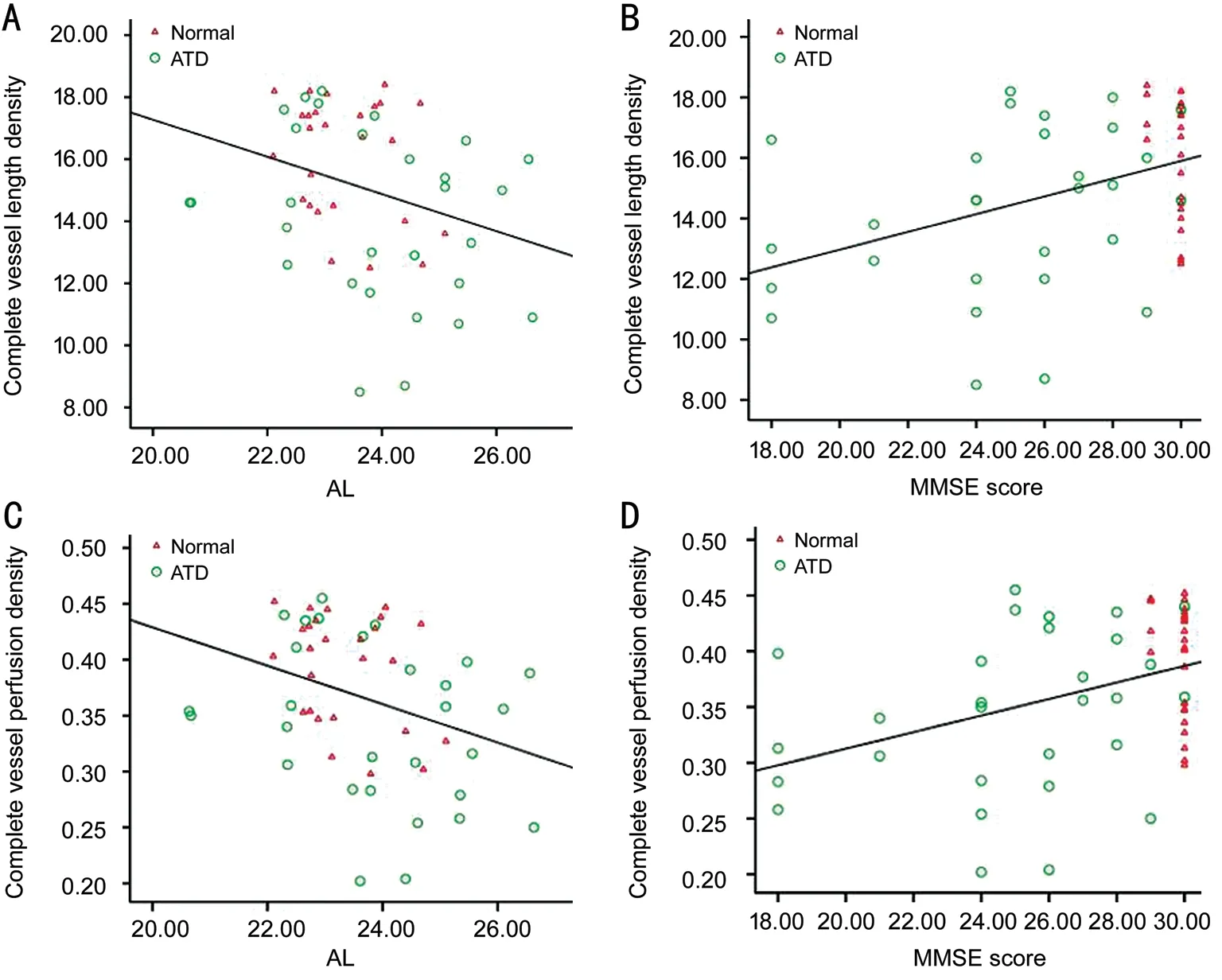
Figure 4 Scatter plots showing the correlations between the complete vessel length density and axial length (A), complete vessel length density and MMSE score (B), complete vessel perfusion density and axial length (C), and complete vessel perfusion density and MMSE score (D).
Statistics AnalysisSPSS 20.0 software (SPSS Inc, Chicago,IL, USA) was used for the statistical analysis of all data. The measured data are reported as means±standard deviations, and the Shapiro‐Wilk test confirmed that all data from each group were normally distributed. Sex and eye distributions were assessed with the Chi‐square test. The independent samplet‐test was used to compare the basic information (age, IOP, ocular axis, CCT, MMSE, MoCA,etc.), vessel length density, vessel perfusion density, FAZ area and choroid thickness parameters between the ATD and normal control groups. The single factor regression analysis was used to determine the correlations between the parameters and basic data. Since MMSE and MoCA scores play an important role in ATD staging, the correlations between the scores for each assessment and various vessel parameters were further evaluated after correcting for other basic factors using a partial correlation analysis. In addition, we performed a multivariate linear regression analysis to explore the relevant factors influencing the vessel density, blood perfusion, FAZ area and choroid thickness.P<0.05 was considered statistically significant.
RESULTS
Comparison of Basic DataThe results are presented in Table 1. Twenty‐nine patients with ATD were enrolled,including 15 males (51.7%) and 14 females (48.3%). Twenty‐six normal healthy individuals were recruited at the sametime, including 10 males (38.5%) and 16 females (61.5%).No significant differences were identified in gender, eye distribution age, IOP, axial length, and CCT between the two groups (allP>0.05). Statistically significant differences in MMSE and MoCA scores were observed between the ATD group and the normal control group (bothP<0.001).

Table 1 The basic clinical data between ATD group and control group
Analysis of Surface Retinal Vessel ParametersWith the exception of the outer temporal and outer nasal regions, the vessel length density and vessel perfusion density in other regions were significantly different between the two groups(allP<0.05). Tables 2 and 3 provide a detailed description of the surface retinal blood vessel parameters in different measurement areas.
A regression analysis was performed on all subjects in this study. Similar to the vessel length and perfusion density in some regions, with the exception of the outer temporal and outer nasal regions, the parameters of patients with ATD were lower than the control group. The complete vessel length density and the complete vessel perfusion density from representative regions were selected for the regression analysis (The independent variables are age, IOP, axial length, CCT, MMSE score and MoCA score). Univariate regression analysis revealed negative correlations between the complete vessel length density in the macular area with age and axial length (β=‐0.099,P=0.027 andβ=‐0.599,P=0.025,respectively). This parameter was positively correlated with the MMSE and MoCA scores (β=0.293,P=0.002 andβ=0.194,P=0.004, respectively). The complete vessel perfusion density in the macular area was negatively correlated with age and axial length (β=‐0.002,P=0.034 andβ=‐0.017,P=0.013,respectively). It was positively correlated with MMSE and MoCA scores (β=0.007,P=0.002 andβ=0.005,P=0.004,respectively). Significant correlations with other factors,including IOP and CCT, were not observed (allP>0.05). The independent variables withP<0.1 in the univariate regression analysis were included in the multivariate regression analysis.The results showed that the complete vessel length density in the macular area was negatively correlated with the axial length (β=‐0.527,P=0.034) and significantly positively correlated with the MMSE score (β=0.275,P=0.002). The complete vessel perfusion density in the macular area was negatively correlated with the axial length (β=‐0.015,P=0.016)and significantly positively correlated with the MMSE score(β=0.007,P=0.003). After adjusting for age and the MoCA score, the multivariate regression analysis revealed that the MMSE score and axial length were the most significant factors influencing the retinal vessel parameters in the superficial layer of the macular area in the two groups (Figure 4).
Foveal Avascular Zone and Choroidal ThicknessThe average FAZ area in the ATD group was 0.42±0.11 mm2and the value in the control group was 0.24±0.08 mm2; the difference statistically significant (P<0.001). A regression analysis was performed on all subjects in this study. The univariate regression analysis revealed negative correlations between the FAZ and the MMSE and MoCA scores (β=‐0.342,P=0.011 andβ=‐0.451,P=0.001, respectively). Significant correlations with other factors, including age, axial length, IOP and CCT, were not observed (allP>0.05). The independent variable withP<0.1 in the univariate regression analysis was included in multivariate regression analysis, and the FAZ was negatively correlated with MoCA score (β=‐0.011,P=0.001).The multivariate regression analysis showed that the MoCA score was the most significant factor affecting the FAZ area in the two groups after adjusting for the MMSE score (Figure 5).The average choroidal thickness in the macular area of the ATD group (203.68±14.20 μm) was thinner than in the control group (240.79±15.08 μm;P<0.001). A regression analysis was performed on all subjects in this study. According to the univariate regression analysis, the average choroidal thickness in the macular area was negatively correlated with age (β=‐0.803,P=0.009) and positively correlated with the MMSE and MoCA scores (β=5.638,P<0.001 andβ=4.053,P<0.001,respectively). Significant correlation with other factors including axial length, IOP, and CCT, were not observed (allP>0.05). The independent variable withP<0.1 in the univariate regression analysis was included in the multivariate regression analysis, and the average choroidal thickness in the macular area was positively correlated with the MoCA score (β=4.053,P<0.001). The multivariate regression analysis showed that the MoCA score was the most significant factor affecting the average choroidal thickness of the macular area in the two groups after adjusting for age and the MMSE score.

Table 2 Comparison of vessel length density in different measurement areas of macular area between ATD group and control group mm‐1

Table 3 Comparison of vessel perfusion density in different measurement areas of macular area between ATD group and control group mean±SD

Figure 5 Scatter plot of the correlations between the FAZ area and MoCA score (A) and between the choroidal thickness and MoCA score (B).
Differences in Flash ElectroretinogramsFlash ERGs were recorded with the RETeval system for all enrolled subjects.The average amplitude of the ATD group (130.97±21.73 μV)was smaller than the control group (149.54±30.90 μV;t=‐2.199,P=0.034). Based on the flash ERGs, patients with ATD have a lower response to the flash in the dark than the control group.
DISCUSSION
The retinal vessel system including radial capillary network near nipples, shallow vessel plexus and three parts of the deep vessel plexus[10], the deep shallow vessel plexus and vessel plexus near the centre concave and macular area of avascular area, called the FAZ[11]. In previous studies, the FAZ area was enlarged in patients with diabetic retinopathy and macular branch vein occlusion, according to an FFA examination.Moreover, the FAZ is extremely susceptible to changes in local blood supply, and an increase in the FAZ area is a sign of ischaemia. Thus, the blood supply of the retina can be measured by observing the changes in the FAZ area[12‐13]. In our study, the FAZ area was significantly increased in patients in the ATD group compared with the control group. Based on this result, the blood supply of the shallow retina is affected by such diseases as ATD, showing a decreasing trend. The results of this study should be confirmed using colour Doppler imaging (CDI).
After discovering statistically significant differences between the aforementioned indicators, our research began to focus on the relevant influencing factors. Basic data were collected for all subjects, including age, IOP, axial length, CCT, MMSE score, and MoCA score. According to the univariate linear regression analysis, the surface vessel parameters, complete vessel length density and complete vessel perfusion density were negatively correlated with age and axial length, and positively correlated with the MMSE and MoCA scores.Age and axial length were the two main factors affecting the surface vessel parameters of the macular area in previous studies[14‐17], and these results were consistent with univariate correlation analysis. Therefore, when we explore the effects of diseases such as ATD on retinal blood vessels, the difference in mean values must be considered when determining the effects of confounding factors. After adjusting for age and MoCA scores, the MMSE score was positively correlated with the parameters, and the axial length was negatively correlated with the parameters in the multivariate regression analysis. The MMSE score and axial length were the most significant factors affecting the surface retinal vessel parameters in the macular area. As one of the diagnostic criteria for clinical ATD, the MMSE score can be used as an index of disease progression.We concluded that the severity of ATD disease progression is significantly correlated with surface retinal vessel parameters in the macular area. In the comparative analysis of FAZ and CT,after considering the combined effects of confounding factors,the MoCA score was significantly negatively correlated with FAZ. MoCA is also one of the diagnostic criteria for ATD, and a low score also indicates an increasing severity of the disease.Therefore, we postulate that the degree of progression of ATD disease is more serious, and the distribution of the vasculature to the surface of the retina in the macular area will gradually decrease.
The MMSE score comprehensively, accurately and quickly reflect the subject’s mental state and level of cognitive impairment.The MMSE score provides scientific evidence for a clinical psychology diagnosis, treatment and neuropsychological research. This score is widely used at home and abroad, and it is the preferred scale for dementia screens[18‐23]. The MoCA score is an assessment tool used to quickly screen for cognitive dysfunction. A screen for mild cognitive impairment (such as amnestic cognitive impairment) and suspected dementia in a single cognitive domain is more sensitive. The cognitive domain is more extensive and comprehensive, the score distribution is more reasonable. The scores for visual space and executive function are improved, the memory test is more reasonable, the number and difficulty of words are increased,and the time of delayed recall is prolonged. MoCA reflects the true state of the patient’s memory. Although the two scoring systems have advantages, experts and scholars at home and abroad indicate that the MoCA score is more sensitive and accurate than the MMSE score[24‐27]. In our study, after adjusting for the confounding effects of multiple factors, the MMSE score was significantly associated with the surface retinal vessel parameters in the macular area, while the MoCA score was significantly associated with the area of the fovea in the macular area. As we mentioned above, the FAZ surface is actively affected by changes in local blood supply. Therefore,is the effect on the retinal blood supply caused by changes in the FAZ area more sensitive than changes in the surface retinal blood vessel parameters? In addition, does this difference in sensitivity coincide with the difference in sensitivity between the MoCA and MMSE scores? In‐depth investigations of big data will be worthwhile. In addition, the differences in statistical correlations may also provide corresponding recommendations for the current clinical diagnosis of ATD.The combination and complementation of the two scoring systems might provide a better method for AD diagnostic screening.
Since software that automatically divides and measures the choroidal thickness using OCT instruments is not available,most research projects still require the observer to manually draw the inner and outer lines of the choroid and use manual calipers to complete all measurements, which inevitably introduces certain measurement errors. All measurements about the choroidal thickness in this study were performed using a self‐designed image measurement software to minimize measurement errors. In our study, the mean choroidal thickness in the macular area of the ATD group was less than the control group, and the mean difference was statistically significant. After correction, univariate and multivariate regression analyses revealed a positive correlation between the MoCA score and the choroidal thickness in the macular area. The choroidal membrane accounts for 80%‐90% of the total blood flow to the eye and plays important roles in providing nutrition to outer layer of the retina and maintaining the structure and function of the retina. Amyloid deposits in the choroid, particularly in the choriocapillaris, have been reported in a histopathological study of a patient with primary systemic amyloidosis[27‐28]. On the other hand, Roybalet al[29]retrospectively analysed 4 patients with amyloid‐induced chorioretinopathy and reported a thicker hyporeflective choriocapillaris band in the OCT images that was caused by the accumulation of amyloid deposits. Because amyloid deposits are typical pathological manifestations of ATD,the decrease in the choroid thickness may be related to choroid atrophy secondary to amyloid angiopathy. Under physiological conditions, both the choroidal circulation and retinal circulation are involved in meeting the metabolic requirements of photoreceptors for oxygen[30]. In addition,scintillation stimulation can increase the retinal vessel diameter and retinal blood flow[31‐35]. It’s obvious show that the retinal blood flow supply is closely related to the normal execution of its function. Researchers have speculated that the degree of progression of ATD is more serious, and the altered blood flow to the outer layer of the retina will decrease. According to several independent studies, patients with ATD present with different types of visual dysfunction, such as decreased colour vision sensitivity and contrast sensitivity defects[2‐4,36]. In our study, a new and convenient RETeval system was used to record flash ERGs, and the amplitude (peak to trough distance,b wave) was used as a primary reference to evaluate the response of cones, rods and Müller cells. The retinal response to flash stimuli decreased in patients with ATD. This result is consistent with our hypothesis about the obstruction of choroid blood flow. Although the research in this area is not extensive,our findings provide a potential insight into the relationship between blood supply and visual function of patients with ATD, but further studies are needed in the future.
This research also has some limitations. First, the analysis of vessel parameters was limited to the inner boundary membrane layer and the inner plexus layer; we were unable to obtain the deeper retinal vessel parameters due to the limitations of the analysis software. Second, as a non‐invasive technique for measuring choroidal thickness, EDI‐OCT has excellent repeatability. However, it only reflects a static structure rather than dynamic changes in blood flow and does not accurately describe the haemodynamic characteristics of the choroid. Therefore, some important information may be lost.Third, some researchers have shown dynamic changes in the choroidal thickness in 24h[37‐39]. Although the imaging data collected from subjects in our study were mainly recorded in the afternoon (14:00‐17:00), errors were not able to be completely excluded. Moreover, we manually measured the choroidal thickness at several sites and thus were unable to completely exclude measurement error and local choroidal changes. These limitations are supported by technological innovations that automatically delineate choroidal regions and measure thickness, and we anticipate that more studies of this topic supported by new technologies will be published in the future. Fourth, the data of flash ERGs varied substantially.Conditions permitting, a larger number of subjects will effectively improve the reliability of the data.
In contrast to the traditional ATD diagnostic approach assessing changes in brain and neural function, we examined the changes in the microcirculation of the retina and fundus of patient with ATD, a degenerative disease, using OCTA in the present study.Our selected subjects tended to be patients with early‐ and middle‐stage ATD, and the changes in the microcirculation observed using OCTA may be auxiliary diagnostic criteria for ATD. Using non‐interventional methods, ATD screening was conducted at the early stage to achieve the early prevention and administer clinical interventions for ATD. On the other hand,a study assessing the long‐term follow‐up of patients with ATD using OCTA also has potential for development and may provide a more convenient and intuitive conclusion regarding the effect of treatments on ATD. OCTA has strong prospects for the clinical diagnosis and follow‐up of ATD.
In conclusion, compared with normal controls, patients with ATD exhibited decreases in the vessel parameters of the surface of retina, an increase in the area of FAZ, and a decrease in the choroidal thickness. These results suggest changes in microcirculation in patients with Alzheimer’s disease. These changes are likely to be related to amyloid deposits caused vascular atrophy or increased blood flow resistance. Moreover, these indicators were significantly correlated with the MMSE score and the MoCA score. The severity of the disease is involved in the process of changes in the microcirculation of the fundus. The flash ERG showed that people with Alzheimer’s disease exhibited a reduced retinal function compared to normal people. OCTA may be an adjunctive diagnostic standard for cognitive disorders and may play important roles in follow‐up and evaluating the efficacy of medications.
ACKNOWLEDGEMENTS
Conflicts of Interest:Li ZB,None;Lin ZJ,None;Li N,None;Yu H,None;Wu YL,None;Shen X,None.
 International Journal of Ophthalmology2021年6期
International Journal of Ophthalmology2021年6期
- International Journal of Ophthalmology的其它文章
- A decrease in macular microvascular perfusion after retinal detachment repair with silicone oil
- Surgical outcomes in acute dacryocystitis patients undergoing endonasal endoscopic dacryocystorhinostomy with or without silicone tube intubation
- Dr. Father Wacław Szuniewicz, a forgotten pioneer in refractive surgery and his work in China
- Deterioration of Avellino corneal dystrophy in a Chinese family after LASlK
- A mutated CRYGD associated with congenital coralliform cataracts in two Chinese pedigrees
- Autophagy dysregulation mediates the damage of high glucose to retinal pigment epithelium cells
