Therapeutic applications of dental pulp stem cells in regenerating dental, periodontal and oral-related structures
Mohammed E Grawish, Mahmoud A Saeed, Nessma Sultan, Ben A Scheven
Mohammed E Grawish, Nessma Sultan, Department of Oral Biology, Faculty of Dentistry, Mansoura University, Mansoura 35516, Egypt
Mohammed E Grawish, Mahmoud A Saeed, Department of Oral Biology, Faculty of Oral and Dental Medicine, Delta University for Science and Technology, Mansoura 11152, Egypt
Ben A Scheven, School of Dentistry, Oral Biology, College of Medical and Dental Sciences, University of Birmingham, Birmingham B5 7EG, United Kingdom
Abstract Dental pulp stem cells (DPSCs) have emerged as a promising tool with great potential for use in tissue regeneration and engineering.Some of the main advantages of these cells are their multifaceted differentiation capacity, along with their high proliferation rate, a relative simplicity of extraction and culture that enables obtaining patient-specific cell lines for their use in autologous cell therapy.PubMed, Scopus and Google Scholar databases were searched for relevant articles related to the use of DPSCs in regeneration of dentin-pulp complex (DPC), periodontal tissues, salivary gland and craniomaxillofacial bone defects.Few studies were found regarding the use of DPSCs for regeneration of DPC.Scaffold-based combined with DPSCs isolated from healthy pulps was the strategy used for DPC regeneration.Studies involved subcutaneous implantation of scaffolds loaded with DPSCs pretreated with odontogenic media, or performed on human tooth root model as a root slice.Most of the studies were related to periodontal tissue regeneration which mainly utilized DPSCs/secretome.For periodontal tissues, DPSCs or their secretome were isolated from healthy or inflamed pulps and they were used either for preclinical or clinical studies.Regarding salivary gland regeneration, the submandibular gland was the only model used for the preclinical studies and DPSCs or their secretome were isolated only from healthy pulps and they were used in preclinical studies.Likewise, DPSCs have been studied for craniomaxillofacial bone defects in the form of mandibular, calvarial and craniofacial bone defects where DPSCs were isolated only from healthy pulps for preclinical and clinical studies.From the previous results, we can conclude that DPSCs is promising candidate for dental and oral tissue regeneration.
Key Words: Dental pulp stem cells; Dentin-pulp complex; Periodontal tissues; Salivary glands; Cell-based therapy; Cell-free therapy
INTRODUCTION
The oral cavity is a complex multi-organic structure, where oral tissues and organs are linked developmentally and functionally.Irreversible damage to any of them is more likely to affect the others, causing extensive malfunction.Tooth decay, periodontal diseases, alveolar bone resorption, and impaired salivary gland (SG) function are conditions that seriously affect oral health.Synthetic materials have traditionally been used as the treatment of choice in prosthetic and restorative dentistry.However, this form of replacement does not regenerate the original biological tissues and other organs, such as SGs, are simply not responsive to mechanical substitution approaches.Thus, tissue engineering (TE) is emerging as a new therapeutic choice for the complete biological regeneration of craniomaxillofacial tissues and organs.Three critical components are required: (1) stem cells; (2) scaffolds; and (3) stimulating factors or inductive signals[1].
Dental pulp stem cells (DPSCs) are neural crest-derived cells with an excellent ability to differentiate along multiple cell lineages (Figure 1).In particular, simplein vitroprotocols can be used to achieve highly efficient osteo/dentinogenic differentiation of DPSCs, making these cells a very attractive and promising tool for the future treatment of dental and periodontal diseases.Among oral-related structures, regeneration of the SGs and bone defects appears to be possible using DPSCs, as researchers over the last decade have made substantial progress in experimental models of partial or total regeneration of both organs[2].Emerging approaches using cell-free therapy with cell extracts or secretome components also exhibit favorable outcomes and when compared to cell-based approaches, the secretome can be relatively easily obtained, quantified and are more stable for long-term storage to be used in various TE fields[3].
In this review, we explored the potential usage of DPSCs in preclinical and clinical trials for the regeneration of different oral, dental and craniomaxillofacial tissues and organs.Therefore, PubMed, Scopus and Google Scholar databases were searched for relevant articles related to the use of DPSCs in regeneration of dentin-pulp complex (DPC), periodontal tissues, SG and craniomaxillofacial bone defects.We present the different strategies used in experimental regenerative models with potential applications in dentistry and oral medicine.Based on data extraction from the different databases, we can conclude that DPSCs may be an ideal choice to be used in experimental models of tissue-engineered reconstruction of organs of the oral cavity, and the paradigmatic cases of the tooth and the SG may constitute the leading edge of a massive research effort on next generation organ replacement therapies.
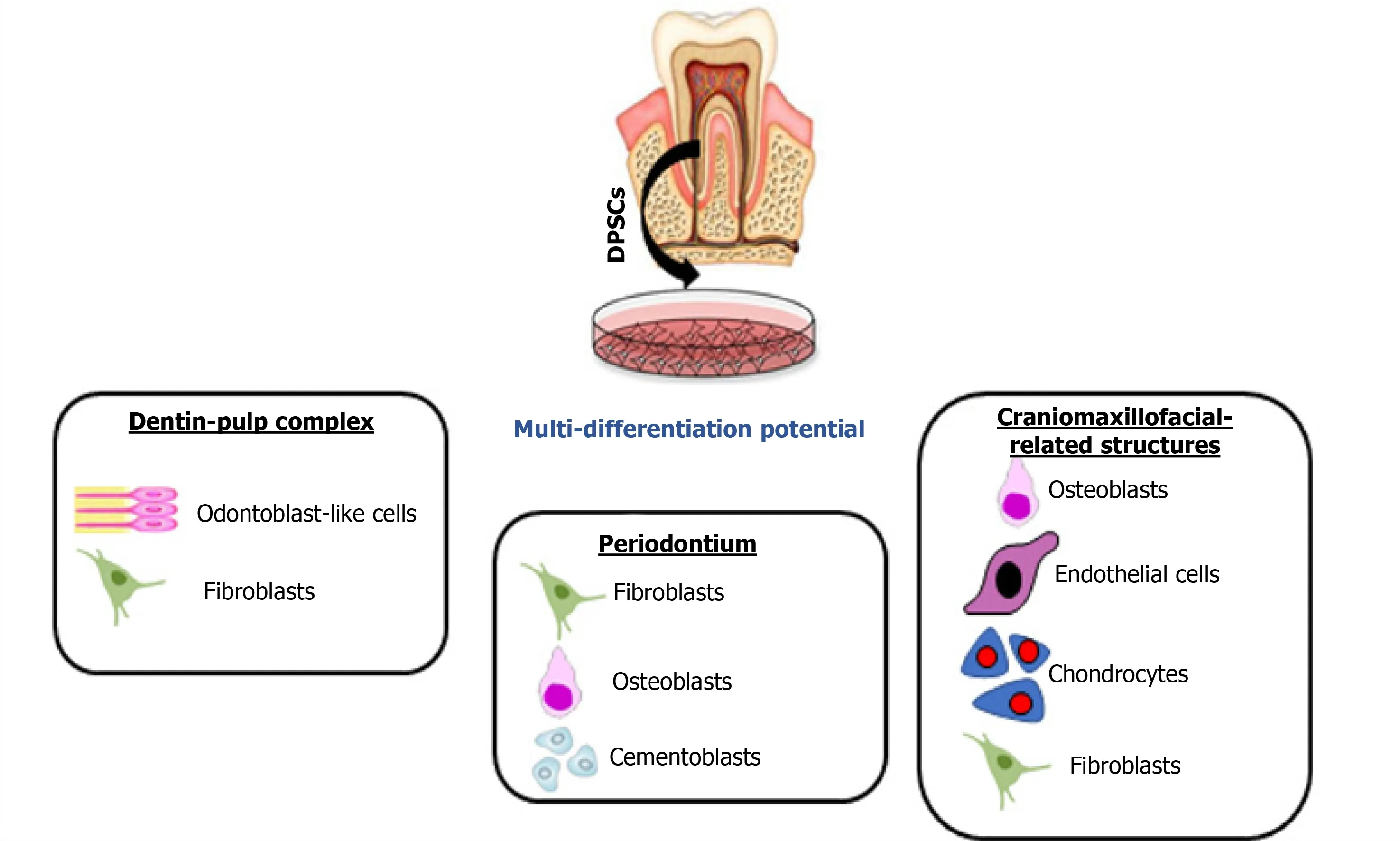
Figure 1 Different types of regeneration possible from dental pulp stem cells.
REGENERATION OF DPC USING DPSCS
Teeth are composed of various dental tissues,viz.enamel, dentin, pulp, and cementum.Dentin and pulp formulate the DPC that preserves tooth viability and integrity[4].On one hand, the pulp provides dentin with moisture, nutriment, and minerals to keep its resiliency and degree of mineralization; therefore, in the case of pulp necrosis, dentin gets dark and brittle with the sequelae of tooth discoloration and fracture.On the other hand, dentin provides a closed protecting barrier for the pulp against bacterial toxins and physical or chemical irritants; therefore, dentin damage or pulp exposure seriously threaten tooth vitality and integrity[5].
Following carious or traumatic injury to dentine-pulp, a complex interplay between host defense response, inflammatory and infective agents and endogenous repair processes occur.DPC is a difficult structure to manufacturein vitroor repairin vivoorex vivo.Embryologically, dentinogenesis starts with the secretion and cross basement membrane transport of signaling proteins from the differentiating ameloblasts during the early bell stage of tooth development.Amongst many cytokines and growth factors, the TGFβ is considered to initiate the dentinogenesis process through inducing odontogenic differentiation of the undifferentiated ectomesenchymal cells of the dental papilla[6].During development and after enamel maturation and tooth eruption, ameloblasts are lost forever; therefore, recreating the dentinogenesis process again in TE without ameloblasts is difficult due to the absence of the initiator.Histologically, the DPC is formed of two different tissues.The outermost layer of the dental pulp (DP) consists of odontoblasts that responsible for the formation of primary, secondary and tertiary dentin throughout life.The odontoblastic processes may extend from the odontoblasts through the whole thickness of dentin and may be embedded in the enamel as enamel spindles, dentin includes various noncollagenous proteins that control the activity of odontoblasts, fibroblasts and DPSCs[7].
Several biomaterials are used to repair the dentin and regenerate DPC, such as calcium hydroxide, adhesive resin cement, mineral trioxide aggregate, Biodentine, and emdogain.Actually, all these materials are developed on empirical bases, rather than on the deep understanding of the histological structure of the DPC or the molecular bases of the pulp self-healing mechanism[8].Consequently, the results of these previous studies showed the inability of these materials to regenerate the DPC, and only disorganized tissues were formed as a response[9].
The idea of regenerating dentin and pulp tissues is based on recruiting DPSCsviaseveral growth factors and scaffolds, what is known as the biological triad of tissue regeneration.The DPSCs have a crucial role in the dentinogenesis process as they are responsible for the reconstruction of the damaged dentin and pulp.These cells work under the influence of chemical/physical signaling mechanisms that induce stem cell differentiation to express the desired phenotype[10].DPSCs were successfully tested in preclinical and clinical studies for DPC regeneration.
Preclinical studies
DPSCs[11-14] and DP progenitor cells[12] have been isolated from permanent teeth pulps.In some instances, the cells were transfected with lentivirus virus-mediated gene transfer of platelet-derived growth factor (PDGF)[15].The strategy of using DPSCs was dependent on scaffold-based and scaffold-free therapies.DPSCs were loaded into the scaffold[12,13] or, incorporated into the scaffold and the combination was injected locally[11,14,15].Nude mice and minipigs were the most selected model for studying the regenerative effect of DPSCs for DPC regeneration in the pre-clinical trials.The cell number most commonly implicated was 1 × 106[11-13] and an 8 × 106[14] cell number was also used (Table 1).
DPSCs-derived from healthy pulp:Loading or injecting a cell suspension of DPSCs.Horibeet al[13] investigated the regenerative capacity of young and aged human (h)DPSCs on ectopic pulp regeneration.They injected 1 × 106cells combined with pepsin-solubilized collagen solution derived from porcine skin into the human tooth root model prior to subcutaneous transplantation into 5-wk-old severe combined immunodeficiency mice.Evaluation was dependent on histologic and histomorphometric analysis, 28 d after transplantation.They concluded that both young and old hDPSCs are promising for clinical applications in DP regeneration.
Wanget al[11] seeded 1 × 106hDPSCs on nanofibrous poly(L-lactic acid) (PLLA) scaffolds, 5.2 mm in diameter and 1.5 mm in thickness.The cell-scaffold constructs were induced in odontogenic media for 2 wk prior to subcutaneous implantation in nude mice with an age 6-8 wk.A cell-scaffold construct cultured in media without odontogenic inducers was used as the control group.The endpoint for this experiment was 8 wk after implantation.The histological examinations of the specimens illustrated formation of new blood vessels and collagen.The immunohistological examin-ation showed high expression of dentin sialoprotein that indicating odontogenic differentiation of hDPSCs.
Panet al[12] investigated the potential of the DP progenitors to regenerate DPC and, besides, they clarified the efficacy of stem cell factor (SCF) on DP progenitors for odontogenic differentiation and regenerative capability.They mixed 1 × 106DP progenitors with 3 mm × 3 mm × 3 mm collagen sponge sheets that were coated with 50 ng/mL SCF aqueous solution for a preclinical study.The other groups used in this study were collagen sponges with DP progenitors, collagen sponges with SCF, and collagen sponges only as a control.The cell-scaffold constructs were implanted into the back of male nude mice for 4 wk.After the endpoint of the experiment, the collagen sponges were dissected and prepared for histological examination.The results of this study revealed a high potentiality of the DP progenitors in DPC regeneration.In addition, using the SCF increased the regenerative capacity of DP progenitors.
Chaudharyet al[14] synthesized nanofibrous sponge microspheres (NF-SMS), which were used to carry hDPSCs into the pulp cavity to regenerate living DP tissues.They removed the DP from 16 molars collected from 2-wk-old rabbits.After that, they injected 8 × 106hDPSCs/NF-SMS complexes pre-cultured under hypoxic condition into the rabbit molar pulp cavities prior to subcutaneous implantation into nude mice with age of 6-8 wk, for 4 wk.The results of this study were evaluated histologically and immunohistochemically.The authors concluded that the hypoxia group significantly enhanced angiogenesis inside the pulp chamber and promoted the formation of odontoblast-like cells lining the dentin-pulp interface, as compared to the control groups, hDPSCs group, NF-SMS group, and hDPSCs/NF-SMS group precultured under normoxic conditions.
Zhanget al[15] explored the influence of the PDGF-BB on hDPSCs proliferation and odontogenic differentiation.They established a PDGF-BB gene-modified hDPSCs using lentivirus delivery vector.The genetically-modified hDPSCs were mixed with a porous calcium phosphate cement scaffold and implanted subcutaneously in nude mice for 12 wk.DPC regeneration was evaluated using histological and immunohistochemical analysis.The conclusion of this study was that the PDGF-BB gene-modified hDPSCs can continuously secrete the PDGF-BB protein enhancing dentin-like tissue formation.The formed dentin-like mineralized tissue showed positive staining for the dentin sialophosphoprotein, similar to tooth dentin tissue, and was surrounded by highly vascularized DP-like connective tissue.
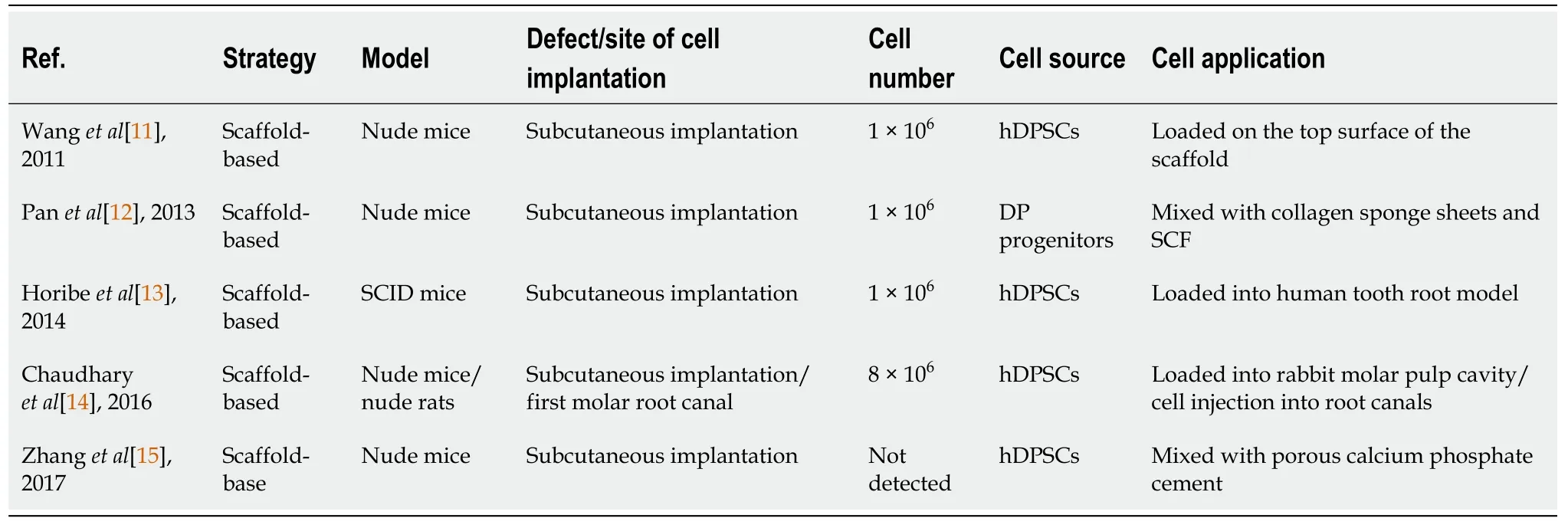
Table 1 Preclinical studies obtained from the PubMed, Scopus and Google Scholar databases related to dentin-pulp complex regeneration using dental pulp stem cells
In another experiment, Chaudharyet al[14] used the maxillary first molars of the nude rats as anin situpulp regeneration model.The entire pulp was removed and the root canals were cleaned and shaped using manual #15-#25 K files.After irrigation with phosphate-buffered saline and 2.25% NaClO and drying the root canals with absorbent points, 8 × 106hDPSCs/NF-SMS complexes primed under hypoxic conditions were injected into the prepared root canals, while those primed under normoxic conditions were injected into prepared contralateral first molar root canals.After 4 wk, the treated maxillary molars were harvested.For comparison, maxillary first molars received no treatment after pulp removal.The histological and immunohistochemical analyses of the specimens revealed that the hypoxia-primed hDPSCs/NF-SMS could fully fill the molar canals through injection and promoted pulp-like tissue formation with intimate integration with the native dentin.
REGENERATION OF PERIODONTAL TISSUES USING DPSCS
The periodontal tissues include two hard and two soft tissues.The two soft tissues are periodontal ligament and gingiva, while the two hard ones are alveolar bone lining the socket and cementum.The principal function of these structures is investing and supporting the teeth to perform their physiologic function.The most common cause for tooth loss in adults is the periodontitis, affecting 10%-15% of adults worldwide as an advanced type.Treatment modalities such as bone grafts[16], enamel matrix derivative[17], platelet-rich plasma (PRP)[18], and guided tissue regeneration[19] allow partial improvements in the architecture and function of the periodontal tissues but fail to completely regenerate this complex structure.Procedures attempting to regenerate the periodontal apparatus have become unconventional and more advanced to include dental tissue-derived stem cell therapy[20].Chiefly, there are two approaches for periodontal tissues regeneration, cell-based and cell-free therapies.The major concern for the cell-based therapy is the complicated implantation process and it includes stem cell injection of cell suspension and stem cell sheet transplantation techniques.Engineering of a cell sheet has been adopted through utilizing cell culture dishes of temperature-responsive surfaces[21], or by culturing the cells in ascorbic acid[22].Even though cultured cells can be used to study the pathogenesis of the inflammatory and immune pathways of periodontal diseases, the complicated host defense system basically responsible for these diseases is not amenable to being reproducedin vitro.Experimental periodontitis is a preclinicalin vivomodel for studying the possible application of cell therapies and biomaterials-targeted for periodontal tissues regeneration.They were considered to be more appropriate mimicking the natural host immune response under microbial loads, which can cause oral pathological environments and periodontal diseases[23].Animals like nonhuman primates, rodents, rabbits, pigs, and dogs have been selected for modeling human periodontitis.Commonly, periodontitis has been induced by placing a silk ligature inside gingival sulcus around the molar teeth to serve as a retentive factor for plaque and microbial proliferation.In addition, alveolar bone loss has been induced by injection or inoculation of human oral bacteria[24].DPSCs were successfully tested in preclinical and clinical studies for periodontal tissues regeneration.
Preclinical studies
Xenogeneic and autologous[25-32] DPSCs or their exosomes (Exo)[32] have been isolated from healthy[25-28,30-32] or inflamed pulps[29].In some instances, the cells were transfected with adenovirus-mediated gene transfer of hepatocyte growth factor (HGF)[27].The strategy of using DPSCs was dependent on scaffoldbased[25,29,30,32], sandwich structure[31], scaffold-free[26-28] and cell-free[32] therapies.Application of DPSCs was in the form of cell injection[26-28,30], cell sheet transplantation[27,28,31], loaded onto the scaffold[25,29] or, incorporated onto the scaffold and the combination was injected locally[32].Miniature pigs were the most selected model for studying the regenerative effect of DPSCs for periodontal tissues regeneration in the pre-clinical trials[26-29].Other animals such as rats[30], mice[31,32] and dogs[25] were also selected for the same purpose.Bone defects of 5 mm width, 7 mm length and 3 mm depth[26,27] and of 5 mm × 6 mm × 5 mm[28] were the most common surgical periodontal defect models created as a site of cell implantation (Figure 2).Ligature-induced periodontitis with three-walled periodontal defects (PDs)[25], PDs adjacent to the root of the second molars[29], periodontitis created using 5-0 silk ligature[32] were also applied as models.Bioroot regeneration was accomplished through subcutaneous transplantation of a sandwich structure constituted by hDPSCs sheet, human treated dentin matrix (hTDM)/hDPSCs and Matrigel/hDPSCs[31].The cell number of 1 × 107was the most commonly implicated[26-29], and 5 × 105[31], 2 × 106[30], 2 × 107[25] cell numbers were also used; in addition, 50 μg[32] of hDPSCs-Exo was indicated for the cell-free therapy strategy (Table 2).
DPSCs-derived from healthy pulp:(1) Loading or injecting a cell suspension of DPSCs.In a scaffold-based trial, Khorsandet al[25] surgically created three-walled PDs with ligature-induced periodontitis in 10 mongrel dogs.The PDs were produced bilaterally at the bone adjacent to the mesial surface of the mandibular first premolar teeth.They investigated the regenerative capacity of autologous dogs' DPSCs on periodontal tissues, with/without Bio-Oss granules.Four weeks after making the periodontitis model, Bio-Oss was engrafted on one side and considered as a control, while on the other side, a combination of 2 × 107autologous DPSCs of passage 3 with Bio-Oss was engrafted and served as the test group.Evaluation was dependent on histologic and histomorphometric analyses, 8 wk after surgery, in terms of cementum, periodontal ligament, and bone formation.The authors concluded that a biocomplex consisting of Bio-Oss scaffold and DPSCs would be advantageous in regeneration of periodontal tissues.Moreover, Linet al[30] investigated the regenerative and therapeutic effects of a daily dose, intragastrically administered 25 mg/kg of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside (THSG)-triggered hDPSCs in the healing of experimental PDs adjacent to the roots of the second molars in male Sprague-Dawley rats.THSG is a water soluble and biologically active component of the Chinese herbal medicinePolygonum multiflorum.The defects of the negative control were treated with Matrigel, while the defects of the positive control received 2 × 106hDPSCs/ Matrigel (vol/vol = 3:1).It was found that THSG revolutionized hDPSCs significantly and shortened the regenerative period of PDs by enhancing new bone formation and increasing expression levels of osteopontin, vascular endothelial growth factor, and proliferating cell nuclear antigen than those in other groups.
In a scaffold-free trial, Maet al[26] evaluated the injection of DPSCs or bone marrow stem cells (BMSCs) forin vivoperiodontal regeneration.The investigators created 12 PDs of 3 mm depth, 7 mm length, and 5 mm width in miniature pigs' interradicular bone of the first molars.The defects were divided into three groups, namely DPSCs, BMSCs and control group, each with four defects.The BMSCs and DPSCs groups were separately injected with approximately 1 × 107human cells in 0.6 mL of 0.9% NaCl, while the defects of the control group were injected with 0.9% NaCl as the cell injection group.Lymphocyte infiltration and impaired sulcular epithelia were evident in the control group but not in the DPSCs and BMSCs groups.Notably, the periodontal bone regeneration capacity was significantly greater for the DPSCs injection group compared to the BMSCs group.
(2) Cell sheet transplantation technique of DPSCs.In a scaffold-based trial, Menget al[31] created a sandwich structure of hDPSCs sheet/hTDM/Matrigel to be subcutaneously transplanted in nude mice for 3 mo.The main objective was to investigate the regeneration potential of hDPSCs, and the characteristics of the above materials for the tooth root regeneration (bioroot).The hDPSCs sheet was obtained by seeding 5 × 105hDPSCs in a culture dish containing complete medium supplemented with 20.0 μg/mL vitamin C.The hTDM was fabricated from healthy extracted teeth and a disk with 5 mm thickness was prepared to be successively decalcified and sterilized.The hTDM/hDPSCs complex was prepared by seeding hDPSCs on hTDM and culturedin vitrofor 2 d, and the Matrigel/hDPSCs suspension was injected into the complex’s pulp cavity.It was concluded that hDPSCs could be used as seed cells for the whole tooth root regeneration, and the sandwich structure constituted by hDPSCs sheet, hTDM/hDPSCs and Matrigel/hDPSCs could be utilized for tooth root regeneration.
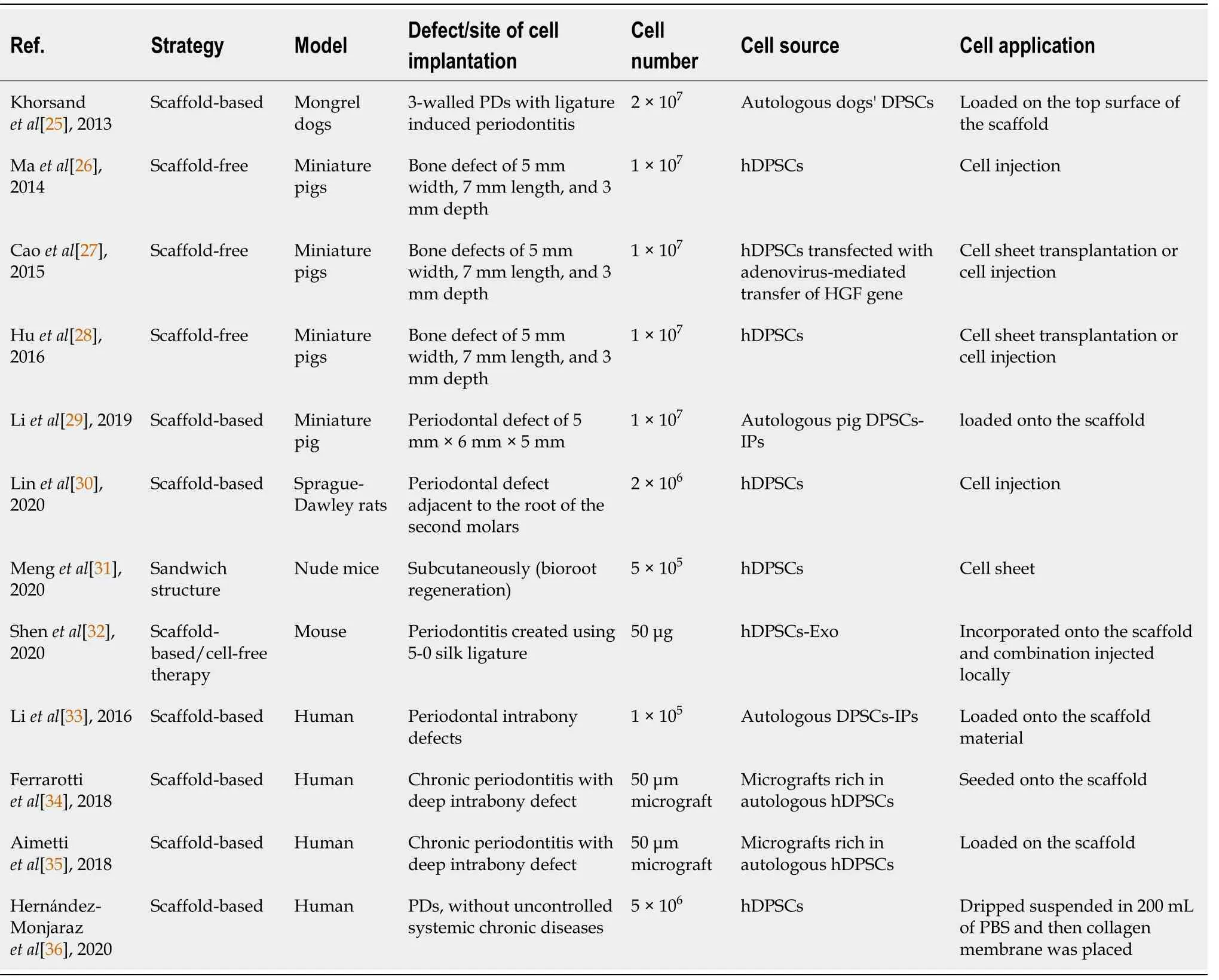
Table 2 Preclinical and clinical studies obtained from the PubMed, Scopus and Google Scholar databases related to periodontal tissue regeneration using dental pulp stem cells
In a scaffold-free trial, Caoet al[27] created 40 PDs of 3 mm depth, 7 mm length, and 5 mm width in miniature pigs, in the mesial region of the mandibular and maxillary first molars.Under good manufacturing practice, hDPSCs were transfected with adenovirus-mediated gene transfer of HGF to evaluate their potential roles for periodontal tissues regeneration.They found that injecting HGF-hDPSCs could significantly improve soft tissue healing and periodontal bone regeneration.In comparison to the injection of dissociated cells, HGF-hDPSCs and hDPSCs sheets showed superior periodontal tissue regeneration.The authors attributed their findings to an increase in blood vessel regeneration and a marked decrease of hDPSCs apoptosis caused by adenovirus-mediated gene transfer of HGF.They concluded that sheets are more suitable for the surgically-managed periodontitis treatments, as they required surgical placement.Moreover, Huet al[28] created 24 PDs of 3 mm depth, 7 mm length and 5 mm width in the mesial region of the mandibular and maxillary first molars of miniature pigs.The hDPSCs were applied using techniques of cell sheet transplantation or cell injection.The complete form of a morphologically cell sheet was produced by inducing hDPSCs with 20.0 μg/mL vitamin C.The cells of the cell sheet contacted with each other tightly and contained five or six layers.Both cell sheet transplantation and hDPSCs injection significantly regenerated soft tissues and periodontal bone.Compared with hDPSCs injection, the hDPSCs sheet had more bone regenerative capacity.
Figure 2 Periodontal bone defect.
Finally, (3) secretome-derived from DPSCs.In a scaffold-based trial, Shenet al[32] created a periodontitis mouse model using 5-0 silk ligature tied around the maxillary left second molar to evaluate the effect of chitosan (CS) hydrogel incorporated with hDPSCs-derived Exo.For Exo treatment, an equal volume of phosphate-buffered saline /CS, hDPSCs-Exo (50 μg), and hDPSCs-Exo/CS was injected locally after ligature removal.It was found that treatment with hDPSCs-Exo/CS alleviated periodontitisviamacrophage-dependent mechanism through miR-1246.hDPSCs-Exo facilitated the way for the macrophages to be converted from a pro-inflammatory phenotype to an anti-inflammatory phenotype, and suppressed periodontal inflammation and modulated the immune response.
DPSCs-derived from inflamed pulp:In a scaffold-based trial, Liet al[29] cultured an autologous miniature pig DPSCs derived from inflammatory dental pulp tissues (IPs).The cells were characterized and loaded onto β-tricalcium phosphate (β-TCP) biomaterial.The compounds were then engrafted into artificially-created PDs of 5 mm × 6 mm × 5 mm in the buccal region of root furcations of the third premolars and first molars.Compared to the β-TCP and control groups, DPSCs-IPs expressed moderate to high levels of CD146 and STRO-1 as well as low levels of CD45 and CD34.The cells have chondrogenic, adipogenic, and osteogenic differentiation abilities and when they were engrafted onto β-TCP, they regenerated bone of the PDs through 3 mo postsurgical reconstruction.
Clinical studies
Autologous hDPSCs-IPs[33], micrografts rich in autologous hDPSCs[34,35] or autologous hDPSCs derived from healthy pulps[36] were the source for a randomized clinical trial (commonly referred to as RCT)[34], a 1-year follow-up case series[35], a quasi-experimental study[35] and a pilot study[33].The strategy of using DPSCs was dependent on scaffold-based only[33-35] as they loaded onto the scaffold[33-35] or dripped suspended in 200 mL of phosphate-buffered saline and then the scaffold membrane was placed[36].Patients diagnosed with combined periodontal-endodontic lesions with pocket depth from 5 mm to 6 mm[33], patients with chronic periodontitis combined with deep intrabony defects[34,35], and patients with controlled chronic diseases and without horizontal PDs or osteoporosis[36] were the models used for cell implantation.Either 1 × 105[33] or 5 × 105[36] and 50 μm micrografts rich in autologous hDPSCs were the cell number/source that was indicated for the scaffold-based strategy (Table 2).
DPSCs-derived from healthy pulp:In a scaffold-based trial, Ferrarottiet al[34] performed a RCT for 29 consecutively enrolled patients with chronic periodontitis presenting one deep intrabony defect and requiring extraction of one vital tooth.They randomly allocated the defects to two groups, test or control ones.They prepared micrografts rich in autologous DPSCs from the DP of the extracted teeth through mechanical dissociation.With the use of minimally invasive surgical technique, the test sites were packed with micrografts seeded onto collagen sponge, whereas the control sites were filled with collagen sponge alone.Their findings exhibited a significantly more probing depth reduction, clinical attachment level gain and bone defect fill in the test sites compared to control ones.It was found that application of DPSCs significantly improved clinical parameters of periodontal regeneration at 1 year after treatment.In addition, Aimettiet al[35] performed a case series with 1-year follow-up for 11 patients with chronic periodontitis superimposed by 11 isolated intrabony defects to explore the potential clinical benefits of the application of DPSCs in the regenerative treatment of deep intrabony defects.Extraction was performed for malposed/impacted teeth to be used as autologous source for DPSCs.The defects were accessed with a minimally invasive flap and filled with DPSCs loaded on a collagen sponge.After 1 year posttreatment, a clinical attachment level gain associated with probing depth reduction and a notable stability of the gingival margin was detected using clinical outcomes supported by the radiographic analysis.In 63.6% of the experimental sites, complete pocket closure was achieved.Moreover, a quasiexperimental study was conducted by Hernández-Monjarazet al[36] for 22 adults with PDs, without uncontrolled systemic chronic diseases.Every participant underwent clinical and radiological evaluations and saliva samples were taken to determine the total concentration of interleukins (ILs), antioxidants, lipoperoxides, and superoxide dismutase (SOD).All subjects underwent curettage and periodontal surgery and then two groups were allocated randomly, namely experimental and control groups.The defects of the control group had a collagen scaffold, while the experimental one had a collagen scaffold treated with DPSCs.The experimental group showed an increase in the bone mineral density of the alveolar bone with a borderline statistical significance.Regarding inflammation and oxidative stress markers, IL1β levels had a level decrease, while salivary SOD level was significantly higher in the experimental group.Their findings suggest that treatment based on DPSCs has both an effect on bone regeneration linked to an increased SOD and decreased levels of IL1β in subjects with PDs.
DPSCs-derived from inflammatory pulp:In a scaffold-based trial, Liet al[33] harvested DPSCs-IPs from 2 patients with periodontal intrabony defects and then they were loaded onto the scaffold material β-TCP and engrafted into the PD area in the root furcation.At 9 mo after surgical reconstruction, DPSCs-IPs were able to engraft and had an effect of regeneration of new bones to repair PDs.The success rate of primary cell culture and growth status was marginally inhibited; however, DPSCs-IPs expressed comparable levels of stem cell markers as well as retaining their ability to multi-differentiate.
REGENERATION OF SGS USING DPSCS
The parotid, submandibular (SMG) and sublingual glands are the three pairs of major SGs.The anatomical architecture of all three glands is virtually identical, with different ratios of serous and mucous cells: an arborized ductal system that opens into the oral cavity with secretory end pieces, or the acini-producing saliva.The acinar cells are surrounded by an extracellular matrix, myoepithelial cells, myofibroblasts, immune cells, endothelial cells, stromal cells and nerve fibers.The ducts transport and modify the saliva before it is excreted into the oral cavity through the excretory duct[37].The aim of regenerative medicine is to replace/regenerate the SG epithelium and restore its secretory function in patients suffering from permanent loss of major SG function, after radiotherapy of head and neck cancer, which is the sixth most common cancer in the world and affects tens of thousands of new patients every year, causing hyposalivation/xerostomia with subsequent difficulties in mastication, swallowing, taste, speech, high patient discomfort and a reduced quality of life.Palliative therapies show a limited effectiveness in relieving the symptoms, such as mechanical stimulation of SG activity using chewing gum, or pharmacological agents such as pilocarpine, as well as saliva substitutes[38].Adult stem cell transplantation strategies have recently shown to enhance clinical outcomes in radiotherapy-induced xerostomia in rodents.Mesenchymal stem cells from adipose tissue are the most promising[39], while those from the labial mucosa, bone marrow or DP have an attractive therapeutic value following successful findings inex vivoandin vivomouse models of SG injury.DPSCs are supposed to be a good source for SG regeneration by being a component of epithelial acinar cells itself or by being a SG mesenchymal component to encourage and support the regeneration process of the SGs.In SG bioengineering, the principal role of DPSCs is to regenerate the salivary stroma or mesenchymal-derived compartment, and these cells are one of the best choices for that purpose because the tooth mesenchyme and the SG mesenchyme share a common neural crest embryonic origin[40].A radiation model was developed to reproduce the hyposalivation induced by head and neck cancer therapy.The ductal ligation model aimed to mimic the obstructive sialadenitis and sialithiasis-induced sialopathy and tissue punch considered as a model of mechanical injury (Figure 3).Systemic disease/medication was devised to correspond to Sjogren’s syndrome[41].
Preclinical studies
Autologous[42,43], xenogeneic[44] DPSCs or their Exo[45] have been isolated from healthy[42-45] DP.The strategy of using DPSCs was dependent on scaffold-based[42], scaffold-free[43,44] and cell-free[45] therapies.Application of DPSCs was in the form of cell injection[42-44].Mice were the most selected model for studying the regenerative effect of DPSCs for SG regeneration in the pre-clinical trials.Other animals such as rat[43] were also selected for the same purpose.Irradiation-induced SG hypofunction[43] and duct ligation-induced atrophy[45] or salivary hypofunction induced by diabetes[44] were the presented study models.The cell number of 1 × 106was the most commonly used[42,43] but 5 × 105[44] cell number was also used, and 0.4 mL[45] of DPSCs-CM was indicated for the cell-free therapy strategy (Table 3).
DPSCs-derived from healthy pulp:(1) Subcutaneous and intraglandular injection of cell suspension of DPSCs.In a scaffold-based trial, Janebodinet al[42] created aninvivomodel to study the differentiation effect of DPSCs to support SG tissue formation.They subcutaneously transplanted either 1 × 106hSG cells alone or 1 × 106hSG cells combined with 1 × 106murine DPSCs.These cells were transplanted with hyaluronic acid (HA) hydrogel scaffold into 2-mo-old male Rag1 null mice to avoid immune rejection against hSG.The cells were injected ventrally onto SMG without penetrating the gland in the form of 100 μL of cell suspension.After 2 wk of transplantation, HA plugs were dissected without involving mouse recipients’ SG tissues, to be investigated with hematoxylin and eosin, periodic acid Schiff and immunofluore-scence staining.Histological evaluation revealed that combination between hSG + DPSCs contributed to the generation of salisphere-like structures.These salispheres expressed markers of terminally differentiated acinar cells and after subcutaneous transplantation in mice, developing a well-irrigated SG-like tissue.These findings provide evidence of the potential use of DPSCs as ecto-mesenchyme for induction and support of SG development.
In a scaffold-free trial, Yamamuraet al[43] established a mouse model in which DPSCs were used as a cell source for treatment of irradiation-induced SG hypofunction.DPSCs were isolated from female C57BL/6J green fluorescent proteinexpressing mice and were differentiated into dental pulp endothelial cells (DPECs).Both SMG of the irradiated mice were injected with 1 × 106DPECs/gland.Eight weeks after irradiation, the effect of DPECs on saliva secretion was evaluated by measuring amounts of saliva secretion.It was found that transplantation of DPSCs are highly effective in maintaining saliva production after irradiation through direct differentiation of into salivary epithelial cells.This study established a mouse model in which DPSCs could be used as a cell source for the treatment of SG hypofunction.Moreover, Narmadaet al[44] injected intraglandular tissues of the SMG in diabetic Wistar rat models once with 5 × 105cells of hDPSCs/250 g in 0.2 mL phosphate-buffered saline solution.The control diabetic rats were injected only with 0.2 mL of phosphatebuffered saline intraglandular.Half of the rats was sacrificed after 7 d, while the remaining rats were sacrificed after 14 d for immunohistochemical evaluation.It was found that transplantation of DPSCs was able to regenerate SMG defects in diabetic Wistar rats by decreasing acinar cell vacuolization.
(2) Secretome from DPSCs.Takeuchiet al[45] collected cell culture supernatant from subconfluently DPSCs culture (DPSC-CM).DP tissues were collected from healthy teeth extracted from individuals.The SMG duct was ligated approximately 2 mm superior to the main body of the gland with a sugita titanium aneurysm clip.The experimental groups were divided into a DMEM group (in which 0.4 mL DMEM was administeredviathe right jugular vein biweekly) and a CM group (in which 0.4 mL DPSC-CM was administered using a similar method); the control group was composed of mice that underwent only the release of ligation.Injection of DPSCs-CM to mice after releasing the SMG main duct ligation significantly elevated the expression levels of precursor cell marker CK5 and acinar cell marker AQP5 after the release.Growth factors contained in DPSCs-CM were considered to be responsible for the effects.This study suggested that DPSCs-CM administration to mice after releasing the SMG main duct ligation may promote acinar cell regeneration of atrophied SGs.
Table 3 Preclinical studies obtained from the PubMed, Scopus and Google Scholar databases related to salivary gland regeneration using dental pulp stem cells
Figure 3 Punch biopsy of submandibular gland.
REGENERATION OF BONE DEFECTS USING DPSCS
Bone fractures appear to occur more often in old age because of bone weakening due to a reduction in calcium content in extracellular matrix and slowing of bone remodeling processes.Current methods of treatment are unable to fully reconstruct bone defects that are caused by fractures, tumors and congenital abnormalities.At present, autogenous or allogeneic bones and artificial materials are used for bone augmentation.However, there are various problems with these grafts, such as the possibility of infection or absorption as well as ethical issues.Alternatively, the periosteum and the bone marrow are considered rich in bone progenitors, so that the bone, since early 1990s, has been one of the approachable goals of clinical TE[46].In addition, osteogenic differentiation of DPSCs is easily inducedin vitroby adding ascorbic acid, dexamethasone, and β-glycerophosphate to the culture medium, along with fetal bovine serum, making these cells a promising tool for bone regeneration[47].Bone defect repair is characterized by three overlapping processes, namely the formation of blood clot, bone formation, and bone remodeling.Osteoprogenitor cells migrate to the wound site during the second phase, proliferate and differentiate into osteoblasts that segregate and induce mineralization.DPSCs are a vital source of osteoprogenitor cells which play a fundamental role in the bone tissue plasticityrelated mechanisms[48-50].
Preclinical studies
Autologous[51] or xenogeneic[49,50,52] DPSCs have been isolated from healthy[50-52] DP.The strategy of using DPSCs was dependent on scaffold-based[52], scaffoldfree[50,51] therapies.Application of DPSCs was in the form of cell sheet transplantation[51], loaded onto the scaffold[52] or, incorporated onto the PRP and the combination was injected locally[50].Animals such as rabbit[52], mice[51] and dogs[50] were selected to study bone regeneration preclinically.Mandibular[50] (Figure 4), calvarial[51] and craniofacial[52] bone defects were presented.The cell number of 1 × 107was the most commonly implicated[50] but 2 × 106[52] was also used (Table 4).
DPSCs-derived from healthy pulp: (1) Loading or injecting a cell suspension of DPSCs.In a scaffold-based trial, Mohammedet al[52] loaded 2 × 106hDPSCs on gel foam scaffold to be fitted in induced temporomandibular joint defect area of 2.5 mm × 2.5 mm in diameter and in depth for the rabbit’s mandible.The histological investigation was performed for qualitative evaluation of bone healing and it was found that newly formed bone with thick trabecular pattern and less osteoid tissue have been observed in the experimental rabbits transplanted with osteogenic-differentiated hDPSCs.The authors suggested the ability of transplanted osteogenic differentiated hDPSCs to produce new osteoid tissue that helps in repairing an induced rabbit mandibular defect in a time and differentiation phase-dependent manner.In an attempt to confirm that bone regeneration was indeed the function of hDPSCs, Chamiehet al[53] tested the efficacy of DPSCs seeded on a collagen scaffold for craniofacial bone regeneration.Plastically compressed collagen gels were loaded with DPSCs (2 × 106cells/mL) which were isolated from molar Wistar rats.A 5-mm diameter calvarial critical-sized defect was created on each side of the parietal bone using a dental bur attached to a slow-speed hand piece operating at 1500 rpm, under irrigation with sterile saline solution; empty defects were created as a negative control for the critical-sized defects.It was found that collagen gel scaffolds seeded with DPSCs improved bone healing and the remodeling process under micro-X-ray computed tomography as well as histological examination.
Liuet al[50] utilized nano-hydroxyapatite/collagen/PLLA as a scaffold for autologous DPSCs seeding to reconstruct critical-size alveolar bone defects in New Zealand rabbit.A 10 mm incision was made and the tissue overlying the diaphysis of the left alveolar bone of incisors of rabbits was dissected.A segmental defect of 10 mm × 4 mm × 3 mm was prepared in the alveolar bone.Cell number of 1 × 106DPSCs was implanted onto the scaffold and transplanted into the alveolar bone defect.The animals were sacrificed 12 wk after operation for histological observation and histomorphometric analysis.It was found that reconstruction of alveolar bone defects using DPSCs seeded on nano-hydroxyapatite/collagen/PLLA had earlier mineralization and more bone formation than achieved with the scaffold alone or even the autologous bone graft.
In a scaffold-free trial, Yamadaet al[49] tried to test the osseointegration around the implant during the healing period using DPSCs.Human DPSCs were obtained from extracted permanent teeth.Adult hybrid dogs with a mean age of 2 years were operated on under general anesthesia.In the mandible region, the first molar and the second and third premolars were extracted, and the healing period was 2 mo.Bone defects were prepared on both sides of the mandible using a trephine bur with a diameter of 10 mm.The defects were implanted with DPSCs or BMSCs (1 × 107cell/mL) plus PRP while the defect-only represented the control group.After 8 wk, an HA-coated osseointegrated dental implant was inserted into the bone regeneration areas and was assessed histologically.The dogs were sacrificed 16 wk after insertion of the dental implant.A histological analysis was performed to obtain a general description of the tissue surrounding the implants.Cavities filled with DPSCs/PRP or BMSCs/PRP were found to show new bone formation at 8 wk with a tubular pattern along with abundant vascularization.This pattern reflected normal bone macrostructure with well-differentiated marrow cavity and cortices, while the cavities in the control group were invaded by vascular and fibrous tissues.Bone-implant contact revealed significant increase in the group treated with DPSCs/PRP or BMSCs/PRP in comparison to controls.
Table 4 Preclinical and clinical studies obtained from the PubMed, Scopus and Google Scholar databases related to bone regeneration using dental pulp stem cells
Figure 4 Mandibular bone defect.
(2) Cell sheet transplantation technique of DPSCs.In a scaffold-free trial, Fujiiet al[51] evaluated bone regeneration by hDPSCs using a helioxanthin derivative and cell-sheet technology.DPSCs were cultured on temperature-responsive dishes after treatment with osteogenic medium for 14 d then the sheets were folded to fit the 3.5-mm calvarial defects of mice.The skin and subcutaneous layer were then closed by 6-0 nylon sutures.Eight weeks after surgery, calvarial defect sites were harvested from euthanized mice.Micro-CT images showed that the DPSC sheets were able to induce bone formation without requiring scaffold or growth factors.Cell sheets are flexible and simple to transplant, so this technology may replace conventional bone graft methods in the future.
Clinical studies
Autologous hDPSCs were the source for a RCT[42] with 3 mo follow-up case series.The strategy of using DPSCs was dependent on scaffold-based only.Patients diagnosed with bilateral bone resorption following impaction surgery of impacted third molars are presented (Table 4).
DPSCs for cell and scaffold-based strategy:D’Aquinoet al[54] utilized DPSCs and a collagen sponge scaffold for oro-maxillo-facial bone tissue repair in patients requiring extraction of their third molars.The patients suffered from bilateral alveolar bone reabsorption distal to the second molar following impaction of the third molar on the cortical alveolar lamina, producing a defect without walls, of 1.5 cm in height.Extraction of the third molar in this clinical condition does not permit spontaneous bone repair and ultimately leads to loss of the adjacent second molar as well.For DPC isolation and expansion, maxillary third molars were extracted.The cells were then seeded onto a collagen sponge scaffold and the biocomplex obtained was used to fill in the injury site left by extraction of the mandibular third molars.At 3 mo after autologous DPSCs grafting, the patients alveolar bone had optimal vertical repair and complete restoration of periodontal tissue back to the second molars, as assessed by clinical probing and X-rays.
CONCLUSION
DPSCs have emerged as a promising tool with a great potential for dental and oral tissue regeneration or engineering.However, many issues and challenges still need to be addressed before these cells can be employed in clinical practice.The full control of differentiation of DPSCs to specific fates is still an important issue, and although DPSCs derivation to certain connective tissue-lineage cells appears to be relatively simple, their differentiation into nerve-tissue lineages still poses important unanswered questions.Moreover, the development of the cell recombination technology required to create next-generation bioengineered replacement organs will necessitate extensive research for the coming years.In this case, DPSCs may be an ideal choice to be used in experimental models of tissue-engineered reconstruction of organs of the oral cavity, and the paradigmatic cases of the tooth and the SG may constitute the leading edge of a massive research effort on next generation organ replacement therapies[2].
 World Journal of Meta-Analysis2021年2期
World Journal of Meta-Analysis2021年2期
- World Journal of Meta-Analysis的其它文章
- Helicobacter pylori infection and susceptibility to cardiac syndrome X: A systematic review and meta-analysis
- Laboratory hematologic features of COVID-19 associated liver injury:A systematic review
- Nonalcoholic fatty liver disease and cardiovascular concerns: The time for hepatologist and cardiologist close collaboration
- Metabolic and biological changes in children with obesity and diabetes
- Viral hepatitis: A brief introduction, review of management,advances and challenges
- Ankle injuries in athletes: A review of the literature
