Nonalcoholic fatty liver disease and cardiovascular concerns: The time for hepatologist and cardiologist close collaboration
Haleh Ashraf, Shahrokh Karbalai, Raika Jamali
Haleh Ashraf, Shahrokh Karbalai, Raika Jamali, Research Development Center, Tehran University of Medical Sciences, Tehran 1938934131, Iran
Abstract Nonalcoholic fatty liver disease (NAFLD) has emerged as the most common chronic liver cell damage worldwide.It is strongly associated with an increased risk of cardiovascular disease (CVD).There are not enough recommendations for screening subjects with nonalcoholic steatohepatitis cirrhosis, who are not candidates for liver transplantation, nor who are asymptomatic with NAFLD without cirrhosis.In the current comprehensive narrative review, we aimed to evaluate the associations between CVD and NAFLD.Distinguishing the mechanisms linking these two disorders offers the opportunity to develop targeted therapies.Moreover, we will discuss screening approaches (whom and how-to) and treatment modalities proposed to reduce cardiovascular risk in patients with NAFLD.
Key Words: Nonalcoholic steatohepatitis; Cardiovascular disease; Screening; Atherosclerosis; Risk scores; Metabolic syndrome
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is considered as the histologic evidence of an accumulation of triglycerides (TGs) in hepatocytes in the absence of a causative factor.It encompasses a pathological spectrum, ranging from steatosis (more than 5% of hepatocytes) to nonalcoholic steatohepatitis (NASH) (fatty infiltration plus necroinflammation and fibrosis) and finally to cirrhosis[1].NAFLD has emerged as the most common chronic liver disease worldwide.It has constantly increasing incidence and prevalence, with a global prevalence of about 25%[2].According to a systematic review and meta-analysis in Iran, the prevalence of total, mild, moderate, and severe types of NAFLD was estimated as 33.9%, 26.7%, 7.6%, and 0.5%, respectively[3].It is strongly associated with an increased risk of cardiovascular disease (CVD), including subclinical atherosclerosis, coronary, cerebral, and peripheral vascular disease, myocardial dysfunction, hypertrophy, and remodeling, valvular heart disease, arrhythmia, and hypertension (Figure 1).The patients show a high risk of fatal and non-fatal CVD events, which occur in 5.2% of subjects.Biopsy-confirmed NAFLD is associated with up to a 64% increased mortality risk compared to the general population, with CVD as the most frequent cause of death (25% of deaths)[4].
In the current comprehensive narrative review, we aimed to review the association between CVD and NAFLD.Distinguishing the mechanisms linking these two disorders offers the opportunity to more considerably develop targeted therapies.Thereafter, we will discuss screening approaches (whom and how-to) and treatments proposed to reduce cardiovascular risk in patients with NAFLD.
PATHOPHYSIOLOGICAL LINK BETWEEN NAFLD AND CVD
NAFLD and CVD attend both presentations of end-organ damage of metabolic syndrome.The specific contribution of NAFLD to increased CVD risk is difficult to discriminate, due to concomitant shared risk factors.Accumulation of dysfunctional visceral adipose tissue in the liver and other organs (such as the pericardium, pancreas, kidneys, or skeletal muscle), subsequent activation of inflammatory pathways, and release of fat-derived toxic metabolites lead to the development of both NAFLD and CVD.This occurs possiblyviamechanisms beyond classic risk factors and metabolic syndrome components[5,6].Epicardial adipose tissue (EAT) produces proatherogenic, proinflammatory, and prothrombotic adipocytokines, which due to its anatomical intimacy with the heart muscle.EAT shares an unobstructed microcirculation with the adjacent myocardium.The underlying mechanisms linking NAFLD to CVD are very complex and simultaneously involve several different pathways, which are described as follows (Figure 1).
Endothelial dysfunction
The main causes of endothelial dysfunction are increased levels of asymmetric dimethyl arginine (ADMA), hyperhomocysteinemia, and systemic inflammation.ADMA is an endogenous antagonist of nitric oxide synthase that disturbs vasomotor regulation of vascular permeability and cause platelet dysfunction.Hyperhomocysteinemia is associated with increased intrahepatic vascular resistance, which impairs nitric oxide formation, and causes oxidative stress and platelet activation.Systemic inflammation associated with NAFLD increases endothelial dysfunction, alters the vascular tone, and enhances vascular plaque formation[5,6].
Atherogenic dyslipidemia
NAFLD changes the serum lipi d profiles, causing high TGs, very low-density lipoprotein (VLDL), apolipoprotein B, and low-density lipoprotein (LDL) levels, dense and smaller LDL particle size and peak diameter, and decreased high-density lipoprotein (HDL) levels.This abnormal serum lipoproteins composition is likely to activate an inflammatory pathway involved in the pathogenesis of atherosclerotic CVD[7].
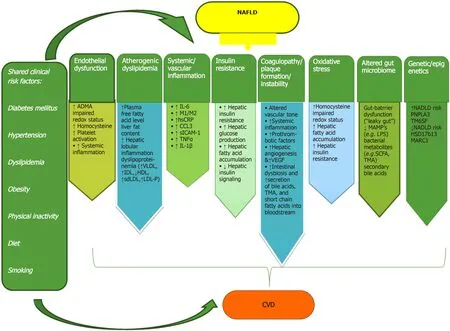
Figure 1 Schematic figure depicting potential pathways for increased risk of cardiovascular disease in patients with nonalcoholic fatty liver disease.
Systemic insulin resistance
Fatty acid accumulation in the liver, subclinical inflammation, oxidative stress, and altered adipokines leads to the suppression of endogenous liver glucose production, and further stimulating insulin resistance (both hepatic and peripheral).The mentioned mechanisms would describe the increased risk of insulin resistance with adverse cardiovascular events[8].
Proinflammatory and pro-oxidative states
Emerging evidence also suggested that serum and liver inflammatory and oxidative markers (including interleukin-6, tumor necrosis factor-α, C-reactive protein, and other proinflammatory cytokines) are increased in NAFLD.These pathways, especially in its necroinflammatory form, might be involved in the pathogenesis of cardiovascular involvement[5,6].
Coagulopathy/plaque formation/instability
Patients with NAFLD have a procoagulant imbalanc e that increases from the less severe (steatosis) to more severe (NASH) stages of disease.This imbalance was caused by the increased serum levels of coagulation factors (VIII, IX, XI, and XII, von Willebrand factor, fibrinogen, and plasminogen activator inhibitor type 1), while anticoagulant factors (like antithrombin III and protein C) are decreased[9].Another aspect that may affect plaque formation and plaque instability in NAFLD patients includes elevated serum concentrations of vascular endothelial growth factor (VEGF) and signs of active angiogenesis, indicative of vascular remodeling.However, the functional role of VEGF in atherogenesis of NAFLD patients is still unclear[9].
Genetic/epigenetics
The role of genetic factors linking NAFLD and CVD is so far unclear.Two missense genetic variants have been detected by genome-wide association reports in NAFLD: Transmembrane 6 super family member 2 (TM6SF2) and patatin-like phospholipase domain-containing protein 3 (PNPLA3).PNPLA3 regulates the physiology and morphology of lipid droplets and appears to be related to TG metabolism.Carriers of PNPLA3 have been shown to have lower serum TG levels but paradoxically increased atherosclerosis, while carriers of TM6SF2 may have reduced levels of serum TG, LDL cholesterol, and total cholesterol with subsequently experience some degree of cardioprotection[10].
Altered gut microbio me
Alterations in the gut microbiota composition, named as dysbiosis, has been identified as a contributing factor for the development of cardiometabolic diseases like NAFLD.However, a NAFLD-specific gut microbiota signature has not been detected yet.Dysbiosis increases mucosal barrier permeability to intestinal microbes and/or microbial products, designated as microbe- or pathogen-associated molecular patterns.Damage-associated molecular patterns (e.g., released from damaged enterocytes), lipopolysaccharide, or peptidoglycans trigger a systemic gut dysbiosis-related inflammatory response[11].
IMPACT OF NAFLD ON CARDIOVASCULAR MORBIDITY AND MORTALITY
Whether NAFLD is an independent risk factor for cardiovascular mortality and other cardiovascular events remains controversial.Most studies demonstrate an association between NAFLD and increased CVD events, especially for those with severe NAFLD/NASH, but the association with overall or CVD mortality is less elucidated[4].A meta-analysis containing 16 observational research studies (9 prospective and 7 retrospective cohort studies) by Targheret al[4] including 34,043 patients with a median 7-year follow-up showed that NAFLD patients had a 64% higher risk of having fatal or non-fatal cardiovascular events (cardiac death, myocardial infarction, need of coronary revascularizations, or stroke) as compared to patients without NAFLD (odds ratio (OR): 1.64; 95% confidence interval (CI): 1.26-2.13).However, a sub-analysis of studies with CVD mortality as the primary outcome, the association between NAFLD and fatal CVD events did not remain statistically significant.
SEVERITY OF NAFLD HISTOLOGY AND CVD MORTALITY RISK
The histologic severity of NASH was associated with the degree of carotid artery intimal medial thickness, independently of conventional cardiovascular risk factors and metabolic syndrome features.Meanwhile, the severity of NAFLD histology did not further increase CVD mortality risk[12].
CARDIOVASCULAR COMORBIDITIES IN NAFLD PATIENTS
Arterial hypertension
Systemic arterial hypertension prevalence varies from 40%-70% among NAFLD patients, and prospective epidemiological studies have demonstrated a 2-3-fold increase in the incidence of arterial hypertension over observation periods of 9 and 5 years, respectively[13,14].
Atherosclerotic CVD
Patients with NAFLD are at increased risk of subclinical atherosclerosis as measured by increased carotid artery intima/media thickness, arterial stiffness, ankle-arm index, aortic pulse-wave velocity, coronary calcification, and endothelial dysfunction as measured by flow-mediated dilation.A meta-analysis which contained 85,395 subjects including 29,493 NAFLD subjects demonstrated higher risk of subclinical atherosclerosis in NAFLD than non-NAFLD patients (OR: 1.60; 95%CI: 1.45-1.78)[15].In addition to subclinical atherosclerosis, patients with NAFLD have a higher risk of clinically significant atherosclerosis, and they may additionally have worse outcomes when experiencing acute events.Haddadet al[16] performed a meta-analysis involving 25,837 patients and reported that NAFLD subjects had a significantly higher risk of clinical coronary artery disease when compared to individuals without NAFLD (relative risk: 2.26; 95%CI: 1.04-4.92;P< 0.001).
Structural heart disease
In addition to functional cardiac alterations, structural heart diseases are also more common among NAFLD subjects.The increase in free fatty acids may result in myocardial steatosis, and subsequent changes in myocardial substrate metabolism, efficiency, and perfusion leading to impaired left ventricular contractility[17].Myocardial remodeling, notably in the left ventricle, which is often accompanied by left ventricular systolic and/or diastolic dysfunction and ventricular hypertrophy is observed in NAFLD subjects[17].The study of VanWagneret al[18] involving 2713 participants showed that NAFLD subjects had higher left ventricle filling pressure, lower early diastolic relaxation velocity, and worse absolute global longitudinal strain than those without NAFLD.NAFLD has also been shown to be independently associated with valvular heart disease, specifically aortic valve sclerosis and mitral annulus calcification[19].NAFLD individuals would experience alterations in the EAT.The thickness of EAT is independently associated with NAFLD and its grades, and also increased risk for coronary calcification (EAT > 3.18 mm)[20].
Cardiac arrhythmias
There is growing evidence that NAFLD patients are at increased risk for cardiac arrhythmias, specifically ventricular arrhythmias, QTc prolongation, and atrial fibrillation independent of overlapping comorbidities and other risk factors.Although the specific mechanisms remain unclear, probable mechanisms are altered electrophysiological myocardial substrate secondary to pro-inflammatory and pro-oxidative states, insulin resistance-induced hypokalemia affecting the ventricular repolarization, myocardial structural changes, and autonomic dysfunction seen in NAFLD[17].The associations between NAFLD and CVD comorbidities are demonstrated in Figure 2.
PREDICTORS OF CVD IN NAFLD
There is a strong association between higher gamma glutamyl transpeptidase (GGT) levels, even within the normal limit and during incident CVD events.In turn, the association of serum alanine aminotransferase (ALT) with CVD outcomes appears more controversial, exhibiting a U-shaped association with total mortality.The mechanism of the stronger association of GGT than ALT with CVD risk remains to be elucidated.GGT may be linked directly in the pathogenesis of atherosclerosis by participating in extracellular catabolism of glutathione and atherosclerotic plaque formation[21].
SCREENING FOR CVD IN PATIENTS WITH NAFLD
Patients with NAFLD can be divided into two groups regarding CVD evaluation.One group encompasses subjects with NASH cirrhosis who are candidates for liver transplantation, and another group comprises asymptomatic patients with NAFLD (without cirrhosis).
In patients with NASH cirrhosis who are candidates for liver transplantation, dobutamine stress echocardiography (DSE) is widely used, but the predicted maximum heart rate may not be achieved, which reduces its reliability; computed tomography (CT) coronary angiography needs a stable low heart rate, is less invasive than conventional angiography, and the negative predictive value is excellent.Myocardial perfusion imaging can also be used to detect myocardial ischemia, but studies have shown contradicting results.Coronary angiography is the gold standard modality for coronary examination; however, it is an invasive test[7].We agree with the algorithm for evaluation of CAD in pretransplant evaluation suggested by Hoganet al[22].It recommended coronary angiography if more than two of the following risk factors are present: Age > 50 years, history of NAFLD, hypertension, family history of cardiovascular disease, type 2 diabetes mellitus, smoking, or known cardiovascular disease.Patients with 1 or 2 risk factors can be taken for DSE, and coronary angiography will be done for patients with abnormal DSE.

Figure 2 Nonalcoholic fatty liver disease is strongly associated with an increased risk of cardiovascular disease comorbidities.
There are not enough recommendations for screening patients with NASH cirrhosis, who are not a candidate for liver transplantation, and also asymptomatic patients with NAFLD (without cirrhosis).According to the American College of Cardiology (ACC)/American Heart Association (AHA) guideline for assessment of cardiovascular risk in asymptomatic adults, once a diagnosis of NAFLD is established, either in the presence or the absence of features of the metabolic syndrome, a thorough clinical evaluation of the patient must be sought including cardiovascular family history, an assessment of physical status such as blood pressure, waist circumference, body mass index, and laboratory parameters, including fasting blood sugar, lipid profile, glomerular filtration rate, and albuminuria.For each patient, the global CVD risk must be calculated using cardiovascular risk assessment tools, among them, in particular, the Framingham risk score[23] and the ACC/AHA tool, and which have been used in patients with NAFLD are valuable to identify those at higher risk of cardiovascular events at 10 years[7].We should keep in mind that the mentioned risk scoring systems are validated for asymptomatic individuals.Therefore, their applicability in NAFLD subjects is questionable.Meanwhile, there are some Iranian risk scores[24,25], but data in Iranian NAFLD patients is also scarce[26,27] and risk scores in these patients are not validated.The Framingham risk score is a traditional risk factor inventory-based scoring system (http://www.framinghamheartstudy.org/risk/coronary.html) that uses readily available variables, such as gender, age, cigarette use, total cholesterol, high-density lipoprotein cholesterol, and systolic blood pressure to estimate the 10-year cardiovascular risk.This score stratifies subjects as low (< 10%), intermediate (10%-20%), or high (> 20%) CVD risk.However, the Framingham risk score has several limitations; primarily, it can overestimate or underestimate risk in understudied populations.Also, it does not predict the risk for heart failure, transient ischemic attack, or stroke[23].Due to these limitations, in 2013, the ACC/AHA Task Force released a new risk assessment tool (http://www.cvriskcalculator.com/) to assess 10-year atherosclerotic cardiovascular disease (ASCVD) risk, called the Pooled Cohort Equation, which uses race, sex, age, total cholesterol, HDL, systolic blood pressure, smoking, treatment for hypertension and diabetes as risk factors[7].
We believe that patients with low-risk by risk scores should be managed with lifestyle modification.Patients with intermediate- or high-risk by risk scores should be referred to a cardiologist for evaluation of CVD.The question remains, however, when is the time for hepatologist and cardiologist close collaboration.Guidelines by the ACC/AHA recommend screening for subclinical atherosclerosis using coronary artery calcifications and carotid artery intima-media thickness in intermediate-risk asymptomatic individuals to modulate the intensity of treatment.Although coronary artery calcifications may have a higher prognostic value in predicting future cardiac events, cost and radiation exposure are their main concerns, and ultrasonography of the carotid artery maybe the initial screening test of choice.Table 1 summarizes methods to screen CVD in asymptomatic patients based on AHA guidelines[7].The proposed algorithm for the screening of CVD in patients with NAFLD is provided in Figure 3.
CVD RISK MEASUREMENT INTERVALS
The timing and frequency of the reassessment of global cardiovascular risk in patients in primary prevention of CVD (including those with NAFLD) are still poorly elucidated.The physician should make decisions on the frequency of remeasuring for cardiovascular risk according to risk measured at baseline.Re-estimation of the risk before 8-10 years is not obligatory for most subjects initially not requiring therapy.However, yearly re-evaluation seems warranted in those with an initial risk of 15% to < 20%.
SCREENING FOR NAFLD IN CVD
Screening for fatty liver in atherosclerotic coronary disease patients seems to be a reasonable approach.The mortality risk is dependent on the presence and extent of fibrosis, and those with simple steatosis were not at increased risk of CVD death.If a cheap, non-invasive, available, and reliable tool to detect high-risk patients (i.e.NASH with some degree of fibrosis) were available, the answer to the above-mentioned question may be yes.Screening for NAFLD using ultrasound in patients with ASCVD may not be clinically possible; patients with ASCVD should be considered a high-risk group, at minimum according to accompanying risk factors.A recent study showed the relationship between NAFLD fibrosis score and cardiovascular risk[28].It suggested that liver fibrosis biomarkers could be applied in primary care cardiovascular risk assessment.
PROPOSED THERAPIES TO REDUCE CVD
While no specific pharmacologic therapy is provided for the liver itself, optimal management of metabolic risk factors is crucial to reduce cardiovascular risk, which means killing two birds with one stone.
Nonpharmacological modalities
The gold standard treatment for NAFLD is lifestyle modification.It includes dietary change, appropriate physical activity, and weight loss approaches.
Getting to ideal body weight:Weight reduction can be achieved by caloric restriction diet, aerobic exercises, and bariatric surgery in morbid obese patients.A total weight reduction of about 5% is suggested for possible reduction in the amount of liver fat content and the improvement of metabolic abnormalities, 7%-10% for decreasing liver cell inflammation, and up to 10% for substantial fibrosis regression.However, weight loss alone is not sufficient for reducing the cardiovascular risk of NAFLD patients.It should always be integrated with further lifestyle modifications[29].
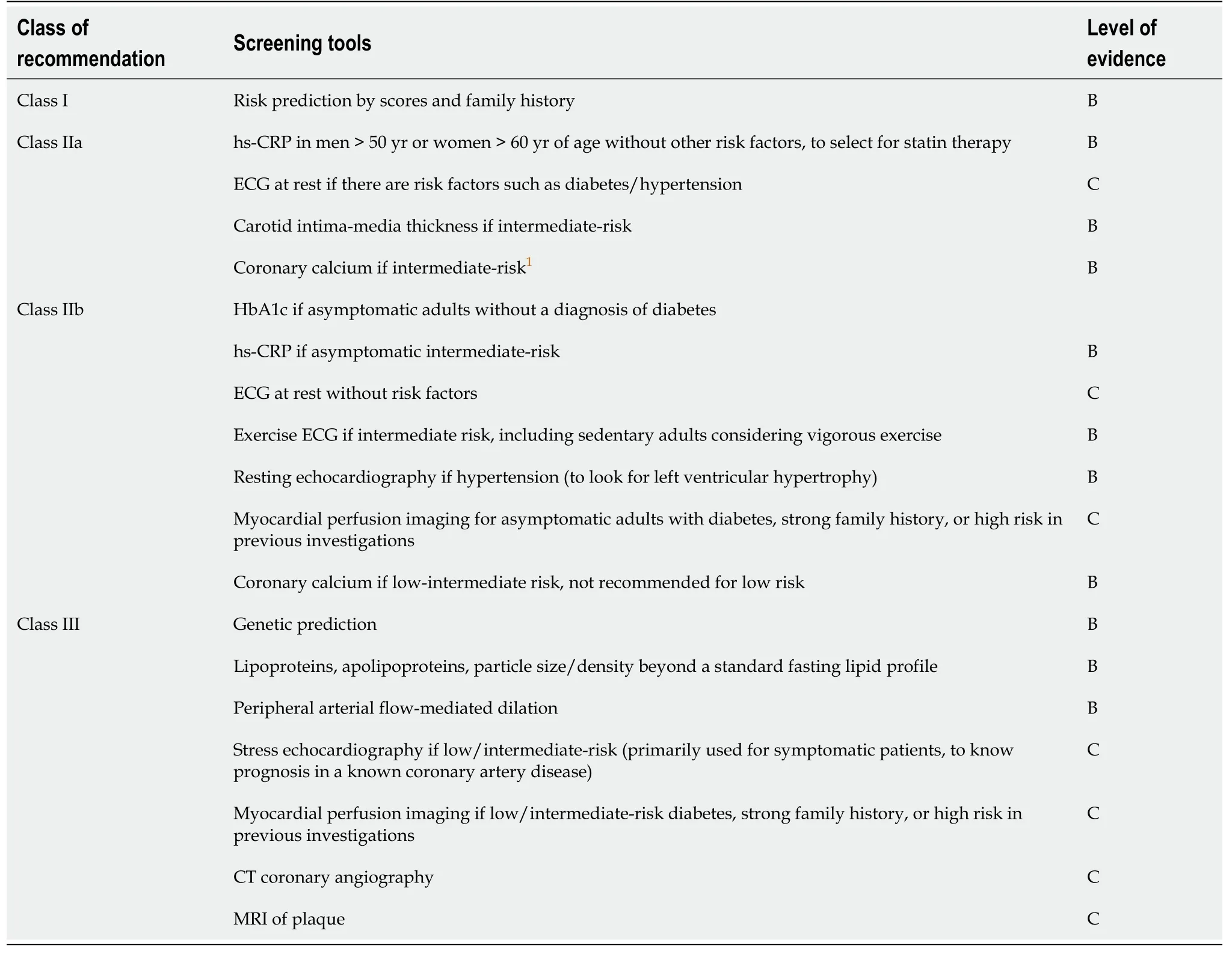
Table 1 Screening of cardiovascular risk in asymptomatic individuals (based on References[6,7])
Exercise: Achieving physical activity at any level or increasing level over the past is better than continuing sedentary behavior.Recommended physical activity consists of at least 2.5 h of moderate-intensity exercise or 75 min of vigorous-intensity exercise per week, supplemented by less sedentary time and at least two sessions of musclebuilding exercise.For improving overall cardiometabolic health, particularly resistance training has beneficial effects by changing body composition which promotes antiatherogenic and anti-inflammatory mechanisms[30].
Dietary changes: A Mediterranean diet rich in vegetables, fruits, legumes, nuts, beans, cereals, grains, fish, and unsaturated fats such as olive oil with and low-carbohydrate diet < 30 g/d are recommended and this special diet strategy has been shown to significantly improve both liver fat content and improve cardiometabolic risk parameters[31].Additionally, ‘when’ and ‘how often’ to eat is also important as well as what we eat[6].Receiving most of the calories before bedtime are associated with a higher risk of NAFLD.At meantime, shifting most of the daily calorie intake for the breakfast or implementing intermittent fasting with energy restriction for about 10-16 h seems to be beneficial for obtaining the ideal body weight[32].Additional lifestyle modifying interventions includes learning strategies to simulate the physiologic response to distress and restorative sleep patterns which have downstream effects on the endocrine pathways relevant in the development of cardiometabolic diseases[33].
Pharmacological interventions
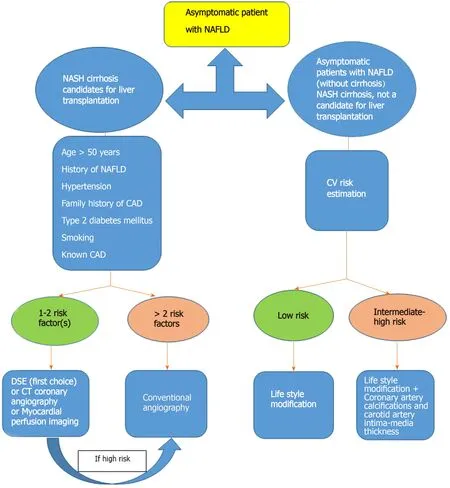
Figure 3 Proposed algorithm for the screening of cardiovascular disease in patients with nonalcoholic fatty liver disease.
Glucose-lowering agents in NAFLD patients with concomitant type 2 diabetes mellitus:Some glucose-lowering medications promote the accumulation of ectopic fat (e.g., insulin/insulin analogs, sulfonylureas), while other antidiabetic medications [e.g., dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 (GLP-1) receptor] decrease the accumulation of ectopic fat without reducing inflammation.In contrast, both SGLT2 (sodium-glucose-linked transporter 2) inhibitors and metformin reduce inflammation, the quantity of ectopic fat and secretion of adipokines.Especially, the novel anti-diabetic drugs such as SGLT2 inhibitors and GLP-1 analogs represent good choices, by providing a favorable influence on the pathogenesis of renal, hepatic and cardiac disease, even in non-diabetic patients[6,34].
Statins:Statins have anti-inflammatory, anti-oxidative, anti-fibrotic, and plaquestabilizing properties, which make them a promising choice to improve vascular and hepatic function among NAFLD patients and reduce cardiovascular risk.The major guidelines on statins have not addressed the specific NAFLD population yet, but it may be reasonable to consider a similar approach as to those with diabetes[5].According to the recommendations of practice guideline provided by the American College of Gastroenterology, American Association for the Study of Liver Diseases, and the American Gastroenterological Association for the diagnosis and management of NAFLD, statins can be used to treat dyslipidemia in patients with NASH and NAFLD[35].The Liver Expert Panel suggests that statin use can be safe in NASH and NAFLD and there is no need for routine liver enzyme measurements.However, checking liver enzymes is recommended before starting statin therapy[36].
Agents directed at the renin-angiotensin-aldosterone system:Angiotensinconverting enzyme inhibitors and angiotensin receptor blockers have been noted as a promising drug in NAFLD patients with hypertension since the renin-angiotensinaldosterone system is involved in the pathogenesis of both NAFLD and CVDs.Although protective effects of renin-angiotensin system blockers on histological fibrosis progression in NAFLD have been demonstrated in several reports, the use of renin-angiotensin system blockers is not recommended in NAFLD patients without hypertension based on the current evidence[37].
Acetylsalicylic acid:In the context of CVD and NAFLD concatenation, acetylsalicylic acid (ASA) is a promising approach.Regarding the benefits of ASA use for primary CVD prevention, there is contradicting evidence; in a recent meta-analysis and systematic review evaluating 13 trials and randomizing (164,225) people to daily aspirin as primary prevention of CVD showed it to contribute to a lower risk of adverse cardiovascular events but a higher risk of major bleeding[38].ASA is recommended as secondary prevention in all patients with established ASCVD including coronary, cerebral, or peripheral arterial disease.Moreover, a recent prospective cohort study suggests that ASA may also have antifibrotic effects in NAFLD, especially in patients who received daily ASA for ≥ 4 years[39].Although, the underlying hepatoprotective benefit is unclear.Meanwhile, reports exists about the beneficial effect of ASA use in inhibition of liver cell fibrosis[40].
CONCLUSION
Patients with NAFLD have a high risk for adverse cardiovascular events and mortality.As such, the diagnosis of NAFLD deserves a thoughtful cardiovascular risk assessment and evaluation for subclinical atherosclerosis to prevent CVD morbidity/mortality.In patients with NASH-related cirrhosis undergoing liver transplantation, screening for significant cardiovascular arterial disease should be done by stress echocardiography and CT coronary angiography, as first-line and conventional angiography is reserved for those with the combination of risk factors for cardiovascular arterial disease.
Recommendation for screening in asymptomatic NAFLD without significant fibrosis is not clear, with each modality having some limitations.When NAFLD is diagnosed, the CVD risk assessment must be sought out and patients with intermediate or high risk or with NASH cirrhosis should be referred to the cardiologist for evaluation of CVD.
The basis of NAFLD prevention and treatment is lifestyle modifications, including weight loss, increased physical activity, and a Mediterranean/low-carbohydrate diet.While no pharmacologic therapy for the liver itself is approved yet, concomitant cardiovascular risk factors should be targeted by using statins, ACE inhibitors and angiotensin-receptor blockers, aspirin, and insulin-sensitizing agents like GLP-1 analogs or metformin and probably SGLT2 inhibitors.
ACKNOWLEDGEMENTS
The authors would like to show their appreciation to the Staff of Sina Hospital Research Development Center for their technical support in preparing the draft.
 World Journal of Meta-Analysis2021年2期
World Journal of Meta-Analysis2021年2期
- World Journal of Meta-Analysis的其它文章
- Helicobacter pylori infection and susceptibility to cardiac syndrome X: A systematic review and meta-analysis
- Laboratory hematologic features of COVID-19 associated liver injury:A systematic review
- Therapeutic applications of dental pulp stem cells in regenerating dental, periodontal and oral-related structures
- Metabolic and biological changes in children with obesity and diabetes
- Viral hepatitis: A brief introduction, review of management,advances and challenges
- Ankle injuries in athletes: A review of the literature
