Laboratory hematologic features of COVID-19 associated liver injury:A systematic review
John L Frater, Tianjiao Wang, Yi-Shan Lee
John L Frater, Tianjiao Wang, Yi-Shan Lee, Department of Pathology and Immunology, Washington University School of Medicine, St.Louis, MO 63110, United States
Abstract BACKGROUND Liver injury is a common complication of infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus.The utility of laboratory hematology data in the diagnosis and risk stratification of patients with coronavirus disease 2019 (COVID-19) has not been comprehensively examined.AIM To address the following.(1) Are the abnormalities in hematologic parameters seen in the general population of patients with COVID-19 also seen in those patients with associated liver injury? (2) Is liver injury in COVID-19 a sign of severe disease and does liver injury correlate with hematologic markers of severe disease? And (3) What is the quality of this evidence?METHODS To address these questions, a comprehensive systematic review was performed.We searched the peer reviewed medical literature using MEDLINE (PubMed interface), Web of Science, and EMBASE for cohort studies that specifically addressed liver injury and COVID-19 without limitation of date of publication or language.A quality assessment of the studies was performed using the Newcastle-Ottawa Scale.RESULTS Thirty-two articles were suitable for inclusion in our systematic review.These included 22 articles with a cohort of COVID-19 patients with liver injury, 5 comparing non-severe vs severe COVID-19 populations in which liver injury was addressed, and 5 other cohort studies with a focus on liver injury.White blood cell count, absolute neutrophil count, absolute lymphocyte count (ALC), and hemoglobin were the parameters most helpful in distinguishing COVID-19 with liver injury from COVID-19 without liver injury.ALC and d-dimer were identified as being potentially useful in distinguishing non-severe from severe COVID-19.Liver injury was more frequently seen in cohorts with severe disease.Most studies were of high quality (24/48, 86%) with 4/28 (14%) of moderate quality and 0 of low quality.CONCLUSION Our study supports the use of select hematologic parameters in diagnosis and risk stratification of liver injury in COVID-19 patients.Although of overall high quality, the current medical literature is limited by the small number of studies with high statistical power and the variable definition of COVID-19 liver injury in the literature.
Key Words: COVID-19; SARS-CoV-2; Laboratory hematology; Systematic review; Coagulation; Liver injury
INTRODUCTION
Rationale
The coronavirus disease 2019 (COVID-19) pandemic, caused by infection by the beta coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been the subject of a vast number of studies in the medical literature; a January 16, 2021 search of the MEDLINE database (PubMed interface) for “COVID-19” identified 92967 results.A recent scoping review of the laboratory hematology literature identified > 400 studies, of which a high fraction were related to the roles of automated hematology, coagulation, and flow cytometry for diagnosis, prognosis, and risk stratification in COVID-19[1].As a result of this work, several abnormalities of complete blood count (CBC) and coagulation testing have been identified as biomarkers of adverse risk in infected patients.These include leukocytosis[2], lymphopenia[2-4], thrombocytopenia[2,5], neutrophilia[2,3,6-9], prolonged prothro-mbin time (PT) and activated partial thromboplastin time (aPTT)[2], and elevated d-dimer[1,2].It is unclear whether this information can be applied to specific subpopulations of patients with COVID-19 disease, such as those with liver injury[10].
Liver injury in COVID-19 has been defined as “any liver damage occurring during disease progression and treatment of SARS-CoV-2 infection”[11].The classification of liver injury in COVID-19 is not systematized due to a lack of international consensus[12-15].Elevated biochemical liver enzymes including aspartate transaminase (AST), alanine transaminase (ALT), and bilirubin (BILI) are a common finding in COVID-19 infection[2,4,6,16,17] and have been reported in approximately 50% of all COVID-19 patients and approximately 75% of hospitalized patients at admission[18-23].COVID-19-related liver injury has been variously defined by increased levels of AST, AST, gamma-glutamyltransferase (GGT), lactate dehydrogenase, alkaline phosphatase (ALP), or total bilirubin (T-BIL) above the upper limit of normal (ULN)[13,19,21,24-28].Whether the elevation of liver enzymes in COVID-19 patients reflects liver injury in all cases is controversial[29], although a study by Bloomet al[30] concluded that elevated liver enzymes in hospitalized patients appeared to be related to true liver injury.The mechanism of liver injury is unclear and may be due to the direct effects of the virus, drug effect, the impact of systemic inflammation, microthrombosis, or some combination of factors[21,31-37].The extent to which COVID-19-associated liver injury contributes to morbidity and mortality is controversial, although the current literature suggests that higher levels of liver enzymes correlate with more severe liver injury[16,38-42].
Objectives
The purpose of this study was to assess the medical literature to answer the following questions.(1) To what extent are previously reported hematologic abnormalities seen in patients with COVID-19-associated liver injury? (2) Based on the combination of outcome data and hematologic findings, is liver injury in COVID-19 a sign of severe disease? Does liver injury correlate with markers of severe disease? And (3) What is the quality of this evidence? These questions are significant, since CBC and coagulation testing are among the most commonly ordered clinical laboratory tests, provide rapid results, and can be performed in virtually any setting, including resource-limited environments in which SARS-CoV-2 testing is unavailable.This is a systematic review that uses specific evidence-based criteria based on “explicit, prespecified, and reproducible methods”[43] to demonstrate where knowledge is lacking and provide guidance for future research[44].
MATERIALS AND METHODS
Format
This study was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
Information sources and search strategy
We conducted an electronic search of Medline (PubMed interface), EMBASE, and Web of Science using the keywords “COVID-19” and synonyms (“SARS-CoV-2,” “2019 novel coronavirus,” “2019-nCoV,” “Wuhan coronavirus,” “novel coronavirus,” “Wuhan seafood market pneumonia virus,” “Wuhan virus”) AND “liver injury.” The search was performed performed on January 13, 2021 without date limitations.This protocol has not been registered.Individual authors were not contacted.
Eligibility criteria
We included the COVID-19 literature related to liver injury indexed by January 13, 2021.There was no restriction of article type in the search; restrictions of article type were applied during the screening process to avoid inadvertently missing relevant articles.
Data collection process
Titles and abstracts of all articles were screened.Following initial screening, the full text of all studies considered for potential inclusion in the analyses were reviewed for suitability.For articles published in Chinese, the full text was evaluated by a member of the research team fluent in that language (Lee YS).Then reviews, meta-analyses, editorials and other opinion pieces, and articles not focused on the study topic were removed.The full text of the articles was reviewed, resulting in another group of papers being eliminated.This resulted in a final group of articles remaining for the qualitative analyses, which included: Studies with a COVID-19 (+) cohort with liver injury provided as a distinct cohort; studies that compared patients with severevsnon-severe COVID-19 disease, in which information about liver injury in these groups was provided; and papers with other study designs that included information regarding liver injury in COVID-19 disease.The content of the included studies was critically appraised by the authors, who are experts in the field of pathology/ laboratory hematology (Lee YS, Frater JL) or clinical science (Wang T).
Synthesis of results
Extracted data and descriptive statistics were entered and performed using Excel (Microsoft, Redmond, WA, United States).For studies in which a COVID-19 (+) cohort with liver injury provided as a distinct group, the following data were extracted: Authors; date of publication; country where study was performed; number of patients in COVID-19 (+) cohort with liver injury; percentage of male patients in COVID-19, (+) cohort with liver injury; setting in which the study was performed; criteria for liver injury used by the study; presence of, and criteria for inclusion in the control group; hematologic parameters evaluated in the study; and results of studies of statistical significance of each hematologic parameter.For studies that compared patients with severevsnon-severe COVID-19 disease, the following information was extracted: Authors, date of publication, country where study was performed, number of patients in the COVID-19 (+) cohort with liver injury, setting in which the study was performed, criteria used by the study to categorize patients as having severe disease, criteria for liver injury used by the study, hematologic parameters evaluated in the study, and summary of the main findings as they pertain to the study topic.For papers with other study designs, the following information was extracted: Authors, date of publication, country where study was performed, setting in which the study was performed, hematologic parameters evaluated in the study, description of study design, and summary of the main findings as they pertain to the study topic.
Additional analyses (quality assessment)
Two authors (Wang T and Frater JL) assessed the study quality of the cohort studies using the Newcastle-Ottawa Scale (NOS)[45].We designated the quality of each study as high (7-9 points), moderate (4-6 points), or low (0-3 points).In situations where the reviewers disagreed on a rating, a third reviewer (Lee YS) adjudicated and provided consensus.
RESULTS
Study selection
Through a search of the databases, we identified a total of 967 potential articles.Following the removal duplicate articles, we screened the titles and abstracts of the remaining articles to further identify potentially relevant articles.After thorough assessment of all full-text articles, 32 studies met the established inclusion criteria and were included in this systematic review (Figure 1).
Study characteristics
A total of 22 studies had a COVID-19 (+) cohort with liver injury provided as a distinct group (Table 1)[26,46-66].
All studies were retrospective and included nine studies from China, five studies from the United States, two studies from Japan, two studies from Iran, and one study each from Germany, Portugal, France, and Italy.The median number of patients in the liver injury cohort of each study varied from 2 to 1784 (median 73.5 patients).A male predominance was identified in all but four studies.In 19 studies, the COVID-19(+) with liver injury group was presented as a single cohort.Inclusion criteria for the study groups were variable.In 15 studies, all patients with a combination of AST, ALT, BILI, and GGT exceeding the ULN were categorized in the liver injury group.In one study, the liver injury group was limited to patients with ALT ≥ 3 times the ULN[53].In the remaining studies, the COVID-19(+) with liver injury groups were stratified into two[48,55] or three groups[60] based on serum liver enzyme levels.In one study[54], a study reporting the results of liver biopsy in two COVID-19 patients with liver involvement and no evidence of pulmonary disease, inclusion in the study was based on the results of the liver biopsies.In another study[56], an autopsy-based report of 11 patients, inclusion was based on positive RT-PCR testing of liver tissue for SARS-Cov-2.The average age of the study group ranged from 41-years-old to 70-years-old.In all but three studies a male predominance was seen in the liver injury group.Most studies drew their study group from hospitalized inpatients, one study was conducted with critical care patients, and two studies were conducted with non-critical care inpatients.
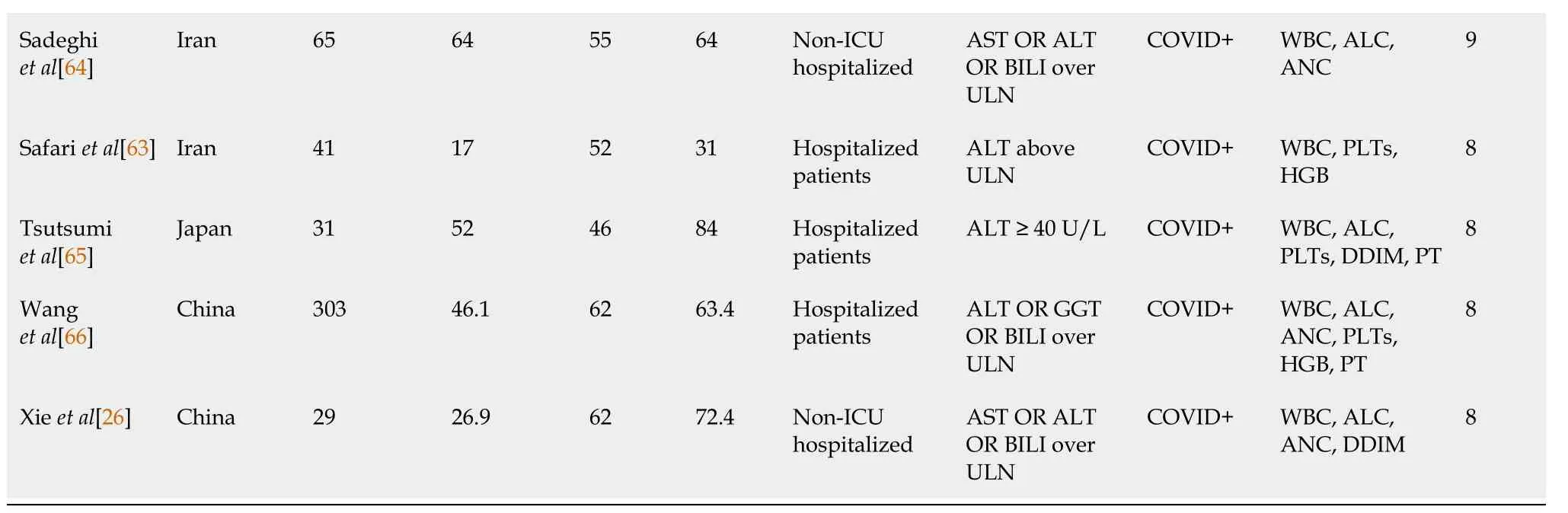
Eighteen studies (4973 patients) in this group contained statistical data that allowed comparative analyses of the following hematologic parameters: White blood cell count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), hemoglobin (HGB), platelet (PLT) count, d-dimer (DDIM), PT, PTT, and international normalized ratio (INR) (Table 2).
Although a direct comparison of the studies is limited by several factors such as heterogenous inclusion criteria and variable sample sizes, the majority of studies in which the inclusion criteria for liver injury were liver enzyme levels > ULN have demonstrated statistically significant differences in WBC, ANC, and ALC between non-liver injuryvsliver injury groups.Although smaller numbers of studies have addressed patients with higher elevations of liver enzymes, there were still statistically significant differences in WBCs between non-liver injuryvsliver injury groups.Limiting the analysis to studies with > 100 patients in the high liver function test (LFT) cohort (n= 10 studies, 4564 patients), statistically significant differences in WBC, ANC, ALC, and HGB were identified between non-liver injuryvsliver injury groups in the majority of studies.These differences appear most pronounced for WBCs and HGB, in which all studies demonstrated statistical significance; and ANC, in which 5/6 studies demonstrated statistical significance.
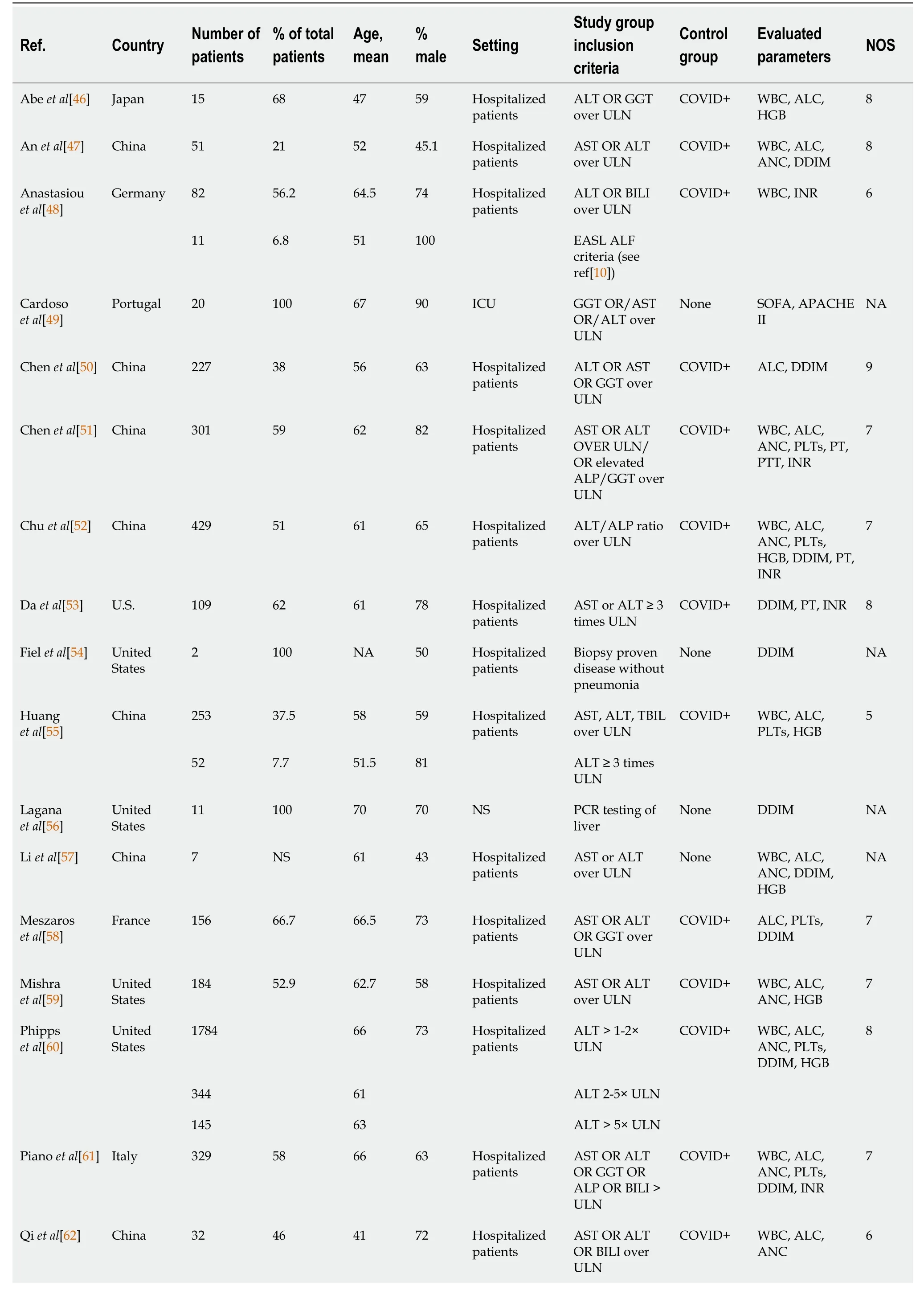
Table 1 Studies with a coronavirus disease 2019 (+) cohort with liver injury provided as a distinct group
ALC: Absolute lymphocyte count; ALP: Alkaline phosphatase; ALT: Alanine transaminase; ANC: Absolute neutrophil count; APACHE II: Acute Physiology and Chronic Health Evaluation II scoring system; AST: Aspartate transaminase; BILI: Bilirubin; COVID-19: Coronavirus disease 2019; DDIM: D-dimer; EASL ALF criteria: European Association for the Study of the Liver Acute Liver Failure Guidelines; GGT: Gamma-glutamyltransferase; HGB: Hemoglobin; INR: International normalized ratio; NA: Not applicable; NOS: Newcastle-Ottawa Scale; NS: Not stated; PLT: Platelet count; PT: Prothrombin time; PTT: Partial thromboplastin time; SOFA: Sequential Organ Failure Assessment scoring system; ULN: Upper limit of normal; WBC: White blood cell count.
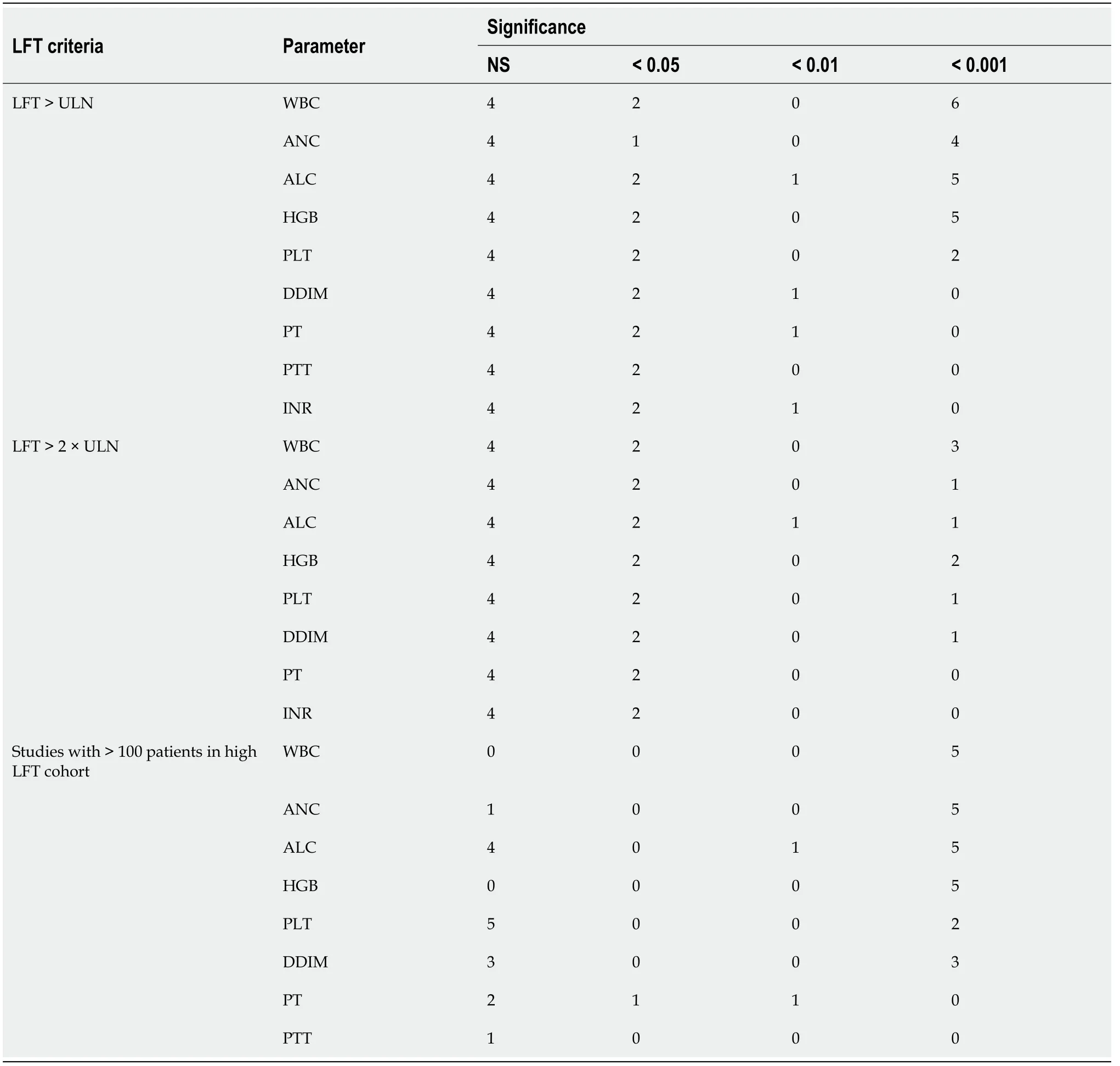
Five additional studies (425 patients), all retrospective, compared COVID-19 patients with severevsnon-severe disease and contained information about liver injury[67-71].The major features of these studies are summarized in Table 3 and provide some additional context to the information in the liver injuryvsnon-liver injury cohort studies about the potential use of hematology data.These include the study of Liaoet al[70], which demonstrated that liver injury correlated with inflammatory cytokine level, ALC, and DDIM; the study by Leiet al[69], which showed that liver injury correlated with disease severity and ALC; and the studies of Caiet al[67] and Jianget al[68], which identified a high percentage of patients with liver failure in the severe disease cohorts, which differed from the non-severe cohorts in ALC and DDIM levels.
Five other retrospective cohort studies (Table 4) likewise demonstrated the potential usefulness of hematology data in COVID-19 disease and liver injury[72-76].These included the study of Caoet al[72] which demonstrated that serum ferritin levels correlated with disease severity and degree of liver injury, as well as showed statistically significant differences in WBC, ANC, ALC, and DDIM between the two groups.Another study correlating elevated liver enzymes/liver injury with increased risk of COVID-19 disease recurrence noted significant differences in PLTs between patients with non-recurrentvsrecurrent disease[73].The study of Dinget al[74], which associated AST and direct bilirubin elevation with increased risk of mortality also noted significant differences in ALC, PLTs, and DDIM between the two groups.Leiet al[75] in a study examining peak levels of AST, ALC, ALP, and TBIL in patients with COVID-19 and liver injury noted that peak levels correlated with ANC, ALC, PLTS, and DDIM.Finally, Luanet al[76], who noted the correlation of COVID-19-related coagulopathy with liver injury, also found an associated significant difference in ALC, ANC, and DDIM between patients with and without coagulopathy.
Additional analysis (study quality)
Twenty-eight studies in our series contained control groups and could be evaluated using the NOS for cohort studies.Overall, 24/28 (86%) studies had a NOS in the high quality (7-9) range, 4/28 (14%) in the moderate quality (4-6) range, and no studies were rated in the low quality (0-3) range.Notably, for 24/28 (86%) papers, there was consensus in the initial evaluation by Frater JL and Wang T.
TabIe 2 Summary of statisticaI data from studies comparing Iiver injury vs non-Iiver injury
DISCUSSION
Summary of evidence
The purpose of this systematic review was to evaluate whether the hematologic parameters of importance in diagnosis and risk stratification in COVID-19 can be applied to the subpopulation with liver injury, and to determine whether the current state of the evidence supports whether SARS-CoV-2 patients should be categorized as having severe disease.A total of 32 studies met our inclusion criteria and were included in the analyses.The overall quality of the studies was strong, with 86% having an NOS ≥ 7.Based on our evaluation, WBCs, ANC, ALC, and HGB appear to the most useful parameters in addition to LFT to distinguish COVID-19 patients with liver injury from those without liver injury.Similarly, studies that analyzed severevsnon-severe COVID-19 cohorts showed that liver injury was present in a high proportion of patients with severe disease, who also had significant differences in ALC and DDIM.Studies focused on liver injury with other study designs likewise supported the association of liver injury with altered WBC, ANC, ALC, PLTs, and DDIM.
Table 3 Studies comparing coronavirus disease 2019 patients with severe vs non-severe disease
Association of liver injury with abnormalities of hematologic parameters: Diagnosis and risk stratification
Automated hematology data and basic coagulation testing is widely available, costeffective, and can be performed with rapid turnaround time.In situations where this data is of proven utility, it can provide value to the clinical team, either alone or as a component of other clinical and laboratory testing predictive models, such as the Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II scoring systems, which are widely used in critical care medicine.
Increased W BC has been associated with severe disease in COVID-19, and in some cases with an increased neutrophil-to-lymphocyte ratio and increase in eosinophils[77,78].In our study 8/12 studies showed a significant increase in W BC in COVID-19 patients with liver injuryvsthose without liver injury.Notably, all evaluated studies with > 100 patients (n= 5) in the liver injury group had a significant difference in WBC (P< 0.001), suggesting that the WBC is a useful addition to LFT for assessing liver function in COVID-19.However, the available data do not appear to support a role for WBC in risk stratification, as only 1/5 studies of non-severevssevere disease demonstrated a significant difference in the WBC.
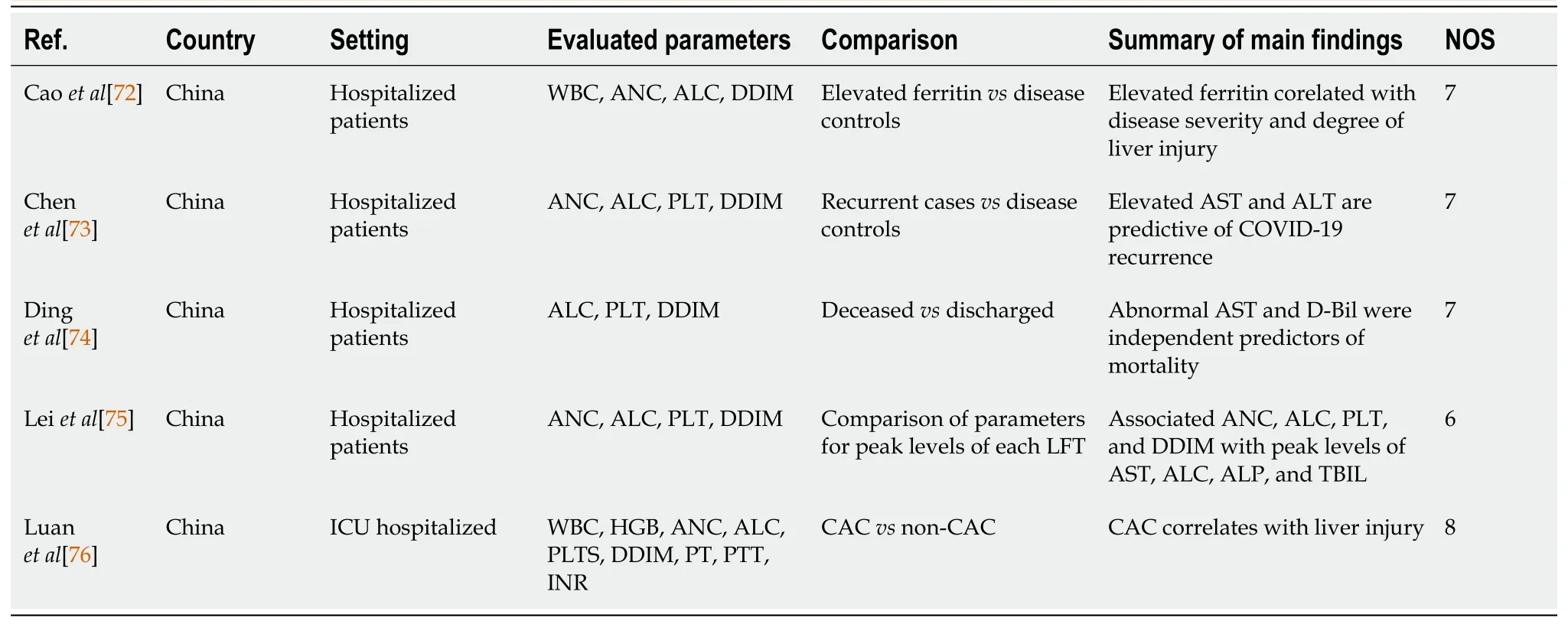
Table 4 Other retrospective cohort studies
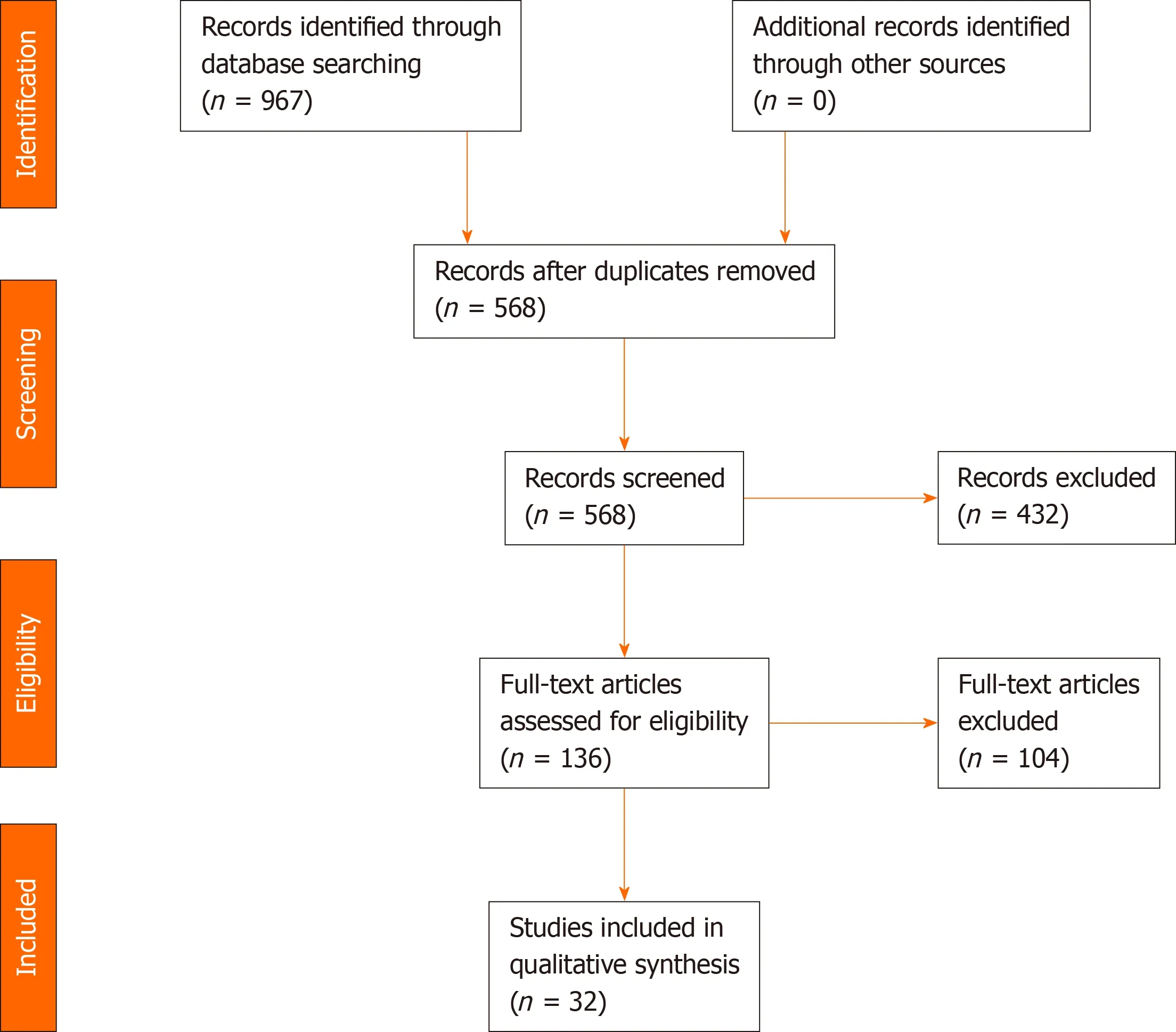
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 flow diagram.
Lymphopenia, believed to be due to a defective host response, has been identified in COVID-19 patients and has been associated with increased risk of severe disease[2-4,79].The overall performance of ALC as a biomarker of liver injury appears mixed, as it is not significantly different from COVID-19 patients without liver injury in 4/10 studies with > 100 liver injury patients.Likewise, 3/5 studies showed the ALC to be associated with severe disease and increased risk of liver injury.
Neutrophilia appears to be associated with cytokine storm and hyperinflammation, which has been implicated as a major factor in the pathophysiology of COVID-19.Accordingly, differences in the ANC have been noted in studies evaluating the roles of hematologic data in more generalized populations of COVID-19 patients[3,6,8,9,50,79].In our study, altered ANC appears to be associated with liver injury in COVID-19, with 5/6 studies with > 100 participants with liver injury having significant differences in ANC compared to those without liver injury.Its usefulness for risk stratification is more difficult to interpret.Although only 1/4 non-severevssevere COVID-19 studies demonstrated a difference in ANC, it has been associated with increased risk of COVID-associated coagulopathy, increased serum ferritin, and increased liver enzymes[72,75,76].Thus, although ANC appears to be a useful maker of liver injury, its potential as a marker for disease stratification is unclear at this point.
Thrombocytopenia has been identified as a maker of severe COVID-19 and has been associated with consumptive coagulopathy[5,79].In our study, the majority of studies of COVID-19 with liver injuryvsthose without liver injury did not identify significant difference in PLTs, with only 2/7 studies with > 100 patients in the liver injury group demonstrating significant differences in PLTS.Likewise, 0/4 studies of severevsnonsevere COVID-19 patients in our analysis demonstrated significant differences in PLTs.These results indicate that PLTs are not useful either for diagnosis or risk stratification in this group.
An association of decreased HGB with severe COVID-19 has been proposed, but is controversial due to the high heterogeneity of available data[80].Interestingly, our data imply a potential role of HGB for assessment of liver function in COVID-19, with significant differences in hemoglobin detected in 5/5 studies of liver injury with > 100 participants.Unfortunately, HGB was analyzed in only 1 study in the severevsnonsevere study group[70], and a significant difference was not identified.Although these results are promising for the use of HGB as an adjunct to detection of liver injury, further work is necessary to determine whether HGB is a predictor of high risk in this population.
Acquired coagulopathies are a major feature of COVID-19, and a subset of patients develop disseminated intravascular coagulation and multiorgan failure[79,81,82].In patients with liver injury, significant differences in DDIM were identified in 3/6 studies with > 100 participants; prolongation of PT was identified in 2/4 studies; and prolonged aPTT was present in 0/1 of these studies.In studies comparing non-severe to severe COVID-19 disease differences in DDIM level were identified in 3/3 studies and PT prolongation was identified in 1/1 study.These findings, although based on only a limited number of studies, imply that coagulopathy in COVID-19 is not limited to patients with liver injury and is associated with more severe disease.
Limitations
There were some limitations to this study.Despite an effort to comprehensively search the peer-reviewed medical literature, publications that did not mention liver injury in the title, abstract, or keywords of the papers may not have been included in the search results.Delays in indexing in the databases may also have contributed to papers not being identified in the search.Although some national and international organizations have worked to precisely define the term COVID-19 liver injury[83,84], the lack of precision, especially in papers published early in the pandemic, makes it challenging to confidently compare the results of individual studies.
CONCLUSION
We conducted a systematic review of the peer-reviewed literature regarding laboratory hematology data and their potential use in diagnosis and risk stratification in COVID-19 patients with liver injury.Based on our interpretation of the evidence, evaluation, WBC, ANC, ALC, and HGB may be of interest in addition to LFT in the distinction of patients with liver injury from those without.Since these markers are cited as markers of severe disease in many studies in the medical literature[1], this implies that COVID-19 patients are at increased risk of severe disease.This claim is supported by our evaluation of the medical literature that discusses liver injury in the context of non-severevssevere disease.Our findings support the conclusion that WBC, ANC, ALC, and HGB may be useful for diagnosis and risk stratification in the subset of COVID-19 patients with liver failure, as they are for the general population of patients infected by SARS-CoV-2.Other hematology parameters, such as PLTs, which are widely used in critical care medicine and are a component of the SOFA score, appear to be less relevant in liver failure patients with COVID-19.Our evaluation of the literature concludes that, although it has a high methodological quality, it is limited by the lack of a clear definition for COVID-19 liver injury, and by the lack of comprehensive reporting of laboratory data in many epidemiological studies.The limited statistical power of a high fraction of these studies, and the lack of statistical analysis of hematologic parameters beyond the univariate t-test are further issues.Further work with larger numbers of patients and more robust data analysis may be of interest to determine the precise role of hematologic parameters in the diagnosis and stratification of COVID-19 patients with liver injury.
ARTICLE HIGHLIGHTS
Research background
Hematology laboratory testing has an established role in risk stratification in patients with coronavirus disease 2019 (COVID-19) disease.Liver injury is common in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.The utility of hematology laboratory data in diagnosis and risk stratification of patients with COVID-19-related liver injury is unclear.
Research motivation
To what degree are the abnormalities in hematologic parameters seen in the general population of patients with COVID-19 also seen in the subpopulation of patients with associated liver injury? Is liver injury in COVID-19 indicative of severe disease.Does liver injury correlate with hematologic markers of severe disease? What is the quality of literature that addresses these questions?
Research objectives
The objectives of this study were: To determine the extent to which previously reported hematologic abnormalities are seen in patients with COVID-19-associated liver injury; to assess whether liver injury in COVID-19 is a sign of severe disease, and the extent to which liver injury correlates with markers of severe disease; and based on the extant literature, to determine the quality of this evidence.
Research methods
This study was conducted as a Preferred Reporting Items for Systematic Reviews and Meta-Analyses-compliant systematic review.We extracted information from cohort studies related to the topic of liver injury in COVID-19 in which laboratory hematology data were included.
Research results
In all, 32 articles were included in the systematic review, which consisted of 22 articles with a cohort of COVID-19 patients with liver injury; 5 comparing non-severe vs severe COVID-19 populations in which liver injury was addressed and 5 other cohort studies with a focus on liver injury.White blood cell count, absolute neutrophil count,absolute lymphocyte count (ALC), and hemoglobin were the parameters most useful to distinguish COVID-19 with liver injury from COVID-19 without liver injury.ALC and d-dimer were potentially useful in distinguishing non-severe from severe COVID-19.Liver injury was more frequently seen in cohorts with severe disease.Most studies were of high quality (24/48, 86%) with 4/28 (14%) of moderate quality and 0 of low quality.
Research conclusions
The use of select hematologic parameters in diagnosis and risk stratification of liver injury in COVID-19 patients appears warranted.The relevant literature is high quality,but is limited by the small number of studies with high statistical power and the variable definition of COVID-19 liver injury in the literature.
Research perspectives
Future studies, preferably with a prospective design, large numbers of patients, and rigorous definition of liver injury would be useful to validate these findings.
 World Journal of Meta-Analysis2021年2期
World Journal of Meta-Analysis2021年2期
- World Journal of Meta-Analysis的其它文章
- Effect of resistance exercise on insulin sensitivity of skeletal muscle
- Is COVID-19-induced liver injury different from other RNA viruses?
- Ankle injuries in athletes: A review of the literature
- Viral hepatitis: A brief introduction, review of management,advances and challenges
- Metabolic and biological changes in children with obesity and diabetes
- Nonalcoholic fatty liver disease and cardiovascular concerns: The time for hepatologist and cardiologist close collaboration
