Short-term effects of intravitreal Conbercept injection combined with laser photocoagulation on macular edema secondary to ischemic retinal vein occlusion
Zheng-Feng Liu, Xing-Rong Wang, Xiao-Yan Zhang, Xue-Mei Pan, Rui-Xue Zhang,Hong-Sheng Bi, Ying Wen
1Medical School of Ophthalmology & Optometry, Shandong University of Traditional Chinese Medicine, Jinan 250355,Shandong Province, China
2Affiliated Eye Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250002, Shandong Province, China
3Shandong Provincial Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Therapy of Ocular Diseases; Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Therapy of Ocular Diseases in Universities of Shandong; Eye Institute of Shandong University of Traditional Chinese Medicine, Jinan 250002, Shandong Province, China
Abstract
● KEYWORDS: Conbercept; laser photocoagulation;macular edema; ischemic retinal vein occlusion
INTRODUCTION
Retinal vein occlusion (RVO) is a vascular disorder that affects the retinal vascular layers[1-4]and choroidal vasculature[1]. Analysis of large-scale population studies from North America, Europe and Asia released the worldwide prevalence of RVO at around 16 million adults in general.RVO may result in impaired blood circulation and retinal ischemia. Severe ischemia of the macula could alter vision,and extensive peripheral ischemia may lead to retinal and iris neovascularization[5]. Among all the complications of RVO,macular edema (ME) is a frequent and sight-threatening one from both the two common subtypes, central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO)[6].Thus, the prevention and treatment of ME secondary to RVO is still an important clinical issue in practice.Vascular endothelial growth factor (VEGF) plays a key role in the pathogenesis of ME secondary to RVO[7]. It is a cytokine produced by hypoxic cells that stimulates vascular permeability and proliferation by binding to endothelial cell receptors.Based on numbers of prospective clinical trials, anti-VEGF therapy has become the most used treatment for ME to RVO.Conbercept (KH902; Chengdu Kanghong Biotech Co. Ltd.,Sichuan Province, China) is a new recombinant fusion protein consisting of the extracellular domain 2 of VEGF receptor 1 and extracellular domains 3 and 4 of VEGF receptor 2 fused to the Fc portion of human immunoglobulin G1, which has high affinity for all VEGF isoforms and placental growth factors[8].Importantly, intravitreal injection with Conbercept represents an excellent option for treating ME to improve visual acuity in RVO with a satisfactory safety profile and efficacy[9].
Laser photocoagulation is another first-line therapy for treating the complications of retinal vascular disease. Theoretically,the combination of anti-VEGF and laser photocoagulation may improve the clinical efficacy on RVO. The current study is aimed to evaluate the short-term (6-month) effects of intravitreal Conbercept injection combined with laser photocoagulation on ME secondary to ischemic RVO (iRVO).
SUBJECTS AND METHODS
Ethical ApprovalThe present research includes a retrospective case series of patients with ME caused by iRVO. Our study was formally reviewed and approved by the Ethics Committee of the Affiliated Eye Hospital of Shandong University of Traditional Chinese Medicine and conducted in accordance with the tenets of the Declaration of Helsinki.Informed consent was waived due to the retrospective nature of the study.
Scanning laser ophthalmoscopy (SLO), fundus fluorescein angiography (FFA), and spectral domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg Engineering,Heidelberg, Germany) were used to diagnose RVO-related ME. Subfoveal choroidal thickness measurement by SD-OCT is a noninvasive method for detecting changes in the choroidal vasculature.
A total of 33 eyes from 33 patients were selected from May 2018 to July 2019. Prior to treatment, each eye underwent comprehensive ophthalmologic examinations, including slitlamp biomicroscopy, best corrected visual acuity (BCVA) and intraocular pressure (IOP) measurements, SLO, FFA, and SDOCT.
The inclusion criteria were as follows: 1) age ranging from 40 to 80y; 2) treatment naïve upon presentation to the hospital; 3)diagnosed with iRVO subtype and had not received intravitreal anti-VEGF or laser photocoagulation before; 4) received comprehensive ophthalmic examinations before and after treatment; 5) diagnosed iRVO no more than half a month. The exclusion criteria were as follows: 1) had a retinal pathology other than ME secondary to RVO; 2) received previous treatment for RVO-related ME; 3) had any significant media opacity or a history of ocular trauma; 4) unable to return for follow-up; 5) had ocular inflammation, fluorescein allergy,or a history of vitrectomy or other intraocular surgeries; 6 the affected or fellow eye had an axial length >26.00 or <22.00 mm.All eyes were treated by intravitreal injection of Conbercept on a 3+pro re nata(3+PRN) basis. Eyes were re-injected if either of the following conditions occurred during follow-up: >2 lines of vision decrease in Snellen acuity or >100 µm increase in central macular thickness (CMT) compared with the previous measurement.
Levofloxacin eye drops were administered four times per day for 2d prior to treatment to prevent infection. All patients were informed of possible adverse effects. After obtaining written informed consent, we performed the procedures with surface anesthesia. All eyes initially received intravitreal injections of Conbercept (0.05 mL/0.5 mg) under sterile conditions through the pars plana by using a 30-gauge needle.
Laser photocoagulation (spot diameter, 200 μm; exposure time, 100-150ms; power, 150-250 mW) was performed on the nonperfused ischemic retina outside the macula and up to the far periphery according to FFA findings obtained after the first injection of all eyes after the hemorrhage absorption
We followed up all eyes 1d after the injection to determine whether complications had occurred. The patients were asked if they experienced any loss of vision. At each follow-up, each patient underwent several examinations, including BCVA and IOP measurement, slit-lamp biomicroscopy, SLO, and OCT.We recorded the patients’ demographics and the results of all examinations at baseline and after each visit. The outcomes of this study included BCVA, CMT, central choroidal thickness(CCT), the number of injections, and complications, if any.
Statistical AnalysisBCVA was measured using Snellen charts, and the measurements were converted into the logarithm of the minimum angle of resolution (logMAR)for data analysis. We used SPSS Statistics 17.0 software for statistical analysis and considered paired-samplest-test.P<0.05 to indicate statistically significant differences.
RESULTS
Thirty-three eyes from 33 patientswere included in this work.The mean age of the patients was 61.5±12.37y (range, 41-77y),9 of the patients (27.27%) had CRVO, and 24 (72.73%) had BRVO. All patients were treatment naïve, followed up on time, and administered treatment on a 3+PRN basis. All the patients required panretinal or sectorial laser photocoagulation before or after the first injection, and none showed neovascular glaucoma. The baseline general characteristics of the patients are summarized in Table 1.
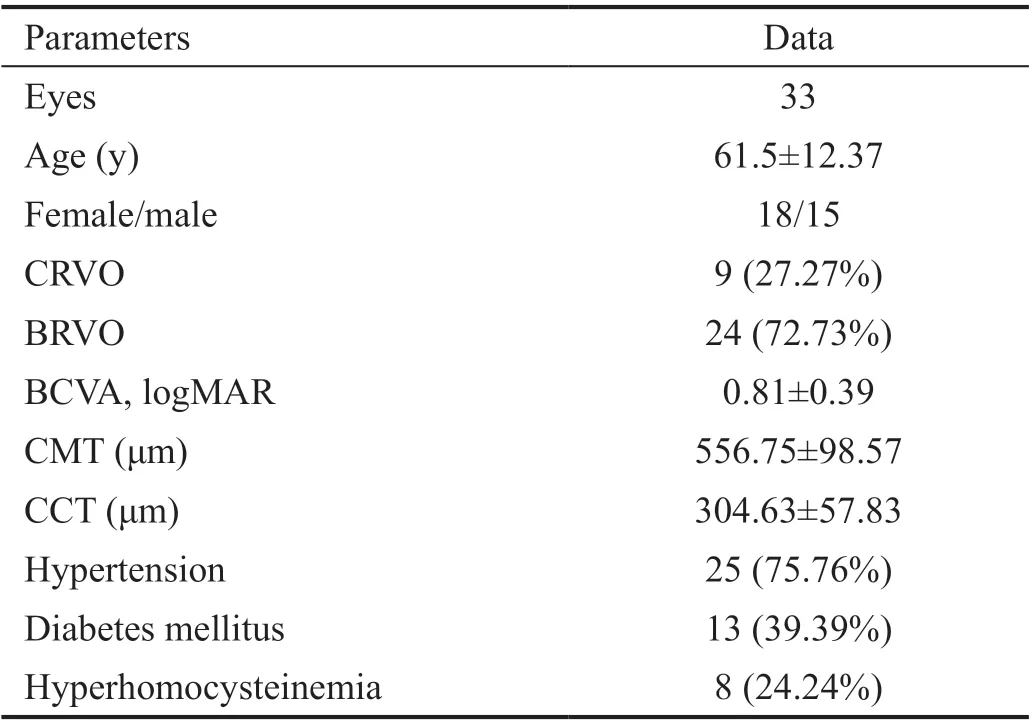
Table 1 Characteristics of the patients
The BCVA at baseline was 0.81±0.39 logMAR, improved significantly to 0.64±0.46 at 1mo (P=0.002), 0.52±0.36 at 2mo(P=0.000), 0.41±0.25 at 3mo (P=0.000), and 0.43±0.29 at 6mo (P=0.000). The CMT at baseline was 556.75±98.57 μm,decreased significantly to 484.78±67.54 μm at 1mo (P=0.002),312.78±59.65 μm at 2mo (P=0.000), 304.78±68.53 μm at 3mo(P=0.000), and 306.85±76.77 μm at 6mo (P=0.000). The CCT at baseline was 304.63±57.83 μm, decreased significantly to 298.42±35.74 μm at 1mo (P=0.72), 272.78±39.06 μm at 2mo (P=0.063), 271.31±45.53 μm at 3mo (P=0.026), and 272.29±39.93 μm at 6mo (P=0.035).
The average number of injections was 3.35. Only conjunctival hemorrhage caused by intravitreal injection was observed as an adverse effect. Approximately 15d after treatment, the conjunctival hemorrhage was gradually absorbed without treatment.
DISCUSSION
The level of VEGF in the vitreous cavity significantly increases after RVO. Overexpression of VEGF and its receptors is closely related to serum protein exudation, retinal thickening,and ME[10]. Intravitreal administration of anti-VEGF agents is an effective treatment for ME secondary to RVO[9]. However,these therapies require frequent follow-up and multiple treatments to maintain improvements in vision. Previous studies have shown that anti-VEGF therapy improves the visual acuity of BRVO patients and promotes reabsorption in the area of the ME[11-12]. Conbercept alleviates ME secondary to RVO by inhibiting inflammation, angiogenesis, and oxidative responses. Earlier studies revealed the molecular mechanism of Conbercept for treating ME secondary to RVO[13]. Sunet al[9]reported that intravitreal injections of Conbercept demonstrate a generally favorable safety and tolerability profile and efficacy in the treatment of ME secondary to RVO. Several studies based on a short-term follow-up also show that intravitreal injection of Conbercept is safe and effective for the treatment of ME secondary to BRVO[14-15]. In our study, the vision of all eyes treated with Conbercept improved and CMT decreased without complications.
VEGF causes blood vessels to expand, increases blood flow, and enhances membrane permeability through the elevated production of nitric oxide; these effects lead to the accumulation of liquid and, in turn, increased choroidal thickness[16]. Inhibition of VEGF by intravitreal injection of anti-VEGF agents may affect the permeability of the choroidal vasculature and choroidal thickness[17]. Choroidal thickness in patients with BRVO has been evaluated in several recent studies, and these works generally show that patients with ME secondary to BRVO have a significantly greater mean choroidal thickness compared with normal eyes[18-19]. Choroidal thickness significantly decreases following anti-VEGF treatment[18-19]. After anti-VEGF treatment, both choroidal and retinal thickness decrease and BCVA is improved significantly[20-21]. Treatment of patients with iRVO with intravitreal bevacizumab leads to reductions in central ME and CCT but poorer visual acuity[22]. In contrast to these studies,Parket al[17]revealed no changes in choroidal thickness after anti-VEGF treatment. The subfoveal choroidal thickness in eyes with ME secondary to RVO decreased significantly within a short period in response to a single intravitreal ranibizumab injection. The study also showed that changes in subfoveal choroidal thickness change are not correlated with RVO subtypes[23]. In our study, all eyes were treated with intravitreal Conbercept injections combined with laser photocoagulation but CCT did not decrease significantly in the first and second follow-ups. We thus speculate that changes in CCT are related to the effects of laser photocoagulation, which could affect the choroidal thickness.
In the present study, laser photocoagulation was performed in the nonperfusion area of the retina in our study. The Brance Vein Occlusion Study Group recommends laser photocoagulation for patients with ME associated with branch vein occlusion[24]. The BRIGHTER study revealed that ranibizumab with or without laser therapy results in statistically significant improvements in BCVA compared with laser alone in patients treated with BRVO for 6mo[25]. Wanget al[26]showed that Conbercept with retinal photocoagulation can effectively improve visual acuity and reduce CMT. In our study, we confirmed the efficacy and safety of Conbercept combined with laser photocoagulation in ME secondary to iRVO.
Several limitations that may affect the interpretation of our results. First, the small sample size, short follow-up period(6mo), and retrospective design of this study present several drawbacks. A prospective study with a larger number of subjects, including iRVO and non-iRVO cases, and longterm follow-up is required to confirm our findings. Second,we only studied the treatment of Conbercept combined with laser photocoagulation for ME secondary to iRVO. The effects of Conbercept with and without the accompanying laser treatment on ME secondary to iRVO should be studied in the future. Third, several patients with diabetes were included in our analysis, and, although they did not have any sign of diabetic retinopathy according to comprehensive ophthalmic examinations, a potential bias may occur. Fourth, because different age groups may exhibit different sensitivities to drugs,age group analysis may also be necessary.
ACKNOWLEDGEMENTS
Foundations:Supported by the Key Research and Development Plan of Shandong Province (No.2017G006033);the Natural Science Foundation of Shandong Province (No.ZR2017LH042); the Development Project of Medicine and Health Science Technology of Shandong Province(No.2017WS073); the Excellent Youth Science Foundation of Shandong University of Traditional Chinese Medicine(No.2018zk26).
Conflicts of Interest:Liu ZF,None;Wang XR,None;Zhang XY,None;Pan XM,None;Zhang RX,None;Bi HS,None;Wen Y,None.
 International Journal of Ophthalmology2021年5期
International Journal of Ophthalmology2021年5期
- International Journal of Ophthalmology的其它文章
- Comprehensive evaluation of intravitreal conbercept versus half-dose photodynamic therapy for chronic central serous chorioretinopathy
- Lipid accumulation and protein modifications of Bruch’s membrane in age-related macular degeneration
- Via pars plana anterior iris enclavation lOL fixation
- Role of microRNA-25 in high glucose cultured Müller glia
- Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HlF-1/VEGFA pathway and promoting PEDF expression
- Expression levels of pro-inflammatory interleukin-8 and certain antimicrobial peptides in concurrent with bacterial conjunctivitis
