Pathophysiological mechanisms underlying gastrointestinal symptoms in patients with COVID-19
Byungchang Jin, Rajan Singh, Se Eun Ha, Hannah Zogg, Paul J Park, Seungil Ro
Abstract
Key Words: COVID-19 ; Gastrointestinal symptoms; Gut microbiota dysbiosis; Impaired barrier function; Serotonin; Angiotensin converting enzyme 2 receptor
INTRODUCTION
Since December 2019 , an acute respiratory infection, referred to as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 ), caused by the novel coronavirus has rapidly spread worldwide[1 -3 ]. In the United States alone, 198589 deaths(60 .3 /100000 ) have been reported due to the coronavirus pandemic from February 13 ,2020 to September 19 , 2020 [4 ].
Based on next-generation sequencing data from patient samples, SARS-CoV-2 is closely associated with two bat-derived SARS-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21 (88 % identity)[5 ]. The binding of the SARS-CoV-2 spike proteins to the host receptor, angiotensin-converting enzyme 2 (ACE2 ), is critical for viral invasion[6]. This viral infection may be asymptomatic or cause symptoms, such as fever, cough, headache, and myalgia[7 -9 ]. Interestingly, up to 40 % of coronavirus disease 2019 (COVID-19 ) patients experience gastrointestinal (GI) symptoms,including diarrhea, anorexia, nausea, vomiting, and abdominal pain (Table 1). In order to provide appropriate medical care to COVID-19 patients, it is necessary to explore pathophysiological mechanisms underlying their GI symptoms.
In this review, we summarized the studies that describe the various GI symptoms in COVID-19 patients and highlighted the likely underlying pathophysiological mechanisms. These insights offer potential new therapeutic approaches for containment of the global inflammatory response. Furthermore, we also shed light on the importance of the altered gut microbiota profile in the possible pathogenesis of COVID-19 .
CLINICAL PRESENTATION OF COVID-19 PATIENTS WITH GI SYMPTOMS
The clinical severity of COVID-19 patients may be stratified into three grades: Placid,ordinary, and grave cases. The incubation period of SARS-CoV-2 ranges from 1 -14 d,but is more commonly 3 -7 d. The typical clinical presentation of SARS-CoV-2 consists of fever, fatigue, dry cough, and shortness of breath. Other common symptoms involve congestion and rhinorrhea, pharyngalgia, myalgias, and diarrhea. In grave cases, the infection culminates in acute respiratory distress syndrome, which is associated with a high degree of mortality. Although the majority of symptomatic SARS-CoV-2 cases present with pulmonary symptoms, extra-pulmonary symptoms are also common, and several case studies have described the presence of digestive symptoms in the SARS-CoV-2 infection.
We identified and analyzed the GI symptoms of COVID-19 patients reported in nineteen published papers, which included diarrhea, nausea, abdominal pain,vomiting, anorexia, and bleeding (Table 1). Out of the nineteen papers, thirteen werefrom China, four were from the United States, one was from Singapore, and one was from Europe. Among GI symptoms, diarrhea was the most prevalent, accounting for 2 % to 33 .7 % of all patients. The median duration period of diarrhea in COVID-19 patients was 4 d with a range of 1 d to 9 d[10 ]. Other frequently reported GI symptoms were anorexia (341 /2914 , 11 .7 %), nausea (253 /2914 , 8 .7 %), vomiting (131 /2914 , 4 .5 %),abdominal pain (90 /2914 , 3 .1 %) and bleeding (5 /2914 , 0 .2 %). GI symptoms were more frequently reported during hospitalization than at the time of admission[11 ]. We have also recently reported a strong correlation between diarrhea and the severity of the disease[12 ]. These data suggest that GI symptoms should be included in the assessment of the disease severity in COVID-19 .
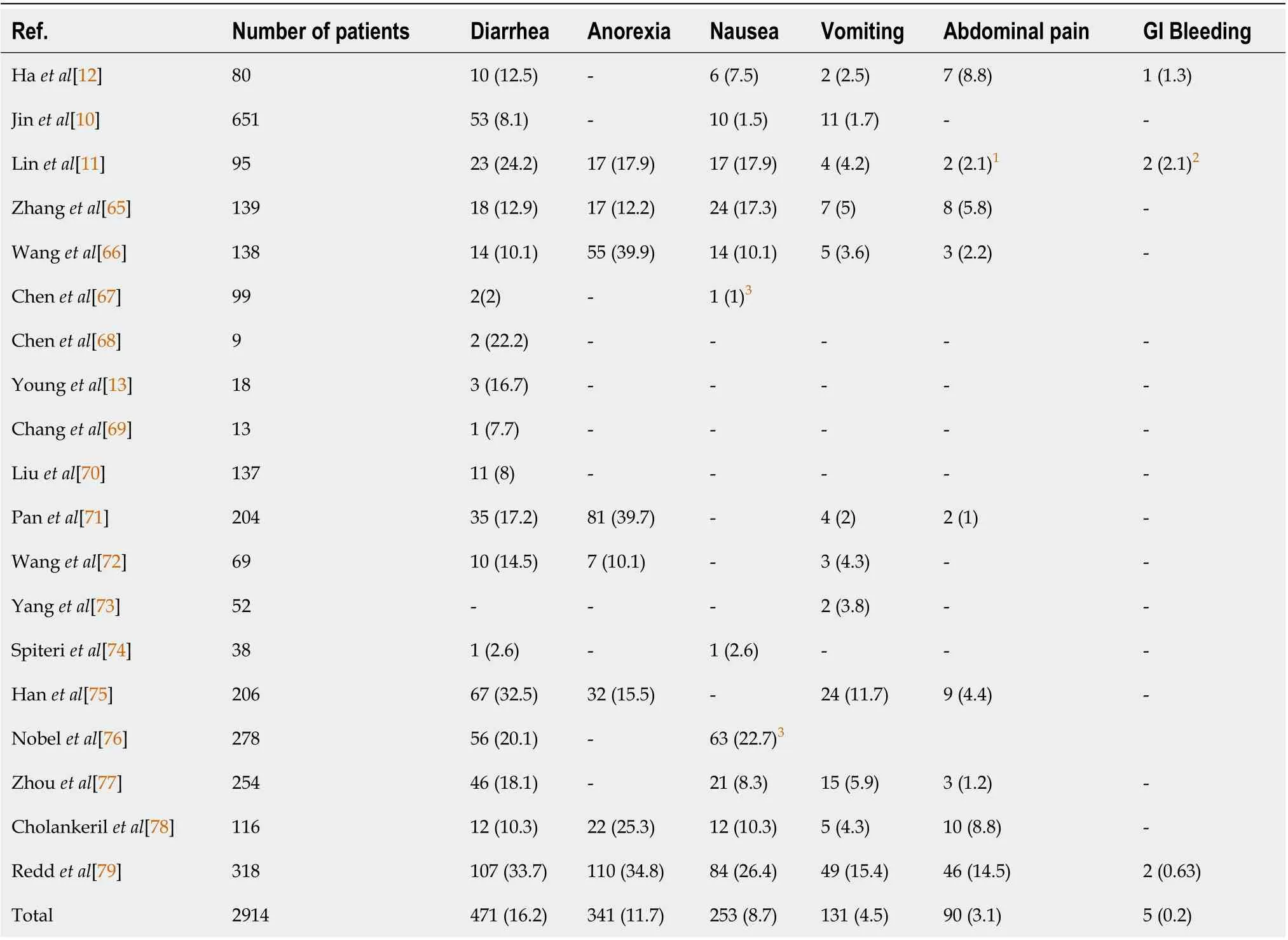
Table 1 Clinical presentation of gastrointestinal symptoms among coronavirus disease 2019 patients, n (%)
Previously, it was shown that RNA from SARS-CoV-2 was found in fecal samples(four out of eight patients) regardless of the presence of diarrhea[13 ]. Furthermore,another study demonstrated that SARS-CoV-2 RNA was found in the feces of 22 /42(52 .4 %) COVID-19 patients with GI symptoms. Among 23 COVID-19 patients without GI symptoms, SARS-CoV-2 RNA was found in the feces of 9 (39 .1 %) patients[11 ].Although the clinical relevance of SARS-CoV-2 RNA in fecal material remains unclear,understanding the biochemical mechanisms behind the SARS-CoV-2 mediated induction of GI symptoms is important to gain further understanding of the pathophysiology of COVID-19 . Therefore, we described potential mechanisms, by which GI symptoms might occur in COVID-19 patients and proposed new therapeutic strategies to modulate the global inflammatory response.
PATHOPHYSIOLOGICAL MECHANISMS FOR GI SYMTOMS IN COVID-19
Intestinal ACE2 receptor mediated impaired barrier function
ACE2 has emerged as a critical regulator of the renin angiotensin system (RAS) by metabolizing angiotensin (Ang) II into the beneficial peptide Ang 1 -7 [14 ]. ACE2 has also been identified as the key receptor for SARS-CoV and SARS-CoV-2 [15 ]. Spike protein treatment led to increased Ang II and pulmonary edema, which was mediated by AT1 R[16 ]. Given the similarities between the SARS-CoV and SARS-CoV-2 spike proteins, a similar mechanism of spike-mediated ACE2 down-regulation most likely underlies tissue damage in COVID-19 by skewing the RAS[16 ].
The pathophysiology of GI symptoms in COVID-19 remains poorly understood.However, evidence points to a role of ACE2 cell-surface receptors and SARS-CoV-2 mediated induced inflammatory processes in the GI tract[17 ]. A vital structural protein of SARS-CoV-2 is the spike glycoprotein (S). It consists of two functional units, S1 and S2 , that bind to the host cell ACE2 receptor by membrane fusion, replicates through replication-transcription complexes, and promotes proliferation by interfering with and suppressing the host’s immune response[18 ]. SARS-CoV-2 is highly concentrated in air droplets exhaled by infected subjects and inhalation of these particles by a noninfected individual may lead to infection of the recipient’s respiratory tractviaACE2 receptors. The respiratory tract is one of the primary sites of viral entry.Interestingly, ACE2 receptors are also highly expressed in the digestive tract making it another potential route of SARS-CoV-2 infection[19 ]. In the gut, ACE2 has a completely different function independent of RAS. ACE2 stabilizes neutral amino acid transporters, such as B0 AT1 and loss of ACE2 compromises intestinal uptake of certain dietary amino acids, such as tryptophan[20 ]. Because tryptophan plays an important role in immunity, ACE2 knockout mice exhibited altered gut microbiota and developed more severe dextran sulfate sodium–induced colitis compared with wildtype control mice[21 ]. These studies implicated ACE2 in SARS-CoV-2 infections in the gut.
ACE2 plays a major role in amino acid transport in the intestinal epithelium, a mechanism linked to the production of antimicrobial peptides, which suggests its role in intestinal barrier maintenance and gut microbiota equilibrium[22 ]. ACE2 controls expression of B0 AT1 in the intestine, which is the primary apical membrane transporter in the intestine that permits Na+coupled uptake of neutral amino acids,such as tryptophan[23 ]. Notably, B0 AT1 substrates, such as tryptophan and glutamine,signal to downregulate lymphoid pro-inflammatory cytokines, maintain the integrity of intestinal tight junctions, activate the release of antimicrobial peptides, and modulate mucosal cell autophagy as defense mechanisms[23 ]. Altered B0 AT1 expression mediated through ACE2 in COVID-19 may be a major contributor to the leaky gut. Thus, it is possible that SARS-CoV-2 mediated disruption of the gut barrier could lead to a systemic elevation of bacterial lipopolysaccharide and peptidoglycan,further worsening GI inflammation. For instance, one study showed that the spike protein of SARS-CoV-2 (S1 ) interacted with the ACE2 complex and the tryptophan amino acid transporter B0 AT1 [24 ]. Furthermore, downregulated intestinal ACE2 -B0 AT1 cell surface expression led to a series of downstream sequelae to promote a leaky gut as well as gut microbiota dysbiosis[23 ,24 ]. Therefore, ACE2 mediated impaired barrier function in combination with microbial dysbiosis may contribute to the cytokine storm seen in patients severely ill with COVID-19 and may also be responsible for their GI symptoms.
Gut inflammation in COVID-1 9 patients with diarrhea
Fecal calprotectin (FC) has evolved as a reliable fecal biomarker allowing detection of intestinal inflammation in inflammatory bowel disease (IBD) and infectious colitis[25 ].Previous studies have shown that COVID-19 patients with diarrhea without IBD had high FC compared to patients without diarrhea, indicating that the infection evokes a significant intestinal inflammatory process[25 ]. Furthermore, FC levels correlated significantly with the pro-inflammatory interleukin - 6 (IL-6 ) serum concentrations,and a murine study showed that deficiency of ACE2 results in highly increased susceptibility to intestinal inflammation induced by epithelial damage[21 ].Collectively, the aforementioned studies highlighted that GI inflammation was overrepresented in patients with COVID-19 that also had functional GI disorders(FGIDs) or post-infection (PI) GI disorders.
Alterations in serotonin metabolism in COVID-1 9 patients
We have reported that plasma serotonin (5 -hydroxytrytamine, 5 -HT) levels were elevated in COVID-19 patients with diarrhea[12 ]. 5 -HT is a hormone and neurotransmitter that has a monoamine structure. 5-HT synthesis begins with the amino acid Ltryptophan, which is converted to 5 -hydroxytryptophan (5 -HTP)viathe rate-limiting enzyme tryptophan hydroxylase (TPH). 5 -HTP is then rapidly decarboxylated by aromatic L-amino acid decarboxylase to produce 5 -HT[26 ,27 ]. 5 -HT either circulates in our body or is absorbed by the cells that express serotonin reuptake transporter to act or decompose, resulting in 5 -hydroxyindoleacetic acid (5 -HIAA)[28 ]. TPH is an enzyme specifically found in 5 -HT producing cells, and there are two different isoforms, TPH1 and TPH2 [29 ,30 ]. TPH1 dependent 5 -HT synthesis occurs in enterochromaffin (EC) cells in GI tract, while TPH2 is involved in 5 -HT synthesis in the central nervous system and enteric nervous system[31 ,32 ].
Since 95 % of total 5 -HT is produced by EC cells in GI tract, 5 -HT has been widely studied for GI functions, especially in GI motility. Many studies have demonstrated that 5 -HT is important for colonic peristaltic reflexes and GI transit[33 -35 ]. Moreover,altered 5-HT levels are closely associated with irritable bowel syndrome (IBS), and it has been shown that platelet-depleted plasma 5-HT levels are increased in IBS patients with diarrhea[36 ]. Therefore, approaches to target 5 -HT signaling have been proposed as a way to alleviate GI dysmotility. A total of seven classes of 5-HT receptors have been identified, and it is well-known that 5 -HT1 , 5 -HT2 , 5 -HT3 , 5 -HT4 , and 5 -HT7 are expressed in the GI tract to influence gut motor function[37 ]. 5 -HT3 antagonists are especially effective in treating IBS with diarrhea[38 ,39 ] and 5 -HT4 agonists are effective in treating IBS with constipation[40 ,41 ].
Previously, we have reported that plasma 5 -HT levels are increased in COVID-19 patients and are directly correlated to the severity of COVID-19 symptoms. Moreover,COVID-19 patients with diarrhea had increased plasma 5 -HT and a lower ratio of plasma 5 -HIAA/5 -HT levels compared to healthy subjects or COVID-19 patients without diarrhea[12 ]. These data suggest that 5 -HT is not being broken down into 5 -HIAA, and 5 -HT remains in some COVID-19 patients’ for a longer duration, resulting in GI symptoms such as diarrhea. Thus, regulating the amount of 5 -HT might be a therapeutic modality for COVID-19 patients with diarrhea.
Gut microbiota dysbiosis in COVID-1 9 patients
From ancient times, viral infectious diseases have been plaguing mankind through a wide-range of clinical manifestations. Moreover, scientific annals depict the occurrence of life-threatening viral diseases that are enumerated as epidemics and pandemics[42 ].Examples include: The flu, polio, Ebola, acquired immune deficiency syndrome and the very recent COVID-19 . In the past several months, COVID-19 has reached pandemic status, exposing the world to eminent danger. Previously, two other similar viral infections including the Middle East respiratory syndrome virus and SARS-CoV have been reported[43 ]. SARS-CoV-2 is an enveloped virus in theCoronaviridaefamily.They harbor single stranded RNA as their genetic material that has positive polarity.Some studies published during the recent pandemic of COVID-19 have provided insight into parameters pertaining to the transmission, susceptibility, clinical presentation and laboratory findings of this potential pathogen[44 ,45 ]. Although respiratory droplets and contact are the prime route of transmission for SARS-CoV2,there have been some instances where prolonged exposure to aerosols with elated concentrations of the virus may facilitate transmission. Symptoms and severity of COVID-19 differ from patient to patient[46 ]. In general, humans of all ages are susceptible. However, individuals with an attenuated immune response including elderly, infants, children below 6 years old, patients with underlying diseases(transplants, cancers, diabetes, asthma, heart ailment, and other peril maladies) are at higher risk.
To inject their genetic material into the host, SARS-CoV-2 pierces the pulmonary epithelial cells of the lower respiratory tract thereby commandeering the host’s cellular machinery[47 ]. Moreover, this process is enhanced by the spike (S) protein that interacts with ACE2 [47 ,48 ]. Thus, the importance of the gut and its microbiome cannot be underestimated. The knowledge in gut research has augmented with a plethora of scientific annals that point towards the role of gut microbes in many degenerative and infectious diseases[49 ]. Gut dysbiosis has been reported in patients with COVID-19 with enrichment of pathogens and depletion of beneficial commensals[17 ]. An inverse correlation between the abundance ofFaecalibacterium prausnitzii(F. prausnitzii) and disease severity has been observed.F. prausnitziihas anti-inflammatory properties,and its depletion has been related to IBS[17 ]. Another study showed the gut microbiome composition was significantly altered in patients with COVID-19 compared with non-COVID-19 individuals irrespective of whether patients had received medication[50 ]. Several gut commensals with known immunomodulatory potential such asF. prausnitzii,Eubacterium rectaleand Bifidobacteria were underrepresented in patients and remained hampered in samples collected up to 30 d after disease resolution[17 ,51 ]. Moreover, this perturbed composition exhibited stratification with disease severity concordant with elevated concentrations of inflammatory cytokines and blood markers such as C-reactive protein, lactate dehydrogenase,aspartate aminotransferase and gamma-glutamyl transferase[17 ]. The depletion of several bacterial species in the COVID-19 cohort was linked to increased concentrations of tumor necrosis factor-alpha, C-X-C motif chemokine ligand 10 , C-C motif chemokine ligand 2 and IL-10 . These studies highlighted the need to understand how gut microorganisms are involved in inflammation and COVID-19 pathogenesis[50 ].
Another study found a signature of active gut viral infection in a subset of patients with COVID-19 even in the absence of GI symptoms, suggesting a ‘quiescent’ GI infection of SARS-CoV-2 [52 ]. The transcriptional activity of viral infection and replication persisted in the gut even after respiratory clearance of SARS-CoV-2. Fecal samples with a signature of high SARS-CoV-2 infectivity harbored a higher abundance of opportunistic pathogens, for instance,Morganella morganii, Collinsella aerofaciens,
Streptococcus infantis, andCollinsella tanakaeiand an enhanced capacity for the biosynthesis of nucleotides and amino acids, along with carbohydrate metabolism, whereas fecal samples with a signature of no SARS-CoV-2 infection had a higher abundance of short-chain fatty acid producing bacteria, for instance,Bacteroides stercoris,Parabacteroides merdae,Lachnospiraceae bacterium, andAlistipes onderdonkii[52 ]. This study provided evidence for active and prolonged ’quiescent’ GI infection even in the absence of GI manifestations and after recovery from respiratory infection of SARSCoV-2 . The gut microbiota of patients with active SARS-CoV-2 GI infection was characterized by enrichment of opportunistic pathogens; loss of salutary bacteria and increased functional capacity for nucleotides, along with increased amino acid biosynthesis and carbohydrate metabolism[52 ].
In addition, bacterial groups belonging to the genus Bacteroides, known to downregulate the ACE2 expression in the murine colon, inversely correlated with fecal SARS-CoV-2 nucleic acid loads. Similarly, SARS-CoV-2 infection of GI epithelial cells has been associated with: (1 ) Lamina propria infiltration of plasma cells and lymphocytes, and edema in the stomach, duodenum, and rectum; (2) Increased levels of FC; (3 ) Higher fecal levels of IL-8 and lower levels of the anti-inflammatory IL-10 when compared with uninfected controls[53 ]; (4 ) SARS-CoV-2 -specific IgA and limited inflammatory cytokines were also present in the stool of select patients with acute COVID-19 ; and (5 ) Gut microbiota dysbiosis. Interestingly, gut microbiota dysbiosis persisted after the resolution of SARS-CoV-2 infection, suggesting that microbiota perturbation may contribute to the persistence of gut dysfunction and symptoms even after the infection has subsided. Indeed, the persistent microbial dysbiosis may contribute to maintaining a chronic state of low-grade GI inflammation, increased intestinal permeability, increased sensory perception, and bile acid malabsorption,which have all been previously associated with symptoms of GI motility disorders.
Post-COVID-1 9 functional GI disorders
Evidence supports the development of FGIDs after a bout of viral, bacterial, or protozoal gastroenteritis or after resolution of an acute flareup of GI inflammatory diseases such as IBD[54 ]. Individual susceptibility to these so-called PI-FGIDs involves genetic predisposition and the presence of pre-existing psychological disturbances such as anxiety and/or depression[55 ,56 ]. PI-FGIDs have also been associated with dysregulation of gut motility, visceral hypersensitivity, microbial dysbiosis, intestinal barrier dysfunction, bile acid malabsorption, and alterations in serotonin metabolism[54 ,57 ]. Current data suggest that the resolution of the SARS-CoV-2 infection may lead to persistent GI dysfunction resembling certain aspects of PIFGIDs[17 ]. Transient non-specific gut inflammation is the common trigger for longlasting symptoms of FGIDs, regardless of the initiating event (i.e., viral, parasitic,bacterial, after resolution of IBD flares)[58 ].
SARS-CoV-2 in stool: Suggesting fecal-oral transmission
Evidence of fecal shedding of viral RNA further supports viral replication in the digestive tract and potentially a fecal-oral route of transmission. Studies showed that more than one-half of COVID-19 patients tested positive for fecal SARS-CoV-2 RNA[59 ]. One study in a group of pediatric patients infected with SARS-CoV-2 had positive rectal swabs for SARS-CoV-2 , even after the nasopharynx was cleared of the virus, suggesting that viral shedding from the digestive tract might be more prolonged than that from the respiratory tract[60 ]. Another study showed that SARS-CoV-2 can infect the enterocytes of bats in an organoid culture system of bat intestinal epithelium[61 ]. One study indicated that infection by SARS-CoV-2 led to an altered fecal microbiome during hospitalization[62 ]. The authors showed depletion of opportunistic pathogens and depletion of commensals during SARS-CoV-2 infection.Coprobacillus,Clostridium ramosum, andClostridum mathewayiwere found more commonly in patients with severe COVID-19 . In contrast, the presence ofF. prausnitziiwas correlated with milder disease. Gut microbial dysbiosis persisted in the majority of COVID-19 patients in spite of clearance of the virus, suggesting that exposure to SARS-CoV-2 might be associated with more long-lasting deleterious effects to the healthy gut microbiome[23 ,62 ]. These studies support the possibility for SARS-CoV-2 fecal-oral route of transmission. Therefore, from both clinical and public health standpoints, it is critical to fully understand all routes of transmission of SARS-CoV-2 . If high levels of infectious viruses are present in the intestinal lumen of infected patients, especially in asymptomatic patients, this poses risks during endoscopy and colonoscopy to gastroenterologists, endoscopy personnel and other patients. For the general public,infectious viral particles in the feces shed by infected individuals, if aerosolized, have great implications in confined environments such as cruise ships, hospitals, individual households, and densely populated housing, such as those in regions with poor sanitation[19 ].
CONCLUSION
GI symptoms are overrepresented in patients with COVID-19 . A proportion of patients affected by COVID-19 may develop PI-FGIDs based on the following pathophysiological mechanisms: Intestinal barrier dysfunction, chronic low-grade intestinal inflammation, altered serotonin metabolism, and gut microbiota dysbiosis. The question of whether gut inflammation is associated with gut microbiota dysbiosis in patients, which may have a central role in the COVID-19 disease progression warrants further investigation. However, there is mounting evidence that gut microorganisms are linked to GI inflammatory diseases, which highlights the urgent need to understand the specific roles of gut microorganisms that are responsible for the immune dysfunction and systemic inflammation in COVID-19 .
The abundance of SARS-CoV-2 viral RNA in stool and the stability of the virus in the environment suggest that fecal contamination may be an important modality for the spread among human hosts. Fecal sources may lead to viral transmission,especially when aerosols are generated. The significance of GI involvement in COVID-19 patients requires attention in clinical practices, such as incorporation of rectal swab testing before discharging patients, as well as the importance of personal protective equipment in the endoscopy setting. These precautions will be imperative in our battle against COVID-19 [63 ].
Considering the critical role of the ACE2 receptor in the pathogenesis of COVID-19 and the potential impact on severity of symptoms in some patients, several therapeutic approaches have been evaluated such as a soluble form of ACE2 (rhACE2 ), ACE2 blockers, TMPRSS2 inhibitors, and Ang 1 -7 receptor agonists. Some of these therapeutic approaches appeared to show promising results and are currently in clinical trials. Another strategy to manage COVID-19 might be to restore the microbiota during the dysbiosis through prebiotic and/or probiotic interventions and dietary nutritional supplementation[64 ].
This review sheds light on the studies that formulate the pathophysiological mechanisms (impaired barrier function, gut inflammation, altered serotonin metabolism and gut microbiota dysbiosis) underlying GI symptoms in patients with COVID-19 (Figure 1 ). To the best of our knowledge we are the first to propose altered serotonin metabolism in the pathogenesis of COVID-19 associated with diarrhea. This novel insight of serotonin metabolism might be a key player underpinning GI symptoms and severity in patients with COVID-19 as altered serotonin signaling modulates the majority of pathological mechanisms in patients with FGIDs.Therapeutic modalities regulating serotonin signaling might offer potential treatment options in a subset of COVID-19 patients. Furthermore, we highlighted the important concept of post-SARS-CoV-2-FGIDs, which warrant future studies to dissect persistent GI symptoms after the clearance of SARS-CoV-2 infection. Scientists and clinicians should be aware of this new clinical scenario, and studies will be needed to further characterize and uncover the pathophysiological mechanisms of this phenomenon.Furthermore, studies are warranted to elucidate the following: (1) the cause-and-effect relationship between changes in relative abundance of gut bacteria and COVID-19 ; (2 )the possibility that the microbiota plays a role in illness severity; and (3 ) the relationship between the host’s immune response (T-regulatory response) to SARSCoV-2 resulting in a high or low cytokine storm.

Figure 1 A simplified diagram of the potential pathological mechanisms for gastrointestinal symptoms associated with severe acute respiratory syndrome coronavirus 2 infection. The figure was created with BioRender.com. SARS-CoV-2 : Severe acute respiratory syndrome coronavirus 2 ;5 -HT: 5 -hydroxytrytamine; EC: Enterochromaffin; ACE2 : Angiotensin converting enzyme 2 .
 World Journal of Gastroenterology2021年19期
World Journal of Gastroenterology2021年19期
- World Journal of Gastroenterology的其它文章
- Celiac Disease in Asia beyond the Middle East and Indian subcontinent: Epidemiological burden and diagnostic barriers
- Biomarkers in autoimmune pancreatitis and immunoglobulin G4-related disease
- Risk of hepatitis B virus reactivation in patients with autoimmune diseases undergoing non-tumor necrosis factor-targeted biologics
- Risk factors and prognostic value of acute severe lower gastrointestinal bleeding in Crohn’s disease
- Changes in the nutritional status of nine vitamins in patients with esophageal cancer during chemotherapy
- Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients
