Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials
Yue Han, Wei-Hua Zhi, Fei Xu, Chen-Bo Zhang, Xiao-Qian Huang, Jian-Feng Luo
Abstract
Key Words: Hepatocellular carcinoma; Systemic therapy; Meta-analysis; Lenvatinib; Firstline; Immune therapy
INTRODUCTION
Liver cancer is the sixth most common cancer globally, accounting for 4 .7 % of all new cancer cases in 2018 , and represents the third most common cause of cancer-related death worldwide behind lung and colorectal cancer[1]. Of the primary liver cancers,hepatocellular carcinoma (HCC) is the most prevalent histological subtype and accounts for 80 %-85 % of cases[2 ]. Surgical resection and liver transplant are associated with the best survival outcomes for patients with HCC, and are potentially curative treatments[3]. Locoregional therapies including arterially directed therapies, ablation,and radiotherapy are also associated with good survival outcomes in patients with unresectable disease confined to the liver[4 ]. However, over 50 % of patients with HCC are diagnosed at an advanced stage or with other characteristics that preclude surgical or locoregional treatment[5]. For these patients, systemic therapy is usually the recommended treatment option[4 ,6 ].
Over the past 3 years, the number of approved first-line systemic therapies for patients with HCC has expanded greatly, and numerous drugs and drug combinations have been evaluated in this setting[7 ]. Between 2007 and 2018 , sorafenib was the only approved systemic treatment for HCC based on the results of the Phase III SHARP trial, which showed a survival benefit for sorafenibvsplacebo[8]. In the decade following the approval of sorafenib, numerous unsuccessful trials of systemic therapies in advanced HCC were conducted until the approval of lenvatinib in 2018 [9 ]. Lenvatinib was approved for first-line use in advanced HCC following the successful outcome of the Phase III REFLECT trial. In this trial, lenvatinib showed a non-inferior overall survival (OS)vssorafenib for the treatment of advanced HCC[10 ].Since the approval of lenvatinib, the immunotherapy drugs nivolumab and pembrolizumab, as well as other tyrosine kinase inhibitors (TKI), have been approved for the second-line treatment of HCC. Most recently, combination therapy with the anti-PDL1 agent atezolizumab plus bevacizumab demonstrated better OS and progressionfree survival (PFS) than sorafenib in the Phase III IMbrave 150 trial[11 ].
The expansion of first-line treatment options for advanced HCC represents a significant advance in the treatment of this disease. However, further data would be useful to inform treatment selection. Most clinical trials of first-line therapies for HCC used placebo or sorafenib as comparators and there are limited data providing a cross comparison of the efficacy and safety of drugs in this setting. Furthermore, although lenvatinib is widely seen as a standard of care in real clinical practice and is a recommended first-line therapy in most international treatment guidelines[4 ,12 ,13 ],there are limited head-to-head data comparing lenvatinib with other systemic therapies. Finally, although historically systemic treatments for HCC were associated with low tumor response rates, recently approved therapies have been associated with response rates > 30 %[14 ]. This has led to renewed interest in tumor response rates in HCC, and investigation of downstaging and conversion therapy strategies. A comparison of response rates for all currently available therapies would therefore be of clinical value.
This network meta-analysis was conducted to systematically review and compare the response rates, survival outcomes, and safety reported by randomized trials of first-line systemic therapies in patients with advanced unresectable HCC, and to provide a comparison between lenvatinib and other systemic therapies in this setting.Two recent meta-analyses have investigated a similar topic to the present study;however, one did not include data on atezolizumab plus bevacizumab and excluded non-targeted therapies[15 ], and a more recent analysis focused on treatment sequencing by investigating survival outcomes only[16 ]. Therefore, although there is some overlap with the present analysis, these studies are complementary to each other. In particular, the present analysis is the first to include data on donafenib, a Chinese drug that has shown a superior OS to sorafenib in a Phase III trial[17 ].Furthermore, our analysis includes data on survival, response rate, and safety, which in combination are important for treatment decision-making, particularly for patients who may be candidates for downstaging. Finally, the present meta-analysis included a sub-group analysis of patients with HBV infection, which is an important population in the Asia-Pacific region and has not been covered by other current meta-analyses.
MATERIALS AND METHODS
The analysis methods and inclusion criteria for this study were specified in advance and the protocol was prospectively submitted for registration in the PROSPERO database on May 26 , 2020 . This report has been written in line with the PRISMA guidelines for network meta-analyses.
Eligibility criteria
This analysis included randomized controlled trials conducted in adult patients (age ≥18 years) with advanced or unresectable HCC not eligible for, or with disease progression after, surgical or locoregional therapies. Eligible studies included patients with Child-Pugh Class A or B liver function, ≥ 1 measurable lesion, and no evidence of untreated brain or meningeal metastases. Eligible studies were also required to report at least an assessment of tumor response, survival [OS, PFS, or time to progression(TTP)], and safety. The analysis excluded studies including patients with Child-Pugh Class C liver function, patients receiving anticoagulation therapy or antiretroviral therapy for HIV, and patients who had received previous systemic treatment. These broad eligibility criteria covered a number of trials reporting negative resultsvssorafenib. Although the analysis therefore includes multiple therapies that failed clinical trials in HCC, this allowed the collection of data for sorafenib from studies conducted over a wide time range, which improved the precision of the analysis.
Information sources, search strategy, and study selection
Studies were identified by searching the following electronic databases: PubMed,Science Direct, and the Cochrane Database, and Excerpta Medica Database Abstracts from the American Society of Clinical Oncology (ASCO) 2020 annual congress were also searched. The search was completed on May 21 , 2020 using the search terms shown in Figure 1.
The Cochrane risk of bias tool for randomized controlled trials was used to assess the quality and risk of bias of studies included in the analysis[18 ].
Data extraction
Data were independently extracted by two evaluators (Luo JF and Huang XQ) and cross-checked. In the case of disagreement, the original documents were checked and the correct data confirmed. General information extracted included journal name,document title, publication time, author, country, region where the lead author was located, and the country and region where the research was conducted. Demographics and baseline characteristics extracted were patient age, gender, Barcelona clinic liver cancer classification, Eastern Co-operative Oncology Group performance status,prevalence of HBV infection, and presence of extrahepatic vascular infiltration and extrahepatic metastasis. Details of interventions extracted included dosage and dose schedule. Efficacy and safety endpoints extracted (where available) were overall response rate [ORR; assessed by response evaluation criteria in solid tumours(RECIST) v1 /1 .1 for all included studies], OS, PFS, TTP, incidence of Grade ≥ 3 adverse events (AE), incidence of treatment interruption due to adverse events (AEs), and incidence of dose reductions due to AEs.
Statistical analysis
For OS, PFS, TTP, and other survival endpoints, hazard ratios (HR) were estimated to compare treatments. For discrete variables such as ORR, and incidence of AEs,estimated risk ratios were calculated to compare treatments. Selection of a fixed effect or random effect model was based on the level of heterogeneity in the data, assessed using the HigginsI2statistic and defined as I2 ≤ 50 % and P > 0 .1 . If no obvious data heterogeneity was found, a fixed effect model was adopted, otherwise a random effect model was utilized. For endpoints reported in a relatively small number of studies (<6), a fixed effects model was adopted.
A network meta-analysis was used to synthesize information from the included studies, and perform direct and indirect comparisons using a method based on the frequency school of Rückeret al[19 ,20 ]. The Q statistic was used to assess the consistency of direct and indirect evidence in the treatment network(s) studied. If no obvious inconsistency (P> 0 .1 ) was found, a fixed effect model was adopted,otherwise a random effect model was utilized.Pvalue, a frequentist analog to the surface under the cumulative ranking curve, was used to rank treatments[21 ]. A funnel chart was used to evaluate publication bias; a symmetrical graph indicates a low influence of publication bias and an asymmetric graph indicates possible publication bias. A post-hoc analysis of all studies reporting data from patients with HBV-related HCC was also included to assess OS, PFS, and safety in these patients.
膀胱痉挛是前列腺电切术和比较常见的并发症,表现为进行性排尿困难、尿频、尿急、夜尿增多等,发生率为40%~100%[7]。膀胱痉挛严重影响了患者的手术舒适度,是护理工作的重点内容[8]。传统的护理方法多重视病情观察以及注意事项的告知,对膀胱痉挛的预防不够。近年来,采用综合性的护理方法预防膀胱痉挛的发生已经成为研究的特点。蔡丽娟等[9]采用综合性护理预防经尿道膀胱肿瘤电切术后的膀胱痉挛发现效果满意,其能明显缓解膀胱痉挛的程度,缩短膀胱痉挛的时间。农小珍的研究显示[3],综合性护理不仅能够减轻前列腺电切术患者术后的膀胱痉挛程度,而且能够减轻患者的心理负担。
All statistical analyses were performed using Rv3 .6 . The Robias toolkit was used for evaluation of literature quality and Netmeta was used for the network meta-analysis.
RESULTS
Studies included in the analysis
In total, 1398 articles were screened: PubMed/MEDLINE, n = 114 ; Science Direct,n=312 ; Cochrane Database, n = 355 ; Excerpta Medica Database, n = 561 ; and the ASCO 2020 abstract book, n = 12 (Figure 1 ). After removing duplicates and top-line screening of abstracts for suitability, a total of 86 articles were reviewed in detail, of which 27 met the full inclusion criteria (Table 1 ). These 27 articles corresponded to 27 different studies (Supplementary Figure 1).
Study characteristics
Of the 27 studies included, 25 investigated targeted treatment regimens (nintedanib,mapatumumab + sorafenib, atezolizumab + bevacizumab, doxorubicin + sorafenib,dovitinib, tigatuzumab + sorafenib, vandetanib, brivanib, linifanib, lenvatinib,nivolumab, sunitinib, sorafenib + erlotinib, sorafenib (two studies), nintedanib,bevacizumab + erlotinib, sorafenib + doxorubicin, sorafenib + resminostat, sorafenib +pravastatin, AEG35156 (a second-generation synthetic antisense oligonucleotide inhibitor of cellular expression of the X-linked inhibitor of apoptosis protein) +sorafenib, sorafenib + gemcitabine and cisplatin, sorafenib + tegafur–uracil, sorafenib+ everolimus, and donafinib) and two investigated combination chemotherapy regimens [oxaliplatin/folinic acid/5 -fluorouracil (FOLFOX4 ) and cisplatin/interferon α-2 b/doxorubicin/5 -fluorouracil] (Table 1 ). Twenty-one of the included studies used sorafenib as the comparator treatment, three used doxorubicin, and three studies were placebo controlled (including the two Phase III studies of sorafenib). The majority of the studies had OS (n= 12 ) or PFS/TTP (n = 10 ) as the primary endpoint, and almost all had reported final data for these endpoints.
Quality assessment
Study design characteristics are summarized in Supplementary Table 1 . In brief, all 27 studies selected for inclusion were randomized controlled studies (20 provided details of the randomization scheme used and seven articles did not specify), seven of the studies used double blinding and 20 were open label, and 24 included a data flow chart. Overall, the quality of the included studies was considered relatively high(Figure 1).
Patient description
All of the studies included patients with advanced HCC who had not received previous treatment. Overall, the total of 10256 patients included in the analysis were predominantly male and had median ages ranging from 49 to 68 years, and most of the studies included > 50 % of patients with extrahepatic metastasis (Table 2 ).
Evaluation of efficacy
Overall response rate:A total of 18 studies reported ORR, including 19 interventions and allowing 20 comparisons (Figure 2 A). No significant heterogeneity was detected between the studies (tau-squared = 0 ; I2 = 0 %; P = 0 .9502 ) and a fixed effect model was selected.Pvalue for ORR showed that lenvatinib was associated with the best ORR among all treatments included in the analysis (P= 0 .9042 ) (Figure 2 B). Atezolizumab +bevacizumab ranked second (P= 0 .8045 ) and nivolumab ranked third (P = 0 .7834 ).Using lenvatinib as the comparator, all treatments included in the analysis had an estimated risk ratio for ORR (RRORR) of < 1 , except for AEG35156 + sorafenib, which had an estimated RRORRof 1 .3451 [95 % confidence interval (CI): 0 .07 -25 .21 ] (Figure 2 B).
Progression-free survival:A total of 15 studies reported PFS, including 15 interventions and allowing 15 comparisons (Figure 3 A). No significant heterogeneity was detected (tau-squared = 0 ; I2 = 0 %; P = 0 .7361 ) and a fixed effect model was selected.Atezolizumab + bevacizumab was ranked first for PFS (P= 0 .9501 ), followed by lenvatinib (P= 0 .9041 ). Nivolumab ranked sixth (P = 0 .558 ) (Figure 3 B). With the exception of atezolizumab + bevacizumab (HRPFS= 0 .90 ; 95 %CI: 0 .64 -1 .25 ), the estimated HRs for PFS for all included treatmentsvslenvatinib were > 1; however, the associated 95 %CI passed through unity for bevacizumab plus erlotinib, linifanib, and FOLFOX4.
Time to progression:A total of 17 studies reported TTP, including 17 interventions and allowing 19 comparisons (Figure 3 C). No significant heterogeneity was detected between studies (tau-squared = 0 ; I2 = 0 %; P = 0 .9028 ) and a fixed effect model was selected. Lenvatinib was ranked first for TTP (P= 0 .9888 ) followed by linifanib (P=0 .9067 ) and sorafenib + doxorubicin (P = 0 .7344 ) (Figure 3 D). When compared with lenvatinib, all other treatments in the analysis had an estimated HRTTP> 1 , although the associated 95 %CI passed through unity for linifanib and sorafenib plus tegafur–uracil.
Overall survival:A total of 24 studies reported OS, including 25 interventions and allowing 28 comparisons (Figure 3 E). No significant heterogeneity was detected between studies (tau-squared = 0 ; I2 = 0 %; P = 0 .9802 ) and a fixed effect model was selected. Atezolizumab + bevacizumab was ranked highest for OS (P= 0 .9651 )followed by vandetanib 100 mg/d (P = 0 .8653 ), donafinib (P = 0 .7958 ), and nivolumab(P= 0 .7701 ) (Figure 3 F). Lenvatinib ranked sixth (P = 0 .6675 ). Atezolizumab +bevacizumab was associated with a lower risk of deathvslenvatinib (HRos= 0 .63 ;95 %CI: 0 .44 -0 .89 ), and the HRosfor most other treatmentsvslenvatinib had associated 95 %CIs that passed through unity.
Outcomes in patients with HBV infection:Ten studies included sub-analyses of patients with HBV infection, including data on the following treatments: atezolizumab+ bevacizumab[11 ], brivanib[22 ], nivolumab[23 ], lenvatinib[10 ], linifanib[24 ], sorafenib[25 ], sorafenib + erlotinib[26 ], sorafenib + resminostat[27 ], tigatuzumab +sorafenib[28 ], and sunitinib[29 ]. A total of three studies reported PFS in patients with HBV infection, including four interventions and three comparisons (Supplementary Figure 2 A). A fixed effect model was selected for the analysis. Lenvatinib ranked first for PFS (P= 0 .8786 ) followed by atezolizumab + bevacizumab (P = 0 .7746 ) and donafinib (P= 0 .2972 ) (Figure 2 B). A comparison of HRs for PFSvslenvatinib is shown in Supplementary Figure 2 B. A total of nine studies reported OS, including ten interventions and allowing nine comparisons (Supplementary Figure 2 C). A random effect model was selected for the analysis. Atezolizumab + bevacizumab ranked first (P= 0 .9751 ), followed by lenvatinib (P = 0 .8308 ) and nivolumab (P = 0 .7732 ) (Supplementary Figure 2 D). Comparison with lenvatinib revealed that atezolizumab +bevacizumab had an estimated HROS< 1 and all other interventions had an HROS > 1 (Supplementary Figure 2 D).
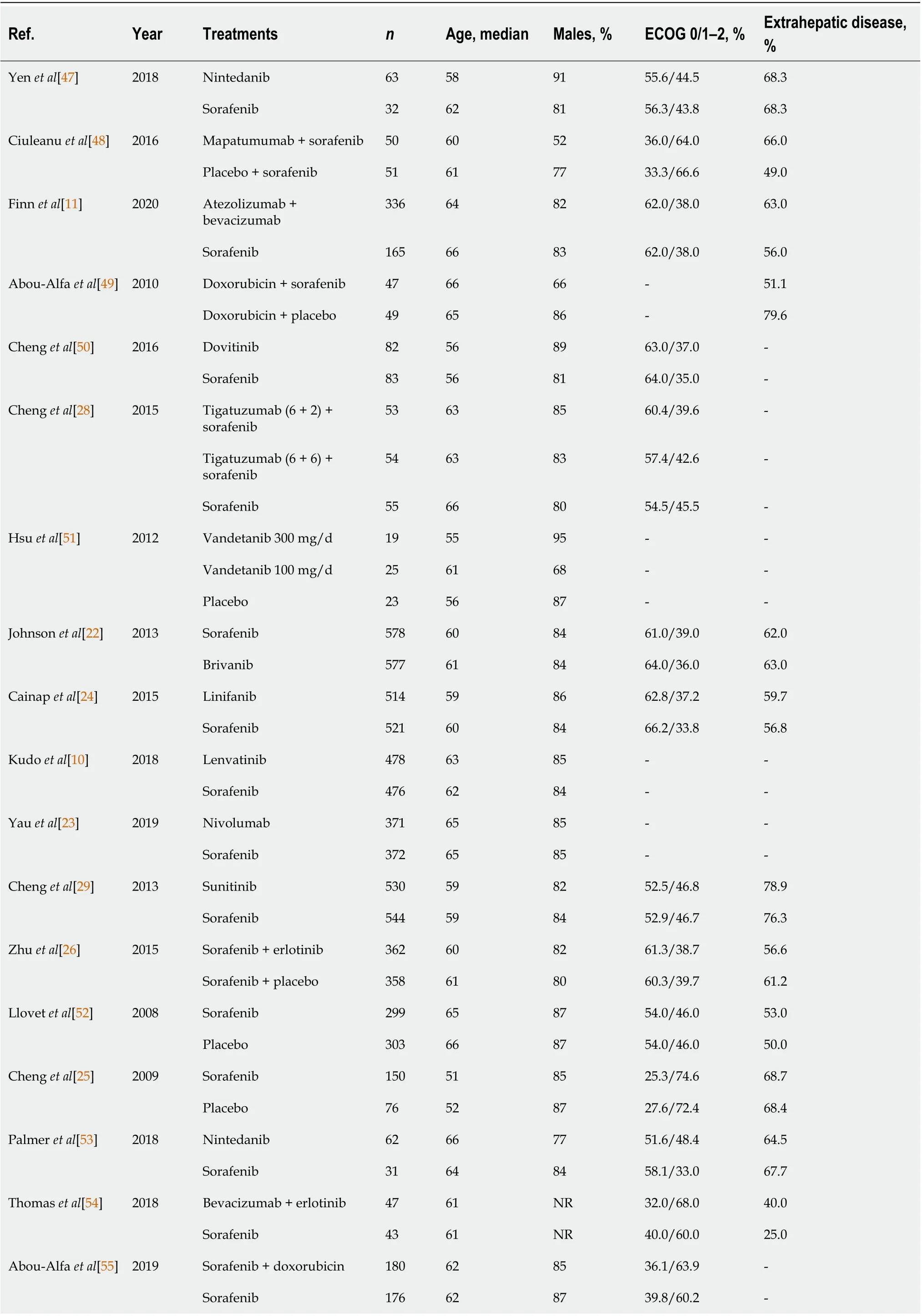
Table 2 Patient characteristics in the included studies
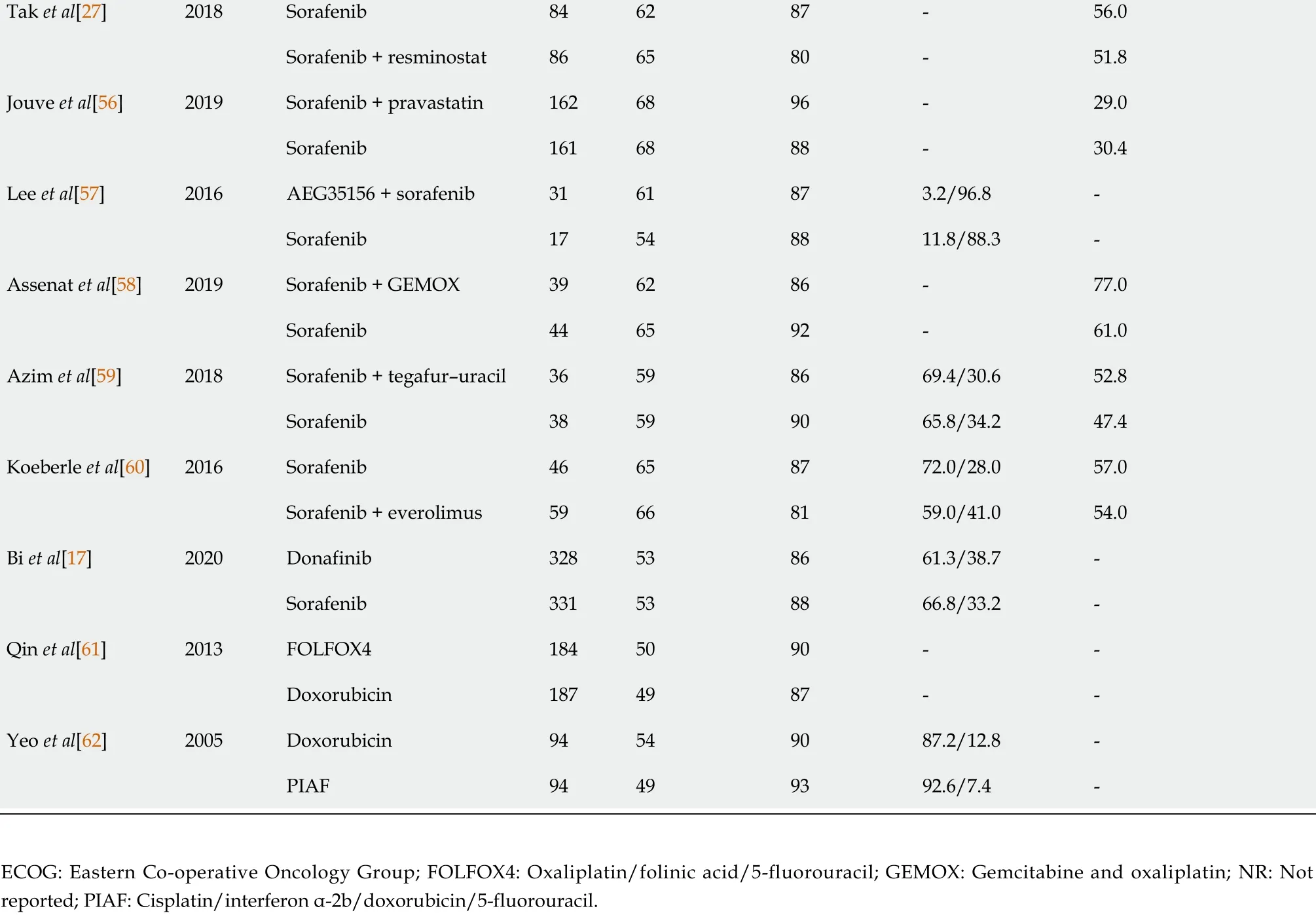
Tak et al[27 ]2018 Sorafenib 846287 -56 .0 Sorafenib + resminostat 866580 -51 .8 Jouve et al[56 ]2019 Sorafenib + pravastatin 1626896 -29 .0 Sorafenib 1616888 -30 .4 Lee et al[57 ]2016 AEG35156 + sorafenib 3161873 .2 /96 .8 -Sorafenib 17548811 .8 /88 .3 -Assenat et al[58 ]2019 Sorafenib + GEMOX 396286 -77 .0 Sorafenib 446592 -61 .0 Azim et al[59 ]2018 Sorafenib + tegafur–uracil 36598669 .4 /30 .652 .8 Sorafenib 38599065 .8 /34 .247 .4 Koeberle et al[60 ]2016 Sorafenib 46658772 .0 /28 .057 .0 Sorafenib + everolimus 59668159 .0 /41 .054 .0 Bi et al[17 ]2020 Donafinib 328538661 .3 /38 .7 -Sorafenib 331538866 .8 /33 .2 -Qin et al[61 ]2013 FOLFOX41845090 --Doxorubicin 1874987 --Yeo et al[62 ]2005 Doxorubicin 94549087 .2 /12 .8 -PIAF 94499392 .6 /7 .4 -ECOG: Eastern Co-operative Oncology Group; FOLFOX4 : Oxaliplatin/folinic acid/5 -fluorouracil; GEMOX: Gemcitabine and oxaliplatin; NR: Not reported; PIAF: Cisplatin/interferon α-2 b/doxorubicin/5 -fluorouracil.
Safety
Grade ≥ 3adverse events: In total, 17 studies reported data on the incidence of Grade≥ 3 AEs, including 19 interventions and allowing 21 comparisons (Supplementary Figure 3 A). No significant heterogeneity was detected between studies (tau-squared =0 ; I2 = 0 %; P = 0 .4493 ) and a fixed effect model was selected. Nivolumab ranked 2 /19 (P= 0 .9351 ), sorafenib ranked 8 /19 (P = 0 .5040 ), atezolizumab + bevacizumab ranked 11 /19 (P = 0 .4167 ), and lenvatinib ranked 16 /19 (P = 0 .2468 ) for incidence of Grade ≥ 3 AEs (higher ranking indicated a lower incidence of AEs) (Supplementary Figure 3 B).
Treatment termination due to adverse events:A total of 13 studies reported the incidence of treatment termination due to AEs, including 13 interventions and allowing 15 comparisons (Supplementary Figure 3 C). A degree of heterogeneity was detected between studies (tau-squared = 0 .1536 ; I2 = 65 %; P = 0 .0573 ) and a random effect model was selected. After ranking all interventions from the lowest to highest incidence of terminations due to AEs, vandetanib 300 mg/d and 100 mg/d were ranked first and second (P= 0 .8036 and 0 .7252 , respectively), sorafenib ranked 5 /13 (P= 0 .5372 ), nintedanib ranked 8 /13 (P = 0 .4251 ), lenvatinib ranked 10 /13 (P = 0 .3907 ),and atezolizumab + bevacizumab ranked 13 /13 (P = 0 .2584 ) (Supplementary Figure 3 D).
DISCUSSION
Following an expansion of first-line systemic treatment options for HCC over the past decade, international treatment guidelines now recommend sorafenib, lenvatinib, and atezolizumab plus bevacizumab in this setting, as well as nivolumab and FOLFOX(off-label use in many countries, but approved by the China National Medical Products Administration) for selected patients[4 ,12 ,13 ]. Numerous other therapies and combinations of therapies have also been unsuccessfully investigated in first-line advanced HCC management. However, most trials of systemic therapy for HCC used sorafenib as the comparator, as it was the only approved systemic therapy available at the time, and this limits the clinicians’ ability to compare currently available treatment options. The present study represents one of the most comprehensive systematic reviews and meta-analyses of first-line systemic treatments for advanced unresectable HCC conducted to date, and compares the treatment outcomes and safety of lenvatinib with multiple other systemic therapies, including immunotherapy(nivolumab) and combined therapy with immunotherapy and a TKI (atezolizumab +bevacizumab).
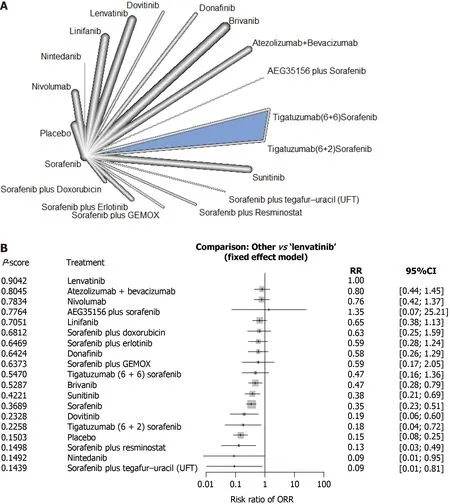
Figure 2 Response rates of first-line systemic therapy in patients with advanced hepatocellular carcinoma. A: Network diagram; B:Interventions ranked by P value with risk ratios and 95 % confidence interval for overall response rate for each treatment vs lenvatinib. CI: Confidence interval;GEMOX: Gemcitabine and oxaliplatin; ORR: Overall response rate; RR: Risk ratio.

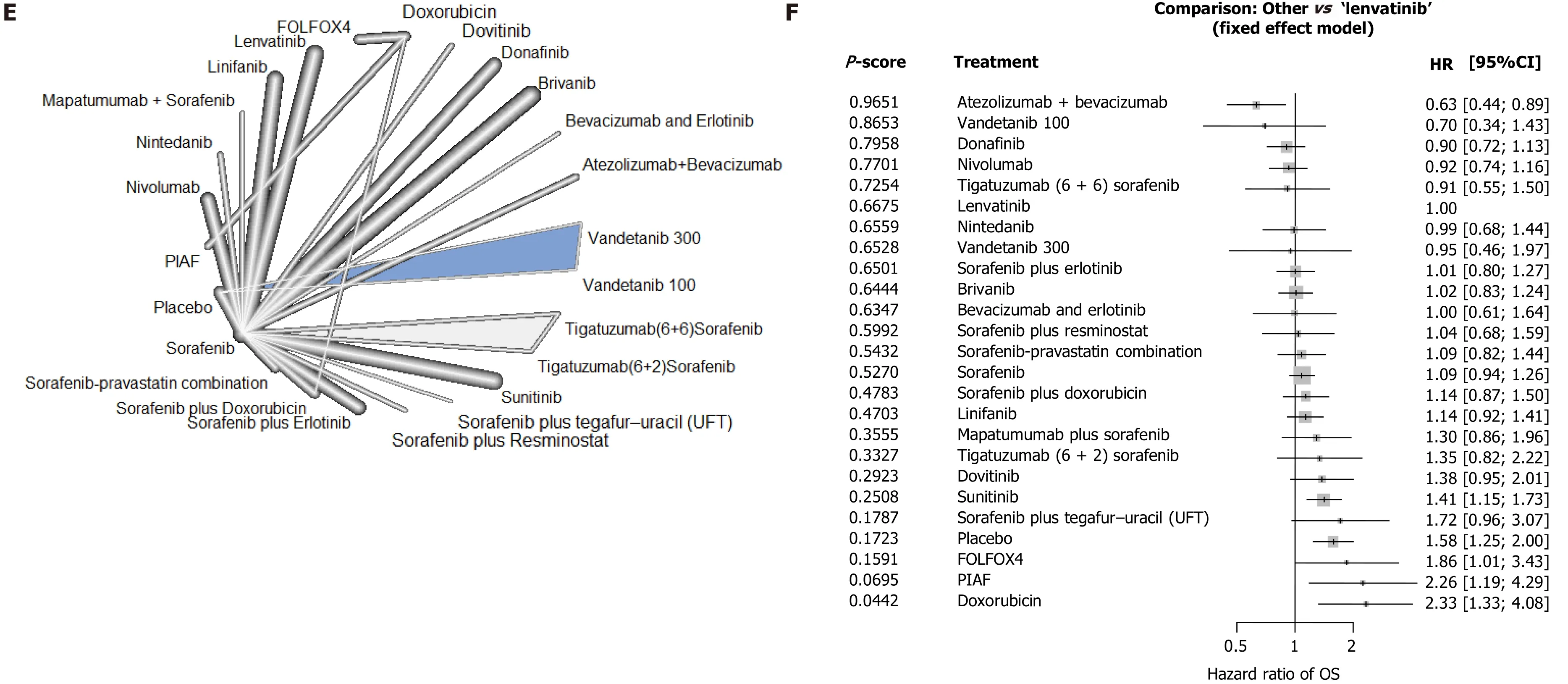
Figure 3 Survival outcomes in patients with advanced hepatocellular carcinoma following first-line systemic therapy. A, C, and E: Network diagrams; B: Interventions ranked by P value with hazard ratios for progression-free survival, D: Time to progression and F: overall survival for each treatment vs lenvatinib. CI: Confidence interval; FOLFOX4 : Oxaliplatin/folinic acid/5 -fluorouracil; HCC: Hepatocellular carcinoma; HR: Hazard ratio; OS: Overall survival; PIAF:Cisplatin/interferon α-2 b/doxorubicin/5 -fluorouracil; TTP: Time to progression.
Our results show that atezolizumab plus bevacizumab is associated with the best OS outcomes of all therapies included in the analysis. This result is supported by findings from a recent meta-analysis that investigated optimal treatment sequencing for HCC and also reported that atezolizumab plus bevacizumab had a higher OS benefitvslenvatinib (HROS= 0 .63 ; 95 %CI: 0 .44 -0 .89 ), nivolumab (HROS = 0 .68 ; 95 %CI:0 .48 -0 .98 ), and sorafenib (HROS = 0 .58 ; 95 %CI: 0 .42 -0 .80 )[16 ]. Atezolizumab plus bevacizumab is the first combined immunotherapy and vascular-targeted regimen to be recommended as a first-line treatment option in the National Comprehensive Cancer Network HCC guidelines[4]. The long OS associated with atezolizumab plus bevacizumab may be related to the ‘long tail’ effect characteristic of immune checkpoint inhibitors, which was also observed in the Phase III Checkmate 459 study of nivolumab. A number of studies have identified several mechanisms by which angiogenesis-related processes can enhance immune checkpoint inhibitors therapy,including vascular normalization, reduction of hypoxia, and increasing tumor infiltrating lymphocytes[30 ]. Although bevacizumab monotherapy failed Phase II trials in unresectable HCC, in combination with atezolizumab it led to superior efficacy compared with bevacizumb monotherapy[31 ]. However, consideration of treatment safety and tolerability is also an important factor in clinical decision-making. Our analysis revealed that atezolizumab plus bevacizumab was associated with the highest incidence of discontinuation due to AEs. This may be associated with the relatively long time to progression and duration of treatment reported for atezolizumab plus bevacizumab, but as treatment discontinuations due to AEs usually involve uncontrolled Grade ≥ 3 AEs, this would likely be a weak association. In addition, the prescribing information for bevacizumab highlights a possible risk of bleeding, and requires termination of bevacizumab at least 4 wk before surgery[32 ]. Therefore, in patients with high risk of gastric esophageal varices and patients with the potential to undergo any surgical procedures, atezolizumab plus bevacizumab should be used carefully, to manage the risk of bleeding events. Atezolizumab plus bevacizumab may be more suitable for patients who are unsuitable for surgery but with good liver function and limited cirrhosis, who have the potential to achieve a long-term survival benefit with systemic therapy.
The results of this meta-analysis show that there is currently not one single systemic treatment for advanced HCC associated with superior outcomes across all outcome measurements (ORR, OS, PFS, and safety). This highlights the importance of individualized treatment selection based on specific treatment goals. For example, a number of studies have shown that lenvatinib or lenvatinib combination therapy[33 ] can allow patients to achieve downstaging and become eligible for surgery[34 -36 ]. For patients with HCC ineligible for surgical intervention at diagnosis, we are of the opinion that treatment selection should be objective based. In patients without serious underlying liver disease and for whom surgery may be possible, systemic treatments with the highest ORR are the optimal choice. Conversely, for patients with poor liver function,underlying liver disease, or local advanced HCC, selection of therapies based on longer OS may provide the most benefit.
In our analysis, lenvatinib had superior short-term efficacy compared with all other systemic therapies investigated. Lenvatinib ranked first for ORR and TTP, and second for PFS after atezolizumab plus bevacizumab. This finding is supported by the results of another recent network meta-analysis presented at the ASCO Gastrointestinal Symposium 2021 that also ranked atezolizumab plus bevacizumab first for OS but lenvatinib first for ORR[37 ]. In addition, although direct comparison of the ORRs(RECIST v1 .1 ) reported for atezolizumab plus bevacizumab and lenvatinib in the IMbrave 100 and REFLECT studies appears to show a moderately higher ORR for atezolizumab plus bevacizumab (27 % vs 18 %)[10 ,11 ], our network analysis provides a more robust comparison of the two therapies by comparing both to sorafenib. There are several possible mechanistic explanations for this finding. First, preclinical studies show that lenvatinib has multiple targets including VEGFR1 -3 , FGFR1 -4 , PDGFRα,RET, and KIT, and this broad spectrum of activity may be one factor explaining the high response rates associated with this therapy[38 ]. Furthermore, lenvatinib is a type V TKI with fast binding and relatively slow dissociation compared with other TKIs[39 ]. In addition to anti-vascular effects, lenvatinib also has a regulatory effect on the immune microenvironment of liver cancer[40 ]. Preclinical research has shown that,compared with sorafenib, lenvatinib has a significant anti-tumor effect in immunodeficient mice, suggesting that lenvatinib may activate immune function by decreasing the number of tumor-associated macrophages, increasing the proportion of activated CD8 + cells[40 ], and increasing activation and infiltration of natural killer cells[41 ].
HBV-related liver cancer is particularly prevalent in Asian populations, especially in China where 69 %-80 % of liver cancers have an HBV etiology[42 ,43 ]. Our meta-analysis of data from patients with HCC and HBV infection suggested that, in terms of OS,atezolizumab plus bevacizumab, lenvatinib, and nivolumab are the three most effective treatments in this patient population. For PFS, lenvatinib ranked first over atezolizumab plus bevacizumab. This finding supports previous meta-analyses that have shown lenvatinib to have a particularly strong anti-tumor effectvssorafenib in patients with HBV-related HCC[44 ,45 ]. It is unclear why lenvatinib may have a particularly good anti-tumor effect in HBV-related HCC, but it may be due to the impact of lenvatinib on the immune microenvironment, as described above. In addition, the China National Health and Health Commission guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition) recommend lenvatinib as a systemic therapy with good efficacy in patients with HBV-related liver cancer[46 ].
This network meta-analysis had several possible limitations. First, the quality of studies included in the analysis had some heterogeneity; for example, the analysis included both large Phase III clinical trials, such as REFLECT and Checkmate 459 , and smaller Phase II clinical studies. Second, there was also heterogeneity in the patient populations included in the analysis, including patients from different geographic regions, of different races, and different proportions of patients with HBV infection.Additionally, it should be noted that second-line therapeutic options for HCC have greatly improved over the past decade. As a result, estimates of first-line OS from older studies are generally shorter than those from more recent studies. However,among the therapies included in this analysis that are currently approved for first-line HCC, only sorafenib has OS data old enough to potentially be biased by this phenomenon. Fortunately, our analysis was based on pooled data from the pivotal study of sorafenib in 2008 and comparator arms of trials conducted between 2008 and 2020 , which limits the potential effect of bias from improvements in second-line therapies[47 -62 ]. Finally, because multiple interventions were included in the analysis,several had data from only one study and therefore a relatively small sample size,which may have led to bias.
CONCLUSION
This network meta-analysis of first-line systemic therapies for advanced HCC revealed that atezolizumab plus bevacizumab is associated with the best OS and PFS, but also with a high incidence of discontinuation due to AEs. The results also showed that lenvatinib is associated with the best ORR of all systemic therapies included in the analysis, as well as a relatively high PFS, particularly in patients with HBV-related liver cancer in whom lenvatinib ranked first for PFS, over atezolizumab plus bevacizumab. Therefore, in patients with unresectable advanced HCC, systemic treatment should be selected based on the individualized treatment goals of each patient.
ARTICLE HIGHLIGHTS


Research conclusions
Our findings suggest that there is no one single first-line treatment for advanced hepatocellular carcinoma associated with superior outcomes across all outcome measurements. Therefore, first-line systemic treatment should be selected based on individualized treatment goals.
Research perspectives
Future research should continue to evaluate new therapeutic strategies for hepatocellular carcinoma in the context of existing treatments, and provide further information to support treatment selection for individual patients.
 World Journal of Gastroenterology2021年19期
World Journal of Gastroenterology2021年19期
- World Journal of Gastroenterology的其它文章
- Receptor for advanced glycation end-products axis and coronavirus disease 2019 in inflammatory bowel diseases: A dangerous liaison?
- Pathophysiological mechanisms underlying gastrointestinal symptoms in patients with COVID-19
- Burden of venous thromboembolism in patients with pancreatic cancer
- Clinical characteristics and outcomes of patients with hepatic angiomyolipoma: A literature review
- Individualized treatment options for patients with non-cirrhotic and cirrhotic liver disease
- Gut microbiota dysbiosis in Chinese children with type 1 diabetes mellitus: An observational study
