Role of bevacizumab intraocular injection in the management of neovascular glaucoma
Fawaz Al Sarireh, Hamzeh Mohammad Alrawashdeh, Khalid Al Zubi, Khalil Al Salem
1Department of Ophthalmology, College of Medicine,University of Mutah, Karak 61710, Jordan
2Department of Ophthalmology, Sharif Eye Centers, Irbid 410739, Jordan
Abstract
● KEYWORDS: bevacizumab; intraocular injection;neovascular glaucoma; rubeosis irides
INTRODUCTION
Iris neovascularization (NV) is the growth of abnormal blood vessels in response to multiple retinal ischemic diseases. Ischemia is responsible for high levels of hypoxia‐inducible factors, which upregulate vascular endothelial growth factors (VEGF) leading to the formation of fibrovascular membranes over the angle structures; these membranes impair the aqueous outflow resulting in neovascular (rubeotic) glaucoma. Controlling the elevated intraocular pressure (IOP) and its effect includes the control of the underlying cause of ischemia and the destruction of the ischemic retinal cells using different methods, such as retinal photocoagulation or cryotherapy, to treat the peripheral retina,besides cyclodestructive procedures, which aim to decrease aqueous production. Moreover, filtering and shunt procedures are employed to improve the drainage and overcome the angle obstruction.
Intraocular use of bevacizumab (Avastin®; Genentech, Inc.,South San Francisco, CA, USA) reduces ocular ischemia and inhibits angiogenesis by decreasing the effect of VEGF as well as the inflammatory effect associated with leakage. Therefore,many short and long‐term studies have been conducted to investigate its efficacy in the management of ischemic ocular conditions[1‐8]. This study aims to evaluate the long‐term outcome after adjuvant intraocular bevacizumab application in patients with neovascular glaucoma, and its effectiveness in controlling the IOP, decreasing the use of antiglaucoma medications and controlling the pain.
SUBJECTS AND METHODS
Ethical ApprovalThirty‐four eyes of neovascular glaucoma patients who received treatment at the ophthalmic clinic in the Al Karak Governmental Hospital and the eye clinic in the Italian Hospital, in the south district of Jordan in the period between June 2017 and May 2018 were retrospectively reviewed. The ethical committee (IRB) at Mutah University approved this study (Ethical code number: 201710, June 4,2017). Inclusion criteria were the presence of neovascular glaucoma, rubeosis, written informed consent, and follow‐up data for 12mo. All eyes with neovascular glaucoma were included regardless of the visual acuity, even eyes with no light perception [NLP; the best‐corrected visual acuities (BCVA) of the eyes were ranging from 6/60 to NLP]. The study protocol adhered to the guidelines of the Declaration of Helsinki, as revised in Tokyo and Venice. For statistical analysis, SPSS IBM 25 version was used.
Off‐label use of intravitreal bevacizumab was not offered for patients with uncontrolled hypertension and renal dysfunction in addition to those with a history of thromboembolic events(cerebrovascular insult and myocardial infarction). Patients who failed to sign an informed consent agreement or were unable to continue 12mo of follow‐up were also excluded.Thirty‐four eyes of 34 patients were included. The possibility of injection repetition and all risks of intraoperative and postoperative complications, including endophthalmitis and retinal detachment, were explained. The off‐label use of intraocular bevacizumab was made clear, after which,informed consent agreements were obtained from patients.
A complete ophthalmic evaluation was performed at the first visit (baseline) and during follow‐ups, including BCVA,slit‐lamp biomicroscopy, IOP measurements (Goldmann tonometer), and angle examination using a Zeiss gonio lens.BCVA was measured with ETDRS charts at four meters at the first two weeks, the first month, and the third, sixth, and twelfth month.
Grading of the iris NV depended on the grading system for rubeosis iridis proposed by Teich and Walsh (Teich, 1981), as follows: 0) No iris NV; 1) Less than two quadrants of NV at iris pupillary zone; 2) More than two quadrants of NV at iris pupillary zone; 3) Grade 2+ less than three quadrants of NV at iris ciliary zone and/or ectropion uveae; 4) More than three quadrants of NV at iris ciliary zone and/or ectropion uveae.
The decision for re‐injection was made and individualized for each patient. Generally, bevacizumab injections were repeated when clinically persistent iris NV was detected by slit‐lamp biomicroscopy with evident IOP elevation. The injection was augmented by laser photocoagulation or cyclodestructive procedures, relying on the degree of retinal ischemia according to fluorescein angiography (FA) and the underlying etiology.Intravitreal injections of bevacizumab were done in the operating room under aseptic conditions. Povidone iodine 5%was used to disinfect the eyelids, eyelashes and conjunctival ocular surface. Oxybuprocaine hydrochloride 0.4% topical anesthetic was installed in the conjunctival sac and a lidspeculum inserted. Patients received intravitreal injection of 1.25 mg of bevacizumab (Avastin®; Genentech Inc.) in 0.05 mL volume 3.5 mm from the limbus. The main outcome was the change in the rubeosis degree and IOP at 12‐month follow‐up. Pain control, numbers of antiglaucoma medications, or additional interventions after injection were the secondary outcomes.

Table 1 Causes of iris neovascularization

Table 2 Previous treatment
RESULTS
In total, 34 eyes receiving bevacizumab for neovascular glaucoma were reviewed. Neovascular glaucoma had been caused by proliferative diabetic retinopathy (n=25), ischemic retinal vein occlusion (n=8) and retinal ischemia caused by detachment (n=1; Table 1). Seventeen of the 34 patients were male (50%) and 17 female (50%). The duration of follow‐up was at least 12mo. The mean age was 56.76y (range: 32‐72y). The 34 eyes received an average of 4.97 injections each(range: 3‐7). Repeated injections were performed in all eyes.All of the eyes had received at least one treatment for neovascular glaucoma at baseline (Table 2). A history of previous laser photocoagulation was found in 17 (50%) eyes and pars plana vitrectomy had been previously performed in 6 (18%), at baseline. Eleven (32%) patients had received intravitreal bevacizumab injection previously. At baseline,all patients were using at least one topical antiglaucoma medication (3.53±0.563).
At baseline, three (8.8%) eyes had rubeosis grade 4, 18(52.9%) had rubeosis grade 3, 12 (35.3 %) had rubeosis grade 2, and one (2.9%) presented with rubeosis grade 1. At the last follow‐up, no rubeosis was detectable in 13 (38.2%) eyes.Partial regression was seen in the remaining 21 (61.8%; Table 3).Mean IOP at baseline was 45.32±7.185 mm Hg (range 29‐57 mm Hg), 95% confidence interval (CI) 22.8‐31.0 mm Hg and decreased to 27.88±5.515 mm Hg (range 17‐42 mm Hg) during the first 2wk; to 29.5±5.848 mm Hg (range 18‐39) after 1mo;to 26.82±5.529 mm Hg (range 18‐40 mm Hg) after 3mo; and to 26.06±5.752 mm Hg (range 17‐43 mm Hg) at 6mo. At 12mo after the first injection the mean IOP was 26.15±5.679 mm Hg(range 17‐41 mm Hg, 95%CI 12.3‐17.2 mm Hg). Reduction of IOP was significant during the first 2wk (P=0.000), at month 3 (P=0.000), month 6 (P=0.000) and month 12 after the first injection (P=0.0000005).
All patients included in this study had been treated previously with panretinal photocoagulation (PRP), intravitreal Avastin,or pars plana vitrectomy (PPV). However, the existence of rubeosis confirmed the insufficiency of previous treatments.Besides, earlier FA done for those patients showed large areas of capillary nonperfusion (retinal ischemia). During the study,rubeosis regression and retinal ischemia were monitored clinically and by FA.
Sixteen eyes (47%) had no further therapy, apart from the intravitreal injections. In 18 (53%) eyes, which were found to have extensive peripheral ischemia on FA within the follow‐up period, further treatment with retinal photocoagulation or retinocryopexy was conducted in addition to bevacizumab injections. Of those eyes, 12 (35%) eyes received retinal photocoagulation, and 6 (18%) eyes had cyclodestructive procedure (Table 4). The significant regression in rubeosis, as well as better adjustment of both IOP and pain, confirmed that these additional procedures enhanced the effect and response to intravitreal bevacizumab injections in the treated eyes.
During the follow‐up period, 5 (15%) patients received 7 injections, 9 (26%) received 6 injections, 4 (12%) received 5 injections, 12 (35%) received 4 injections and 4 (12%)received 3 injections (Table 5).
At presentation, all the patients had a degree of pain. By the 12thmonth of follow‐up, 24 (71%) were pain‐free and 10(29%) continue to complain about pain. A subjective numerical rating scale from 0 to10 was used to categories patients according to the severity of pain (Table 6).
One patient stopped antiglaucoma therapy. Topical antiglaucoma continued to be used in the remaining patients with a decrease in the number of medications. Patients using three medications or less numbered 32 (96%) at the last follow‐up, while at the beginning, 19 (56%) patients were on four antiglaucoma medications (Table 7).
No progression of rubeosis was observed, and no intraoperative or postoperative complications were encountered during the period of follow‐up.
DISCUSSION
Anti‐VEGF has some role in managing neovascular glaucoma due to its anti‐inflammatory effect along with its ability to inhibit the neovascular activity and decrease capillary permeability. Bevacizumab is a humanized, full‐length monoclonal antibody directed against all the biologically active isoforms of vascular endothelial growth factor (VEGF‐A)[9‐10], andits safety for intraocular use has been confirmed[11‐12]. Many short‐term studies have shown the fast and dramatic effect of intraocular bevacizumab on decreasing the leakage from iris vessels and reducing iris and angle NV due to ischemic retinal disorders, such as diabetic retinopathy and retinal vein occlusion[13‐15].
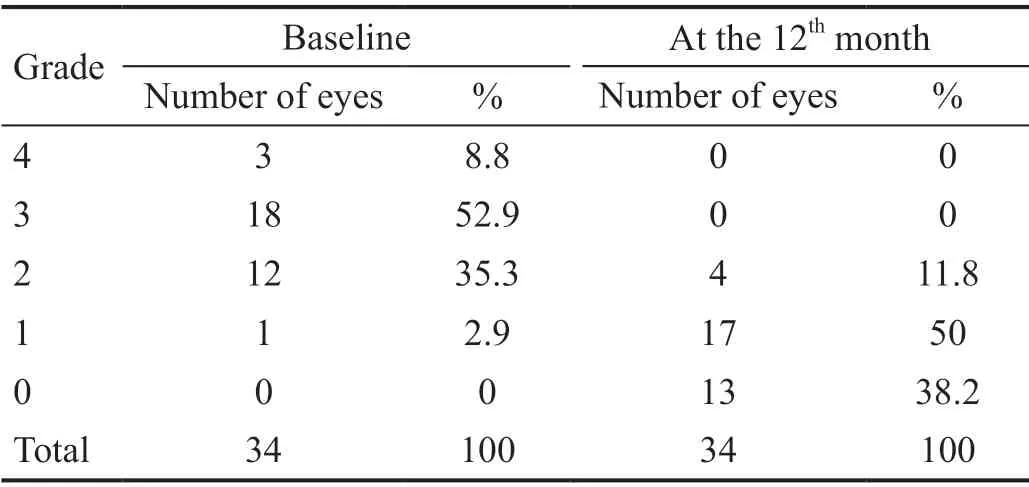
Table 3 Grade of rubeosis at baseline and 12mo
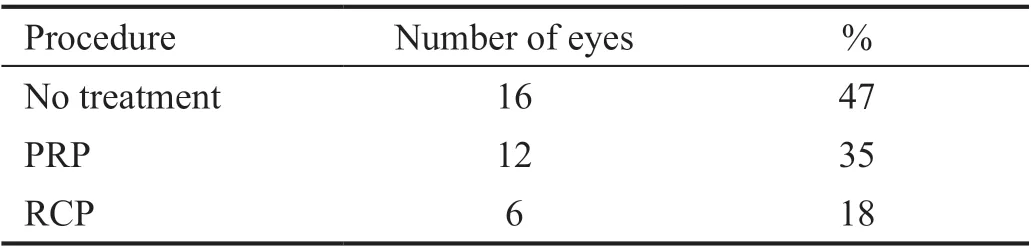
Table 4 Further procedures

Table 5 Total number of injections

Table 6 Pain

Table 7 Number of medications
Many of these studies involved a small number of participants or covered short periods of numbers of follow‐ups[1,3,5].
These studies, and many others, failed to show the dosage of bevacizumab and the proper timing for the adjunctive treatment methods. This study, as did other studies, repeatedly showed the response of iris NV, due to different ischemic retinal diseases, to anti‐VEGF treatment and investigated the long‐term safety of this therapy regarding the ocular and systemic complications during long‐term follow‐up.
Understanding the intraocular bevacizumab pharmacokinetics is crucial in determining the appropriate therapeutic plan. The regression of iris and retinal NV starts after two days following intraocular injection. The action of bevacizumab continued for about four weeks after the intracameral route, and 6‐8wk after the intravitreal route[16‐20]. Since the effect of intravitreal injections of bevacizumab lasts longer, in both anterior and posterior segments, than in intracameral injections, all patients in this study underwent only intravitreal injections of bevacizumab to cover the anterior pathology for a longer period.
There is no general agreement on the dosage of intraocular bevacizumab needed to treat neovascular glaucoma or whether it needs to be titrated according to the degree of rubeosis or ischemia[7,21‐22]. The dosage used ranged from 1.0 mg to 1.5 mg. In this study, the dose of 1.25 mg was used based on many studies regarding safety and efficacy[23‐26]. A rapid and significant(30% drop from the primary) drop in IOP was noticed using the 1.25 mg dose in 22 (65%) eyes after two weeks and,accordingly, this dose seems to be adequate to decrease VEGF activity transiently.
Depending on the stage of the underlying disease and previous treatment, it is important to deal with each patient individually.Sixteen (47%) eyes continued to be treated by bevacizumab injection and topical antiglaucoma without further intervention.Eighteen eyes (53%) required additional interventions, of which 12 eyes (35%) received retinal photocoagulation and six eyes (18%) received retinocryopexy (Table 4), which helps in decreasing the production of VEGF in eyes with uncontrolled IOP and persistent rubeosis. The adjunction between bevacizumab injection and other procedures to decrease the production of VEGF helps in extending the therapeutic window to give more time for these additional procedures to reveal their effects. Long‐term control of IOP and pain and a reduction in the number of antiglaucoma agents was possible during the follow‐up period. A decrease in the number of medications was found in 32 (94%) eyes, while pain control was achieved in 24 (71%).
This is a retrospective study with several limitations,such as the lack of a control group, the inability to have a standardized protocol regarding the timing and type of further intervention, a categorization of NV stage, and the necessary number of injections, besides being examiners dependent on concerning the decision for re‐injection and adoption of further intervention. Nevertheless, it showed that the angiogenic activity, vascular permeability and inflammatory process associated with retinal ischemia could be decreased by combining the repeated bevacizumab injections and other procedures. Managing neovascular glaucoma depends on reducing the inflammatory and angiogenic activity by anti‐VEGF, in combination with other destructive procedures, to have a faster and more sustainable effect.
The combination of inhibitors of angiogenesis drugs with other procedures can be adapted to treat different stages of neovascular glaucoma. In the early stages of NV, the adjunctive use of bevacizumab with the other procedures can prevent the development of more advanced complications,such as adherent synechiae. In advanced stages, the use of bevacizumab can decrease the angiogenesis‐associated effects,such as leakage and inflammatory processes, helping to control the angiogenesis and improve the response to further destructive procedures, leading to better IOP control. As we treat these cases, we should remember that the anti‐VEGF agents alone do not change the primary cause of ischemia,and the recurrence of NV is inevitable if the primary cause of retinal ischemia is not targeted. In unresponsive cases, more aggressive cyclodestructive procedures or filtering procedures may be needed.
In conclusion, the intravitreal injection of bevacizumab can be used as an adjunctive inhibitor of angiogenesis in patients with neovascular glaucoma. The earlier the initiation of treatment in the early stages of rubeosis, the better the chance of controlling angiogenesis. In severe cases of rubeosis, this therapy can be combined with other conventional procedures to reduce the IOP and have better pain control.
ACKNOWLEDGEMENTS
Conflicts of Interest: Al Sarireh F,None;Alrawashdeh HM,None;Al Zubi K,None;Al Salem K,None.
 International Journal of Ophthalmology2021年6期
International Journal of Ophthalmology2021年6期
- International Journal of Ophthalmology的其它文章
- A decrease in macular microvascular perfusion after retinal detachment repair with silicone oil
- Surgical outcomes in acute dacryocystitis patients undergoing endonasal endoscopic dacryocystorhinostomy with or without silicone tube intubation
- Dr. Father Wacław Szuniewicz, a forgotten pioneer in refractive surgery and his work in China
- Deterioration of Avellino corneal dystrophy in a Chinese family after LASlK
- A mutated CRYGD associated with congenital coralliform cataracts in two Chinese pedigrees
- Autophagy dysregulation mediates the damage of high glucose to retinal pigment epithelium cells
