Expression levels of pro-inflammatory interleukin-8 and certain antimicrobial peptides in concurrent with bacterial conjunctivitis
Alaa El-Dien Shawky Hosny, Zeinab El-Demerdash El-Bazza, Mohammed Abdelhalim Ramadan, Maha Ahmed Shafik, Mahmoud Ahmed Shafeek, Rania Abdelmonem Khattab
1Microbiology and Immunology Department, Faculty of Pharmacy, Cairo University, Kasr Al-Aini 11562, Cairo, Egypt
2Drug Radiation Research Department, National Center for Radiation Research and Technology, Atomic Energy Authority,Cairo 9621, Egypt
3Ophthalmology Department, Faculty of medicine, AL-Azhar University, Cairo 11754, Egypt
Abstract
● KEYWORDS: interleukin-8; human beta defense-2;human beta defense-3; infectious conjunctivitis; real-time polymerase chain reaction
INTRODUCTION
Infectious conjunctivitis is an inflammation of the mucosa of the conjunctiva due to infection with bacteria, fungi,and/or viruses. Conjunctivitis in most cases is a minor eye infection but sometimes develops to a severe ocular issue.This depends on the immune status of the patient as well as the microbial etiology which depends on the lifestyle, region,and age[1-2]. In concurrent with these factors, it may be a good chance for bacteria to cause infection.
In bacterial conjunctivitis, mono and multi-microbial infections are involved, including pathogenic and opportunistic ones;gram-positive bacteria such asStaphylococcus, Streptococcus,CorynebacteriumandBacillusspecies, and gram-negative bacteria such asHaemophilus, Pseudomonas, MoraxellaandAcinetobacterspecies[1,3].
Bacterial epidemiology commits the use of broad-spectrum antibiotics in the empirical treatment of eye infections, which on prolonged use with misuse of antibiotics lead to developed and/or acquired resistance to antibiotics[4]. This can account for the necessity of tracking the immune response changes as a reflection of changes in etiological agents.
Most infections can be curbed in their initial phase by the human immune system. Serious infection arises when the immune system is flooded by a vast number of bacterial invaders or highly pathogenic strains or efficient bacterial virulence mechanisms. Adaptive immunity comes into play after several days, which is mostly based on specific antibodies. In contrast, the innate immune system, which is activated by conserved bacterial surface structures work within hours to combat the invading bacteria. These prevent bacteria from proliferating to an extent that would enable them to overgrow and ultimately breach through the protective epithelial layers[5-7].
On the epithelial cells in the ocular surface, the toll-like receptors (pattern recognition immune receptors) recognize microbial residues. Upon recognition, a chain of immune reactions occurs leading to signals that regulate different genes expression[8]. The inflammatory response is part of the tissue primary defense mechanism. Many of the cytokines are released at the beginning of cell activation. The released cytokines participate in antigen recognition and regulation of the length and intensity of the innate and specific immune response, recruiting cells towards the conflict zone. Cytokine share functions with antimicrobial peptides (AMPs)[7,9].
The pro-inflammatory interleukin-8 (IL8) is an important chemotactic cytokine, a key mediator in neutrophil recruitment and activation in infected tissues, cell signaling and clearance of pathogens[10]. AMPs represent a major arm of the innate immunity on epithelia, where they are secreted mostly by keratinocytes. Most AMPs are cationic and interact with the negatively charged bacterial surface. The most important AMPs belong to the defensin class, which can be further divided into alpha and beta-defensins and the cathelicidins.They contribute to the diverse killing mechanisms that act simultaneously to eliminate bacteria within the phagosomes of neutrophils and other phagocytes[5]. They have a broad spectrum of antimicrobial properties and they are active against gram-positive and gram-negative bacteria. They exert their action by disrupting the surface of microorganisms. In gram-negative bacteria, they bind the anionic portion of the lipopolysaccharide molecule, and in gram-positive bacteria,they interact with teichoic acids or with anionic groups present in the peptidoglycan molecule[7,11]. They can distinguish between the target membranes and the host, which make the AMPs mostly cell-selective[8,12]. The studies carried on the immune response in keratoconjunctivitis, although they may be replicated, they help us to better understand the disease abrupt changes, the transition of etiological agents, and provides better treatment options.
The present study focused on investigating the gene expression of the pro-inflammatory IL8 in concurrent with the AMPs;human beta defense-2 (HBD2) and human beta defense-3(HBD3) in conjunctiva epithelial impression cytology (CIC)samples collected from bacterial conjunctivitis patients compared to healthy controls.
SUBJECTS AND METHODS
Ethical ApprovalThe samples were taken according to the Ethical Board of Al-Hussein University and consistently with the Principles of the Declaration of Helsinki. Written consents were taken from all participants.
PatientsA total of 64 eyes of 64 patients who were presented with conjunctivitis at the outpatient clinic of Al-Hussein University Hospital, Cairo, Egypt were enrolled in the study.The control group consists of 20 healthy participants without any ocular problems, they were age and sex-matched with the patients.
Clinical ExaminationPatients with a chief complaint of red-eye, discharge, or sticky eyelids or eyelashes who were given a diagnosis of conjunctivitis were eligible for the study.All patients were subjected to a complete ophthalmological examination to ensure conjunctivitis as well as healthy control to ensure healthy eye tissues[13-14].
Collection of SamplesFor all enrolled patients, in cases of bilateral eye involvement, the eye with more significant signs or symptoms was used for specimen collection. If both eyes were equally affected, then the first affected eye was used for the study. From each participant (healthy controls and patients), two samples were collected, a microbiological sample from the conjunctiva sac and a CIC sample both from the same eye.
The microbiological samples from the conjunctiva sac were collected with a sterile Rayon tipped swab by rubbing them over the conjunctiva very carefully without touching the cornea. The swabs were then inserted into brain-heart infusion broth (Oxoid, UK) tubes.
The CIC samples were collected using a sterile gridded cellulose-acetate filter paper with a pore size of 0.45 μm(Millipore Corporation, Bedford). The sterile filter-paper was trimmed into D shape strips (5 mm width) under a sterile condition and then further sterilized by gamma radiation. The strips were sterilized at a dose of 25 kGy using Gamma Cell 40 (Canada Ltd.) located at the National Center for Radiation Research and Technology (Cairo, Egypt).
The technique of CIC sampling is as follows: the sterile D shape stripe filter paper is applied on the conjunctiva using sterile forceps and smoothed onto the conjunctiva by applying gentle pressure, remaining in contact with the conjunctiva for approximately 5-10s and then removed in a peeling motion.To obtain the maximum yield of RNA, each area was sampled twice using separate filter-papers applied sequentially to the same site, after peeling, the filter papers were stored in RNAlater buffer (Qiagen, USA) at -80°C until RNA extraction[12,15].The conjunctivitis clinical features grading was given as mild,moderate, and severe: 1) mild, presences of mucopurulent discharge with mild redness; 2) moderate, presences of mucopurulent discharge with moderate redness; 3) severe,presences of heavy mucopurulent discharge with severe redness and in some cases with edema of lids.
Exclusion CriteriaPatients with a history of symptoms greater than one week in duration, patients who used systemic or local anti-inflammatory and antibiotics within the past week, patients who were uncooperative with sample collection(although giving a consent) and those without written consents,patients CIC sample with poor RNA yield (less than 45 µg/mL) and microbiological swab sample without bacteria were excluded from the study.
Identification of Causative AgentsThe identification of the causative microorganisms was done within 48h of sample collection. Standard culture techniques, specific media and biochemical tests were carried out according to Bergey’s manual of determinative bacteriology.
Reverse Transcription-quantitative Polymerase Chain Reaction
Extraction of RNA and complementary DNA synthesisTotal RNA was isolated from CIC samples individually using commercial kit RNeasy and QIAshredder columns (Qiagen,USA) according to the manufacturer’s instructions. The eluted RNA was transferred to a new eppendorf tube. The purity(A260/A280 ratio) and the concentration (A260) of RNA were obtained using spectrophotometry (dual-wavelength Beckman,USA). For each sample, the total RNA was reverse transcribed into cDNA using the QuantiTect Reverse Transcription kit(Qiagen, USA) according to the manufacturer’s instructions.
Real-time quantitative polymerase chain reactionIt was used to measure the relative gene expression of IL8, HBD2 and HBD3 and the endogenous control glyceraldehyde-3-phosphate dehydrogenase (GADPH) using QuantiTect SYBR Green polymerase chain reaction kit (Qiagen). Real-time quantitative polymerase chain reaction (qPCR) amplification and analysis were performed using an Applied Biosystem with software version 3.1 (StepOne™, USA).
The qPCR assay with primer sets was optimized at the annealing temperature. The reaction master mix and the running conditions for qPCR were carried out according to the manufacturer’s instructions. An appropriate negative(non-template control and reverses transcriptase control)and endogenous positive control (GADPH) were run in each experiment. The gene’s primers are shown in Table 1.
For the 20 healthy controls and 64 patients, the qPCR reactions were performed with IL8, HBD2, HBD3, and endogenous control GADPH primer pairs on the cDNA prepared from the CIC samples. The cDNA synthesis quality was assessed with the endogenous control GADPH primers.
Calculation of Relative Quantification Relative ExpressionThe relative quantification (RQ) was calculated according toApplied Biosystem softwareusing the following equation:ΔCt=Ct gene test - Ct endogenous control; ΔΔCt=ΔCt sample-ΔCt healthy; RQ=2-ΔΔCt; The RQ is the fold change compared to the healthy.

Table 1 The primers sequence of the studied genes
Statistical AnalysisThe qPCR data were statistically analyzed using IBM SPSS 20.0v software (USA). The statistical significance was set atP<0.05 and a highly significant set atP≤0.001. The independentt-test was used to statistically compare the gene expression of the IL8, HBD2 and HBD3 studied in patients CIC samples with those obtained from healthy controls.
The Chi-square testwas used to compare two qualitative parameters. The one-way ANOVA test was used to statistically compare the gene expression of the IL8, HBD2, and HBD3 toward the severity of the infection and the number of isolates per specimen. The linear correlation coefficients were used to assess the significant relation between two quantitative parameters in the same group. The Bonferroni test was used for multiple comparisons. All data were represented as means and standard deviations (SDs) of two independent experiments performed in triplicate.
RESULTS
This study included 20 healthy controls and 64 patients with active bacterial conjunctivitis. The demographic data of controls and patients are given in Table 2.
Microbiological StudyOut of the 20 healthy controls, six patients’ microbiological samples did not give growth and fourteen samples gave growth upon culturing. All isolates were mono and multi gram-positive bacteria. For the 64 patients,112 bacteria were isolated. They were in mono and multipopulation. Gram-positive bacteria were the predominant over gram-negative. The microbiological results and the severity of the clinical features are listed in Table 2, as well as the detailed microbial profile is shown in Figure 1.
Gene Analysis Regarding IL8, HBD2, and HBD3The RT-qPCR analysis was carried out for the pro-inflammatoryIL8 and AMPs HBD2 and HBD3 expression in a total of 20 CIC samples from the healthy controls and 64 CIC samples from active bacterial conjunctivitis. A quantitative evaluation of the up regulation of IL8, HBD2, and HBD3 in bacterial conjunctivitis has been conducted.
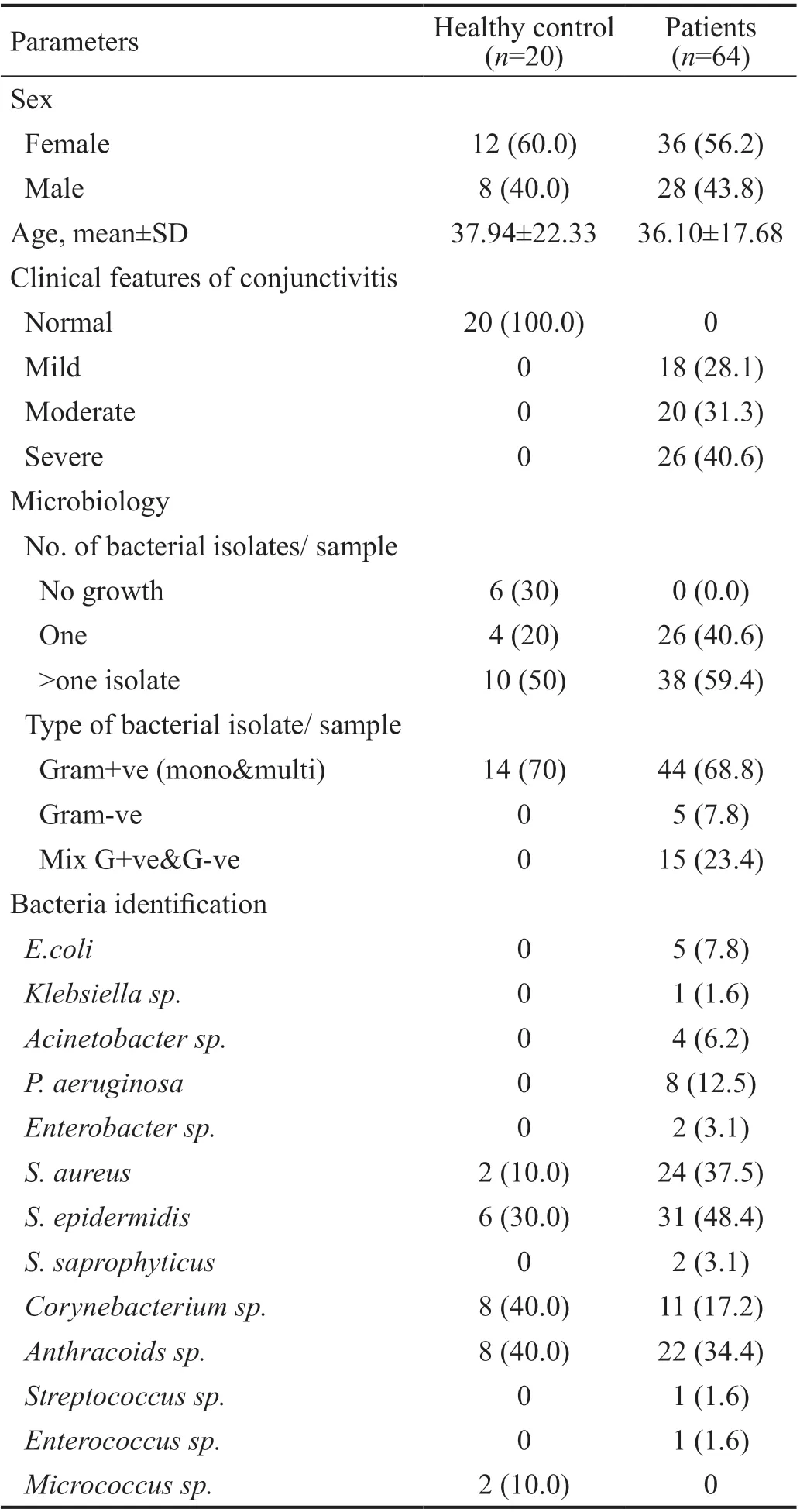
Table 2 Demographic, clinical and microbiological data of the patients and healthy controls n (%)
Gene Expression of IL8, HBD2, and HBD3The gene expression of IL8, HBD2 and HBD3 showed low levels in all the healthy controls, 0.91±0.22, 0.93±0.22 and 1.02±0.22,respectively. On the other hand, their expression was up regulated in bacterial conjunctivitis with a high statistical significance (P<0.001). In the patient group, the ranges of upregulation for IL8, HBD2, and HBD3 were from 1.19 to 15.28 fold, 0.63 to 11.77 fold, 0.66 to 10.98 fold, respectively (Figure 2).The patients were divided according to the type of bacteria causing conjunctivitis, into three groups: 44 patients with gram-positive bacterial conjunctivitis (mono and multi), 5 patients with gram-negative bacterial conjunctivitis, and 15 patients with mixed bacterial conjunctivitis (gram-negative and gram-positive bacteria).
In gram-positive bacterial conjunctivitis, the up-regulation of IL8, HBD2, and HBD3 were 6.2±3.66 fold (max: 15.28, min:1.37), 2.73±1.37 fold (max: 6.23, min: 0.63), and 3.06±1.76 fold (max: 10.98, min: 1.09), respectively. In gram-negative bacterial conjunctivitis, the up-regulation of IL8, HBD2, and HBD3 were 3.17±1.58 fold (max: 5.57, min: 1.59), 7.58±3.15 fold (max: 11.77, min: 4.33), and 2.18±1.43 fold (max: 4.12, min:0.66), respectively. In mixed bacterial conjunctivitis, the upregulation of IL8, HBD2, and HBD3 were 4.64±3.25 fold(max: 10.57, min: 1.19), 4.89±2.41 fold (max: 10.96, min:1.26), and 2.82±2.09 fold (max: 6.58, min: 0.69), respectively.There was a statistically significant increase in the expression of the antimicrobial peptide HBD2 (7.58 fold) with gramnegative bacterial conjunctivitis (P<0.001). Although it was not statistically significant during gram-positive infection, there was a relative increase in the expression of the inflammatory IL8 compared to other types. For the antimicrobial peptide HBD3, it was approximately up-regulated in the same range in the three types of bacterial conjunctivitis (Figure 3).
Correlation Between the IL8, HBD2, and HBD3 Upregulation Levels, in Each Type of Bacterial ConjunctivitisAccording to the type of bacteria causing conjunctivitis, the correlation between the IL8, HBD2, and HBD3 with each other was studied in each group separately.
In the gram-positive bacterial conjunctivitis group, there was a direct statistically significant correlation between the inflammatory IL8 and HBD2 (P<0.05). In the gram-negative bacterial conjunctivitis group, there was no correlation detected between the three genes. In the mixed bacterial conjunctivitis group, there was a direct statistically significant correlation between the AMPs HBD2 and HBD3 (P<0.05; Table 3 and Figure 4).
The data shown in Table 4 represent a statistically significant relation between the type of bacterial conjunctivitis and HBD2 up-regulation (P<0.05). However, there was a non-statistically significant difference between the up-regulation of HBD3 in the three types of bacterial conjunctivitis. Although it was not statistically significant, a further increase in the inflammatory IL8 up-regulation in gram-positive bacterial conjunctivitis than that in gram-negative bacterial conjunctivitis or in the mixed bacterial conjunctivitis was observed. Moreover, a non-statistically significant increase in the mixed bacterial conjunctivitis compared to that in gram-negative bacterial conjunctivitis (P=0.094) was observed.
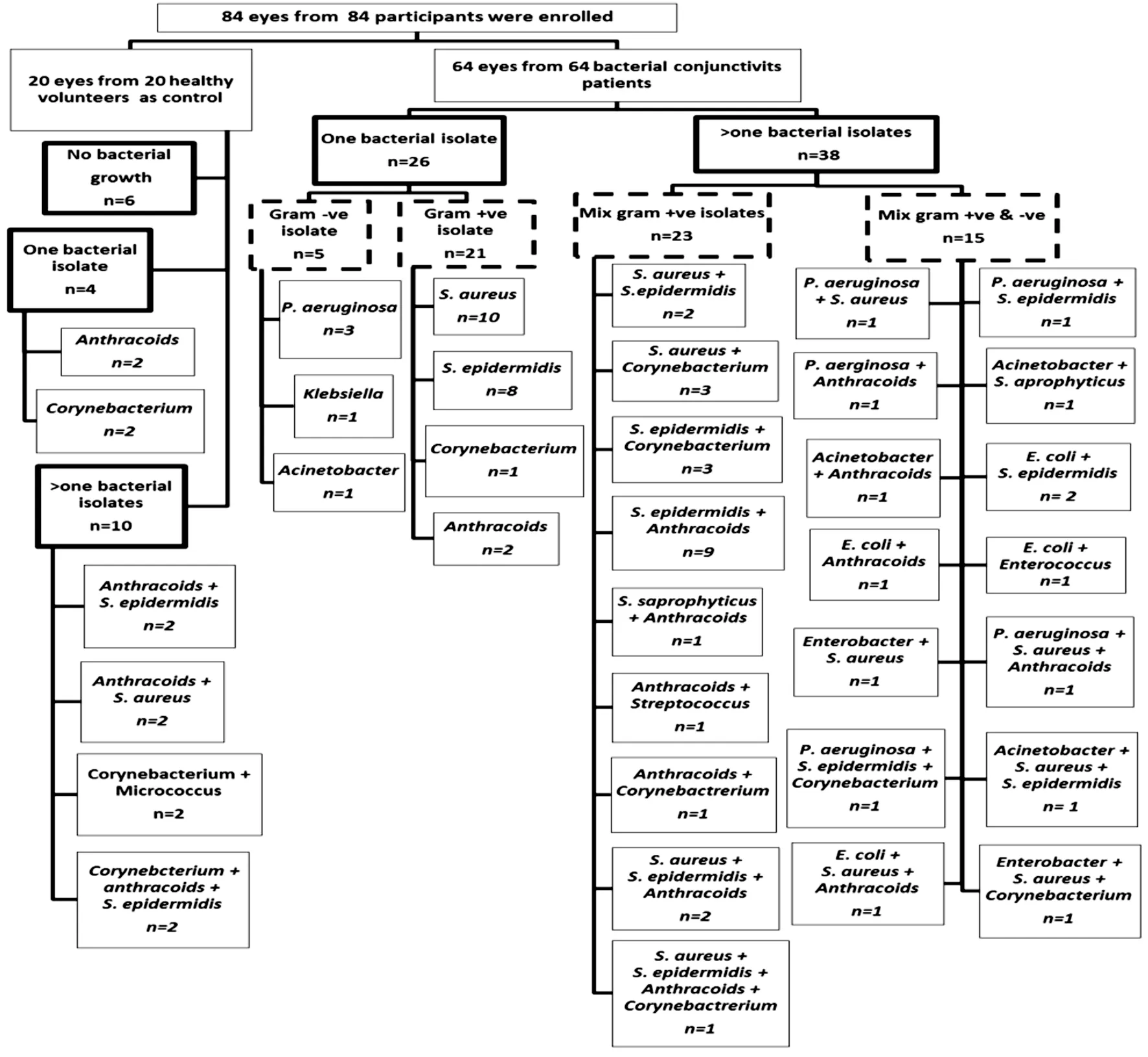
Figure 1 Flow chart of healthy control and patients enrolled in the study overall culture results per microbial profile.
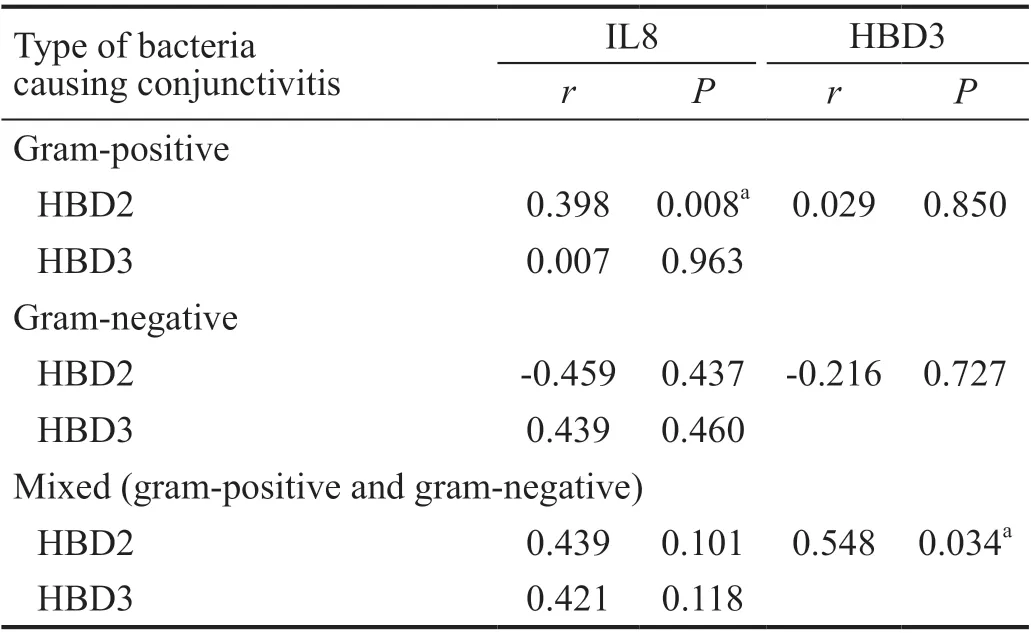
Table 3 The correlation between the up-regulation of IL8, HBD2,and HBD3, in each type of bacterial conjunctivitis
Relation Between IL8, HBD2, and HBD3 Up-regulation Levels and the Number of Bacterial IsolatesThe patients were divided according to the number of isolated bacteria per sample (number of isolated bacteria from the infected eye),into two groups; one bacterial isolate /sample (26 patients) and>one bacterial isolate /sample (38 patients). The up-regulation of the inflammatory IL8 and the AMPs HBD2 and HBD3 were not affected by the increase in the number of isolated bacteria from the infected eye (P>0.05).
Relation Between IL8, HBD2, and HBD3 Up-regulation Levels and Clinical Features of Bacterial ConjunctivitisThe patients were divided according to the severity of the clinical features, into three groups: mild (18 patients), moderate(20 patients) and severe (26 patients). The up-regulation of the inflammatory IL8 and the antimicrobial peptide HBD2 was significantly up-regulated in the severe clinical features(P<0.05) compared to mild and moderate features. Although it was not statistically significant, the antimicrobial peptide HBD3 up-regulation was relatively expressed more in patients with severe clinical features (Figure 5).
DISCUSSION
The conjunctival epithelium as a part of the ocular surface represents an active barrier against infectious agents, playing a vital role in the cellular immune response. The infectious agents may be commensal, pathogenic and mutualistic[16-17].
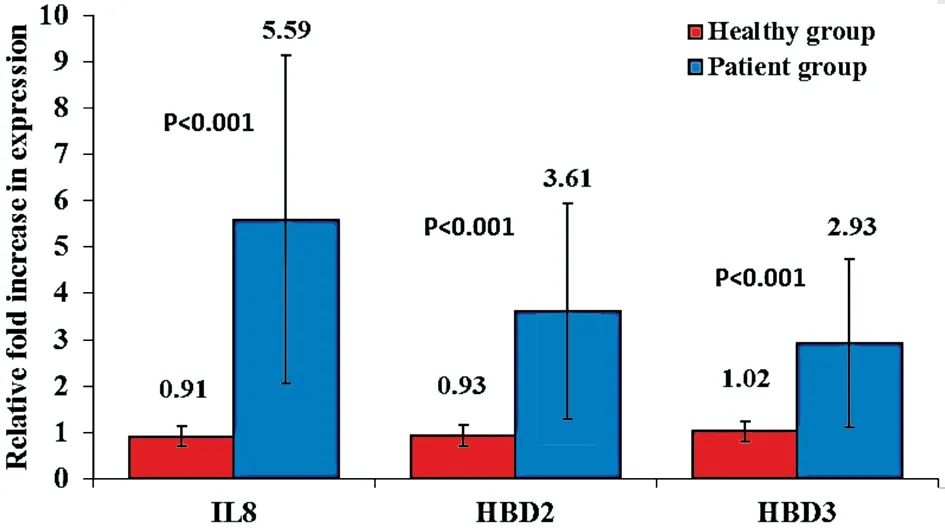
Figure 2 The increase in expression of IL8, HBD2, and HBD3 in the CIC samples of the patients with bacterial conjunctivitis compared to healthy controls.
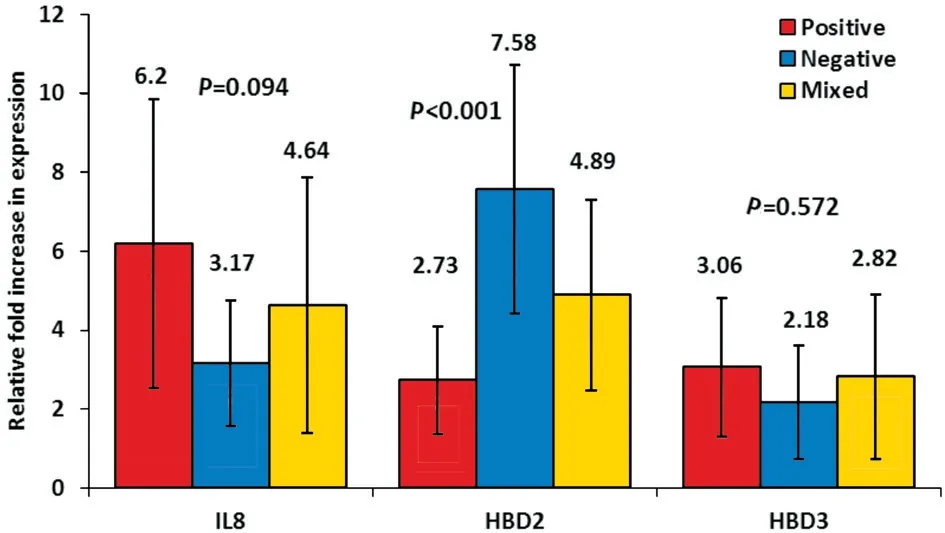
Figure 3 The relation between the IL8, HBD2, and HBD3 fold increase in expression and the type of bacteria causing conjunctivitis.
Bacterial conjunctivitis is the major cause of red-eye worldwide(90%), and the bacterial toxins may contribute to the development of obstructed terminal ducts in meibomian gland dysfunction[18]. This makes studying the immune response at the ocular surface fundamental to understand the infection and provide better treatment options for patients.
The present study is concerned with conjunctivitis infections caused by bacteria. The microbiological culturing showed that 70% of normal conjunctiva floras from the healthy controls were harbored with bacteria, 50% of the isolates were present in the form of a mixed population of gram-positive bacteria,and no gram-negative bacteria were detected. On the other hand, the microbiological culturing of patients’ specimens showed that 100% of the patients were infected with bacteria.The isolated bacteria distribution and proportion were variable,but gram-positive bacteria (mono and multi) (68.8%) were the major contributor over gram-negative bacteria (7.8%) and mixed (gram-positive and gram-negative) bacteria (23.4%).Staphylococcusspecies were the most frequent infectious agents either separately or in a mixed population. It was surprising that gram-positive bacilli were seriously involved in bacterial conjunctivitis.

Figure 4 Gram-positive and mixed bacterial conjunctivitis A: The direct correlation between the up-regulation of IL8 and the upregulation of HBD2 in the 44 patients with gram-positive bacterial conjunctivitis. r=0.398, P=0.008. B: The direct correlation between the up-regulation of HBD2 and the up-regulation of HBD3 in the 15 patients with mixed bacterial conjunctivitis. r=0.548, P=0.034.
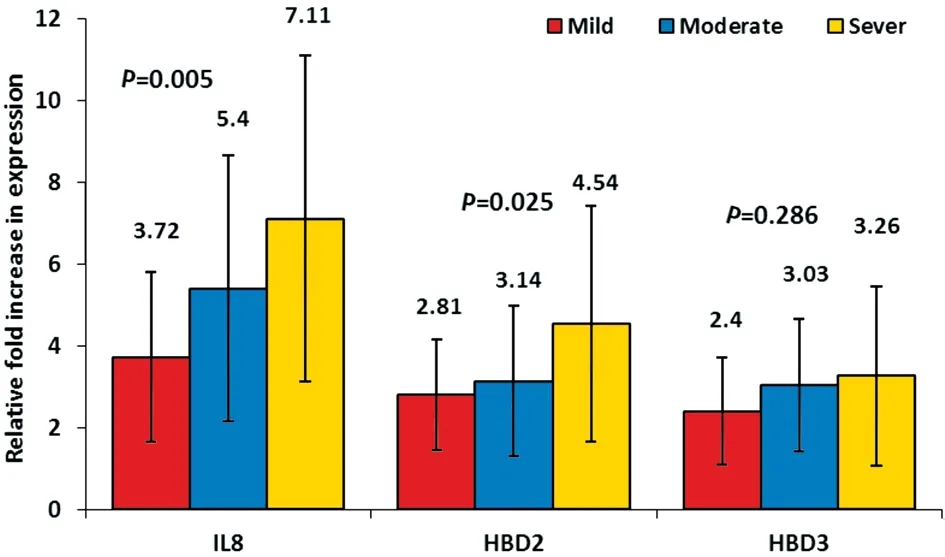
Figure 5 The relation between the IL8, HBD2, and HBD3 fold increase in expression and clinical features of conjunctivitis.
The findings of the current study are in agreement with most literature on conjunctival swabs from healthy and patients,especially in the Egyptian population. However, there were some differences in causative pathogens, which may be due to the little updates of the changes in the etiology of causative pathogens other than what has been reported in the few recent clinical studies carried out on eye infections[1,3-4,13].
The recent emphasis on the immune defense, the little data on how immune effectors contribute individually or collectively to host defense and the development of resistance mechanisms in infectious agents, both confirm the persistent need to pursue changes in the immune response to infections. In thisstudy, the authors were concerned with the ocular immune response to bacterial conjunctivitis. They focused on the quantitative expression of the pro-inflammatory IL8, HBD2 and HBD3 (major subtypes of AMPs) genes during bacterial conjunctivitis.

Table 4 The relation between the type of bacterial conjunctivitis and up-regulation of IL8, HBD2, and HBD3
It is documented in the literature that in the ocular surface, the recognition of infection agents by toll-like receptors initiates early immune response inducing the production of proinflammatory cytokines and consequently activates AMPs by different intra-signaling pathways[17,19].
In the present study, quantitative expression of pro-inflammatory IL8, HBD2 and HBD3 were detected in 64 human conjunctival epithelial cells during bacterial conjunctivitis. As well these genes were detected in 20 healthy controls. The CIC samples of patients were collected during the acute stage of infection(within 7d of infection).
The impression cytology technique was applied using a sterile gridded cellulose-acetate filter paper with a pore size of 0.45 μm, which is a non-invasive technique to collect 2 to 3 layers from the conjunctiva epithelial cells. It was ideal since it was important to collect cells with an optimum yield of RNA(≥45 µg/mL) and preserve the constituents of the cells and prevent any degradation[20].
In this study, during the active bacterial conjunctivitis, a statistically significant up-regulation in the expression of the pro-inflammatory IL8, HBD2, and HBD3 compared to healthy control (P<0.001) was observed. The present findings are in agreement with previous studies[7,21-26].
Consistent with IL8 up-regulation in this study, it is well documented in the literature that pro-inflammatory IL8 is activatedin vivobyS. aureusandP. aeruginosain human conjunctival epithelial cells through different signal transduction pathways[27-28]. The ability of an organism to cause infection depends on the production of toxins that stimulate the inflammatory cascade[18].
Matching the current findings, for HBD2, it is reported that it is extremely effectivein-vitroagainst gram-negative bacteria such asE. coliandP. aeruginosa. However, it possesses bacteriostatic properties against gram-positive bacteria[6-7,29-30].Its activity is sensitive to salt concentration whichin vivoshows decreased potency in the presence of tears[13].
The multiple tasks of HBD3 in the different ocular tissues are compatible with its up-regulation in all cases of bacterial conjunctivitis, demonstrated in this study. It is reported that HBD3 hasin-vitroandin vivobactericidal activity againstS. aureusandP. aeruginosa. This effect is salt-independent,whichin vivoshows an active antimicrobial potency in the presence of tears[6,31-32]. Besides, it has been connected to the initiation of the adaptive immune response through its capability to activate dendritic cells in conjunctiva and other ocular tissues[33].
As regards the isolated bacteria during infection, bacterial conjunctivitis patients were grouped into: gram-positive bacterial conjunctivitis (44 patients), gram-negative bacterial conjunctivitis (5 patients), and mixed bacterial conjunctivitis(15 patients). The gram-positive bacterial conjunctivitis group contained infections with mono and multi bacteria, the gramnegative conjunctivitis group contained infections with one isolate of bacteria as no multi gram-negative isolates were detected in this group, and the mixed bacterial conjunctivitis group contained infections with mix gram-negative and grampositive bacterial isolates. To the best of our knowledge, this is the first study to detect gene expression in mixed bacterial conjunctivitis.
Concerning IL8, a non-statistical significant difference in its expression during different types of bacterial conjunctivitis infection was detected. It was observed, more than 3.0 fold increase in expression during gram-positive infection compared to gram-negative infection. Also, more than 1.4 fold increase in the expression during the mixed-infection compared to gram-negative infection.
Despite that, it is widely known that lipopolysaccharide is a potent inducer of cytokine release from a variety of host cell types than peptidoglycans and teichoic acids[34]. This can be explained by the increased pathogenicity and virulence of gram-positive bacteria in the last decay[4,35].
As for HBD2, It was up-regulated with a high statistical significance in gram-negative bacterial conjunctivitis showing 7.58 fold (P<0.001). This data are in agreement with previous studies[36-37]. The data in current study also show that HBD2 expression was higher in the mixed bacterial conjunctivitis than in gram-positive bacterial conjunctivitis, 4.89 and 2.73 fold increase, respectively. This corroborates its increased expression against gram-negative bacteria compared to grampositive bacteria.
As regards HBD3, its up-regulation was mostly the same for the different types of bacterial conjunctivitis. The up-regulation was 3.06, 2.18 and 2.82 for gram-positive, gram-negative and mixed bacterial conjunctivitis, respectively. This shows a relative equal expression against different infectious agents.The current findings showed less than 0.9 fold increase in the expression of HBD3 during gram-positive infection than gramnegative and mixed infection. The present result confirms the broad-spectrum activity of HBD3 and its expression in all types of bacterial conjunctivitis.
This result is in agreement with previous literature[36,38-39].However, it was partially in controversy with Otriet al[11],where they found that HBD3 increased by 10-fold during gram-positive and 4-fold during gram-negative infections although this is non-statistically significant.
In line with the previous result, it was observed in the gramnegative bacterial conjunctivitis group that HBD2 was upregulated in 5/5 patients higher than HBD3. In the mixed bacterial conjunctivitis group, it was up-regulated in 13/15 patients higher than HBD3, and on the other hand for the grampositive bacterial conjunctivitis group, it was up-regulated in 15/44 higher than HBD3. These data highlight the intimate relation between HBD2 up-regulation and the detection of gram-negative bacteria in the site of infection.
With regards to the type of bacteria causing conjunctivitis,the authors analyzed the correlation between the proinflammatory IL8 up-regulation and the AMPs HBD2 and HBD3 up-regulation. It was found that in gram-positive bacterial conjunctivitis, there was a direct correlation between the up-regulation of IL8 and HBD2 (P<0.05) and for HBD3 it was proved to be non-significant. In gram-negative bacterial conjunctivitis, although non-statistically significant, it was observed that IL8 up-regulation was relatively inversed with HBD2 up-regulation. In the mixed bacterial conjunctivitis,there was no correlation detected between their up-regulation.Moreover, concerning the type of bacterial conjunctivitis,the correlation between HBD2 and HBD3 up-regulation was analyzed. It was found in gram-positive bacterial conjunctivitis non-statistical significant correlation between the two AMPs.In gram-negative bacterial conjunctivitis, although nonstatistically significant, an inverse correlation between the upregulation of the two AMPs was observed. However, in mixed bacterial conjunctivitis, as expected, a positive correlation between HBD2 and HBD3 up-regulation was observed (P<0.05).This reveals the synergism between different AMPs to combat different bacterial types participating in the same infection.
In view of the above, the differences in the up-regulation and correlations between the genes reflect the repercussions of the type of bacteria causing the infection. The chief target of gene regulation is to resolve the infection.
In this study, the authors tried to discern if there was any specific gene up-regulation related to the number of isolated bacteria. Contrary to expectations, no relation among IL8,HBD2, and HBD3 up-regulation and the number of isolated bacteria was detected. This may be justified through bacteria virulence factors, bacterial competition on nutrition access,space competition, the rapid growth rate of one bacterium over the other bacterium, and the production of their antimicrobials against each other which aids in supporting the host defense[40-41].
As regards the patients’ clinical features, a statistically significant up-regulation of IL8 and HBD2 (P<0.05) with severe clinical features was observed, these data are in concurrent with other studies[18,37]. But in controversy with a previous study that stated that the degree of ocular irritation is not directly related to cytokine concentration variations[42]. As expected,the current data ensure the involvement of IL8 in infectious conjunctivitis clinical features and the contribution of HBD2 in controlling the inflammatory response.
The HBDs aim to complement their actions, where HBD2 acts by binding to negatively charged membrane phospholipids,inducing efflux of intracellular components causing cell death,but as for the HBD3, two mechanisms have been reported,destruction of bacterial membrane and interaction with lipid II precursor[36,43]. Besides, it is now apparent that synergy between host defense proteins (such as lactoferrin and lysozyme) and AMPs as well as between these peptides may help overcome the effect of salt[44]. The present work supports the hypothesis that bacteria require millions of years to become resistant to AMPs[5].
Overall, the present data suggest differential regulation of HBD2 and HBD3 in the patients’ conjunctiva epithelial cells in bacterial conjunctivitis infection, both depending on causative bacteria. Importantly, in addition to the well-documented antimicrobial properties of HBDs, the evidence is rapidly increasing on their role as potent immune modulators capable of enhancing inflammatory processes and hence potentially contributing to the onset and/or persistence of inflammation at the site of infection.
It is worth noting that the present study has some limitations.First, the focus was made on aerobic bacterial isolates. Second,all the isolates recovered from each specimen were studied.The causative agents of the infection could not be defined,although all the required precautions have been taken while collecting the specimens to avoid contamination. These were common limitation issues with other researchers[35,45]. Third,the patients with gram-negative infections were five only and the mixed infections were fifteen only, which are relatively low numbers to rely on and partially influencing the results obtained. Fourth, IL8, HBD2 and HBD3 expression were only measured at the onset of disease; they were not continuously monitored at different time-points.
Finally, it must be conceded that the milieu of the typical bacterial infection site is not simple to reflect, owing to the presence of contributors in the immune response; host neutrophils, macrophages, mast cells, lymphocytes, platelets,all of the other cell types found in the tissue itself[46]. Taking these additional factors into account will help to further understand the disease and the development of new antibiotics.In conclusion, AMPs work in a network and cytokines regulate signals for effective response. They are up-regulated in response to microbial infection. The authors confirm the up-regulation of pro-inflammatory IL8, HBD2, and HBD3 as part of the immune response in the ocular surface to curb the infection process, the production of both AMPs HBD2 and HBD3 to fight infection in different concentrations.
ACKNOWLEDGEMENTS
Conflicts of Interest: Hosny AES,None;El-Bazza ZE,None;Ramadan MA,None;Shafik MA,None;Shafeek MA,None;Khattab RA,None.
 International Journal of Ophthalmology2021年5期
International Journal of Ophthalmology2021年5期
- International Journal of Ophthalmology的其它文章
- Comprehensive evaluation of intravitreal conbercept versus half-dose photodynamic therapy for chronic central serous chorioretinopathy
- Lipid accumulation and protein modifications of Bruch’s membrane in age-related macular degeneration
- Via pars plana anterior iris enclavation lOL fixation
- Role of microRNA-25 in high glucose cultured Müller glia
- Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HlF-1/VEGFA pathway and promoting PEDF expression
- lris manipulation during phacoemulsification: intraoperative and postoperative complications
