lris manipulation during phacoemulsification: intraoperative and postoperative complications
Eric R. Williams, Jennifer L. Patnaik, D. Claire Miller, Anne M. Lynch, Richard S. Davidson,Malik Y. Kahook, Leonard K. Seibold
Department of Ophthalmology, Sue Anschutz-Rodgers Eye Center, University of Colorado School of Medicine, Aurora,CO 80045, USA
Abstract
● KEYWORDS: phacoemulsification; iris manipulation;inflammation
I NTRODUCTION
Worldwide, cataracts are the leading cause of blindness and cataract surgery continues to be the most commonly performed ophthalmic procedure in developed countries[1-2]. From 2012 to 2014, at least 2.5 million outpatient cataract procedures were performed in the United States alone[3]. It is estimated that 1%-3% of all cataract procedures are associated with small pupils[4]. Cataract surgery among eyes with small pupils is a known risk factor for multiple complications both intraoperatively and postoperatively[5-7].To combat this, several techniques have been developed to manage intraoperative miosis at the time of surgery.Current techniques include topical pharmacologic agents,intracameral pharmacologic agents, manual pupil stretching,and mechanical pupil expanders[4,8-9]. Two common mechanical pupil expansion devices are the Malyugin ring (MicroSurgical Technology, Redmond WA) and iris hooks[8-9]. There are a variety of iris hooks available that are generally placed through additional small, limbal paracenteses with multiple variants of iris hook placement[10-11]. The Malyugin ring is a square singlepiece injectable iris expander that creates 8 iris contact points,which allows it to create a rounded as opposed to square pupillary opening[8-9,11].
Despite widespread use and demonstrated safety and efficacy,the use of mechanical pupil expanders has been associated with increased operative times as well as postoperative complications[12]. These include iris sphincter tears, corneal edema, and postoperative anterior uveitis[12-13]. However,limited data have been published regarding outcomes of phacoemulsification surgery involving mechanical pupil expansion compared to routine and complex cases without the use of these devices. The objective of this study was to further characterize clinical outcomes including intraoperative and postoperative complications associated with the use of mechanical pupil expanders.
SUBJECTS AND METHODS
Ethical ApprovalThis study was obtained from the Colorado Multiple Institutional Review Board and the protocol adhered to the tenets of the declaration of Helsinki.
This was a retrospective cohort study of all patients who underwent phacoemulsification with intraocular lens implantation (phacoemulsification) surgery between January 1, 2014 and June 30, 2017 at the University of Colorado Sue Anschutz-Rodgers Eye center in Aurora, Colorado. Data were extracted from the electronic medical record (EMR)by trained research assistants and then entered into a secure web application, REDCap electronic data capture tool[14].Patients with a history of uveitis and/or history of chronic topical steroid use, who underwent planned combination surgeries [minimally invasive glaucoma surgery, pars plana vitrectomy (PPV), penetrating keratoplasty, Descemet’s stripping automated endothelial keratoplasty, or Descemet’s membrane endothelial keratoplasty], or did not complete at least 28d of follow-up were excluded from the study (both intraoperative and postoperative analyses). Patients that required an additional procedure within the three months after phacoemulsification (either planned or unplanned) were excluded from postoperative analyses.
Data CollectionFor all cases, baseline patient demographics and preoperative ocular characteristics were collected including tamsulosin use, traumatic cataract, mature cataract,intraocular pressure (IOP), visual acuity logMAR (VA), and axial length. Intraoperative data including use of mechanical pupil expanders, capsular tension rings (CTRs), Trypan blue or similar anterior capsule staining technique were collected in addition to the occurrence of posterior capsule rupture (PCR)with or without vitreous loss (VL), retained lens fragments,zonular dialysis (ZD), or choroidal hemorrhage. The total surgical time was also collected from operative records.Postoperative data including IOP, VA, use of topical steroid therapy, presence and grade of inflammation, and presence of cystoid macular edema (CME) were collected through extraction from the EMR at subsequent visits.
Surgical TechniqueCataract surgeries were performed with standard phacoemulsification technique using either an Alcon Infiniti or Centurion machine (Alcon, Ft Worth, TX, USA)and clear corneal incisions. The vast majority of cases were performed under topical anesthesia, unless otherwise indicated by surgeon discretion.
Complex phacoemulsification with iris manipulation was defined as cases requiring one of the following: Malyugin ring expansion device (6.25 mm or 7.0 mm), iris hooks (Alcon,Ft Worth, TX, USA), other less common pupil expanders such as the Oasis iris expander (OASIS medical, San Dimas,CA, USA), or stretch pupilloplasty. The use of these devices was determined at the discretion of the surgeon. Those eyes that required a technique listed under complex without iris manipulation and also required one of the iris manipulation techniques were only analyzed in the complex with iris manipulation group.
Complex phacoemulsification without iris manipulation was defined as cases requiring one of the following: anterior capsule staining with Trypan blue (DORC, Zuidland, the Netherlands) or capsular support devices such as CTR(Morcher GmbH, Stuttgart, Germany) or capsular hooks(Morcher GmbH, Stuttgart, Germany). The use of these devices was determined at the discretion of the surgeon.
Non-complex phacoemulsification cases were those cases that did not require any techniques described above in either of the other two study groups.
OutcomesOperating times: defined as time out of the surgery suite minus the surgery start time as noted in the nursing documentation.
Intraoperative complications: complications were extracted from the surgeon’s operative note. Patients that required anterior vitrectomy or subsequent PPV were excluded from postoperative complication analysis since these patients typically have more postoperative complications.
Postoperative complications: postoperative IOP was measured at day 1, week 1-3, and month 1-3 and summarized at each visit, and as a mean change from baseline levels, as well as rates of IOP spikes (defined as either greater than 10 mm Hg from preoperative value, or greater than 20 mm Hg from preoperative value). Preoperative best-corrected VA and bestcorrected postoperative VA from months one to six were collected. The change in VA from baseline was also evaluated.Refractive surprise was analyzed based on expected refractive error and actual refractive error and was compared based on a +/-0.5 diopters difference and 1.0 diopters difference.Postoperative inflammation was defined as presence of cell/flare that was still present at the one-month postoperative visit, a drop regimen requiring increased or prolonged topical steroids (i.e., drops longer than 4wk, extended four time per day regimen), or recurrent inflammation that required restarting topical steroids from postoperative month one to month three.Postoperative CME was defined as the presence of cystic intraretinal macular fluid on macular optical coherence tomography(OCT) imaging. This diagnostic test was ordered based on the discretion of the surgeon.

Figure 1 Inclusion and exclusion criteria.
Statistical AnalysisBasic frequencies, means with associated standard deviations, and medians were used to describe clinical characteristics and outcomes between the three surgical groups (non-complex phacoemulsification, complex phacoemulsification with pupil manipulation, and complex phacoemulsification without manipulation). Each of the three groups were compared to the other groups for all statistical testing. Statistical comparisons were conducted with general linear and Logistic regression modeling with estimating equations to account for the correlation of patients who had two eyes included. Multivariate modeling for outcomes of interest included potential confounders that were significant in the univariate analyses.P-values <0.05 were considered significant. SAS version 9.4 (Cary, North Caroline, USA) was used for all analysis.
RESULTS
A total of 6990 phacoemulsification cases were recorded from January 1, 2014 to June 30, 2017. Of these, 5772 cases were included in the intraoperative analysis cohort, and 5726 were included in the postoperative analysis cohort(Figure 1). Within the intraoperative cohort, there were 4905 non-complex phacoemulsification (84.9%), 367 complex with iris manipulation (6.3%), and 500 complex without iris manipulation cases (8.6%). Within the complex with iris manipulation cohort, there were 85 cases that also received a maneuver categorized in the complex without iris manipulation group. Of these 85 cases, the majority (61%) were capsular staining alone. Of the total cases, 1218 were excluded from both the intraoperative and postoperative cohort because of planned or unplanned combined surgery, a history of uveitis,follow-up less than 1mo, and/or chronic steroid use prior to surgery. An additional 46 patients were excluded from the postoperative analysis group due to subsequent intraocular surgery within three months after phacoemulsification.
Baseline patient demographics and ocular characteristics are detailed in Table 1. The mean age of non-complex, complex with iris manipulation, and complex without iris manipulation were 69.6, 72.9, and 67.1y, respectively, and were significantly different across all group comparisons. The percentage of tamsulosin use was significantly higher in the complex with irismanipulation cohort (24.2%), compared to the non-complex and complex without iris manipulation cohort (4.0% and 3.2%,respectively). Additionally, eyes in the iris manipulation cohort were significantly more likely than both other groups to be male and have axial length <23 mm. There were no significant differences in preoperative mean IOP across the three groups.Preoperative mean VA was 0.295, 0.482, and 1.070 in the noncomplex phacoemulsification, complex with iris manipulation,and complex without iris manipulation respectively (P<0.0001 for all three comparisons).

Table 1 Baseline patient demographics and ocular characteristics
Complication outcomes for each group are listed in Table 2.The incidence of any intraoperative complication of interest in the complex with iris manipulation and complex without iris manipulation groups were 4.1% and 5.2% respectively,compared to 0.8% in the non-complex phacoemulsification(P<0.0001 comparing each complex group to noncomplex phacoemulsification). The adjusted odds ratio of any intraoperative complication in the complex with iris manipulation cohort was 4.3 (95%CI: 2.2-8.3,P<0.0001)compared to non-complex phacoemulsification cases. The adjusted odds ratio of any intraoperative complication in the complex without iris manipulation cohort was 2.4(95%CI: 1.0-5.6,P=0.040) compared to non-complex phacoemulsification. Posterior capsular rupture rates were significantly greater in complex with iris manipulation cases(1.4%) compared to noncomplex cases (0.5%). VL and zonular dialysis occurred more frequently in complex with iris manipulation and complex without iris manipulation cases compared to non-complex phacoemulsification (2.7%,3.6%, and 0.2% respectively,P<0.0001). Mean surgical timewas significantly different across all groups and was longest in complex with iris manipulation (32.5min) followed by complex without iris manipulation (29.1min) and noncomplex cases (18.8min). Cumulative dissipated energy (CDE) was significantly increased in both complex cases compared to noncomplex phacoemulsification. Additionally, complex phacoemulsification without iris manipulation had significantly increased CDE when compared to complex with iris manipulation.
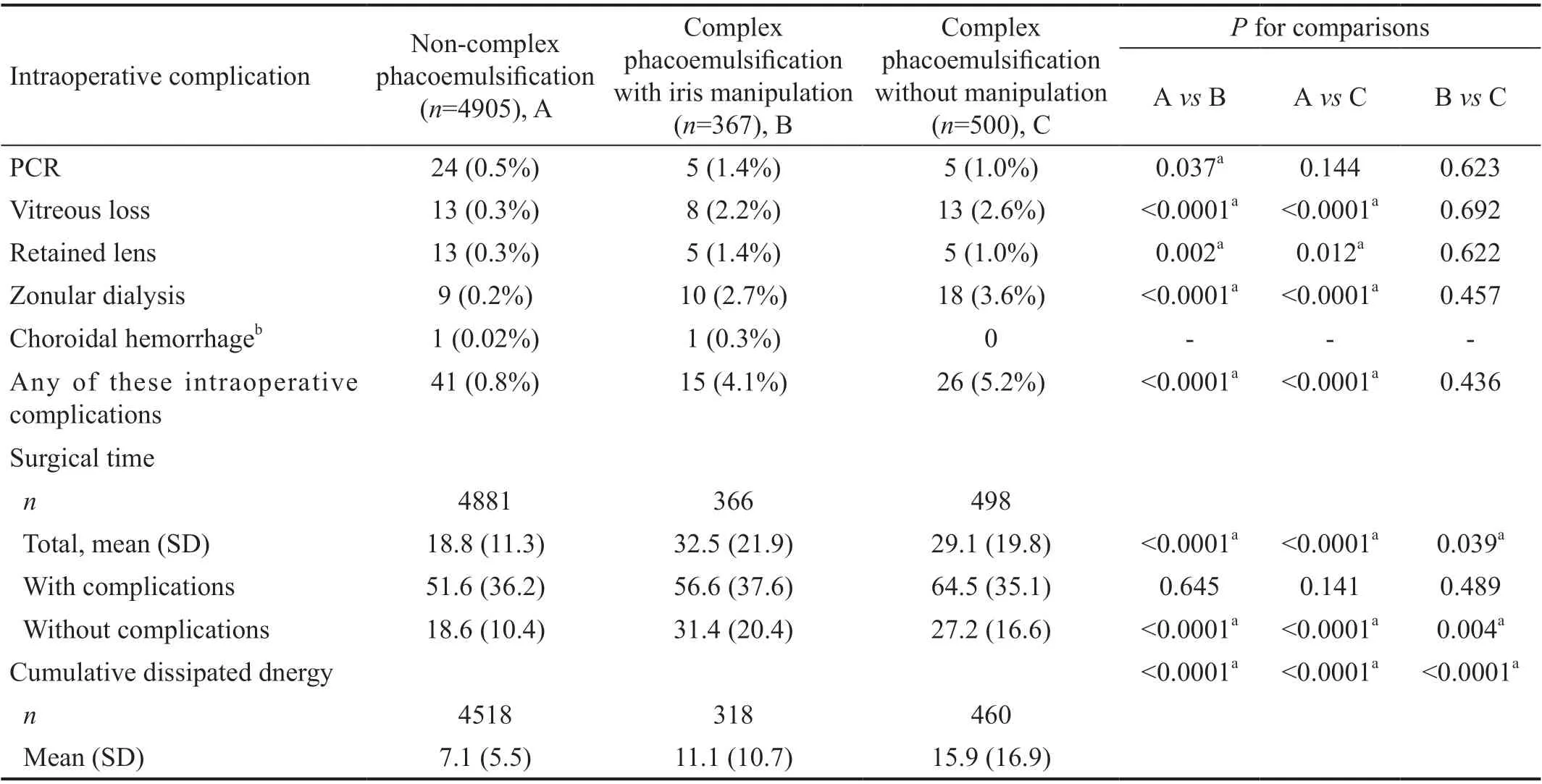
Table 2 Intraoperative complications
The postoperative cohort consisted of 4877 non-complex phacoemulsification (85.2%), 359 complex with iris manipulation (6.2%), and 490 complex without iris manipulation cases (8.6%). Postoperative outcomes are listed in Table 3. Postoperative IOP spikes >10 mm Hg from baseline occurred in 7.5% of complex with iris manipulation cases compared to 3.1% of non-complex phacoemulsification cases(P=0.001). Postoperative IOP spike >20 mm Hg from baseline was 1.8% in complex with iris manipulation and 1.6% in complex without iris manipulation compared to 0.4% in noncomplex phacoemulsification (bothP=0.001 when compared to non-complex phacoemulsification). The corrected distance postoperative VA was significantly better in non-complex cases (0.077) compared to both complex with iris manipulation(0.147), and complex without iris manipulation (0.169), bothP<0.05 when compared to non-complex phacoemulsification.Rates of postoperative inflammation were significantly greater in complex with iris manipulation cases (5.6%) compared to non-complex cases (2.8%,P=0.006), but the rate for complex phacoemulsification without manipulation (4.1%) did not significantly differ compared to either of the other groups. The adjusted odds ratio of postoperative inflammation in complex with iris manipulation cases compared to non-complex phacoemulsification was 2.3 (95%CI: 1.3-4.0,P=0.005), and was 2.7 (95%CI: 1.3-5.7,P=0.009) compared to complex without iris manipulation. The rate of CME and refractive surprise was similar between all study groups postoperatively.
DISCUSSION
This study compared non-complex phacoemulsification,complex phacoemulsification with iris manipulation, and complex phacoemulsification without iris manipulation to evaluate the effects of mechanical pupil expansion on intraoperative and postoperative outcomes. We found that after adjusting for multiple baseline variables, the risk of any intraoperative complication was significantly increased in complex cases with or without iris manipulation. Additionally,after similar adjustments for baseline variables, complex phacoemulsification with iris manipulation more than doubled the odds of increased postoperative inflammation when compared to both non-complex phacoemulsification and complex phacoemulsification without iris manipulation. Both complex with and without iris manipulation led to IOP spikes of >20 mm Hg postop. day 1; however, only complex cases involving iris manipulation led to increased spikes >10 mm Hg.Other studies have discussed the increased risk of inflammation associated with iris manipulation in cataract surgery[8,12-13,15-16].Nderitu and Ursell[12]demonstrated in a study of 9552 eyes(409 of which required either Malyugin ring or iris hooks)that there was a significantly increased risk of anterior uveitis and corneal edema with the use of the Malyugin ring (6.7%)compared to no pupil expanders (2.6%), or iris hooks (1.1%).Similarly, Taipaleet al[16]compared outcomes from 536 total eyes undergoing phacoemulsification with or without a pupil expansion device. Eyes with a pupil expansion device had significantly greater amounts of intraocular inflammation at postoperative 28d compared to those without. This difference did not remain significant at follow-up of 3mo.
In contrast to the previous work above, our study was the first to our knowledge to compare complex phacoemulsification with iris manipulation to other phacoemulsification cases deemed complex but without iris manipulation. These results lend further evidence that direct iris manipulation, as opposed to just the complexity of the phacoemulsification case, is associated with postoperative inflammation. This effect cannot be explained by increased CDE in our series because complex cases without iris manipulation had statistically significantly increased CDE compared to complex with iris manipulation.Multiple studies have asserted that postoperative inflammation is related to iris sphincter tears[8,12-13,15]. However, a recent study by Aketaet al[17]demonstrated that iris damage regardless of severity leads to increased cytokine levels in the aqueous humor. These results in combination with our study suggest that manipulation of the iris with or without sphincter tears is enough to lead to increased aqueous humor cytokines, and thus postoperative inflammation.
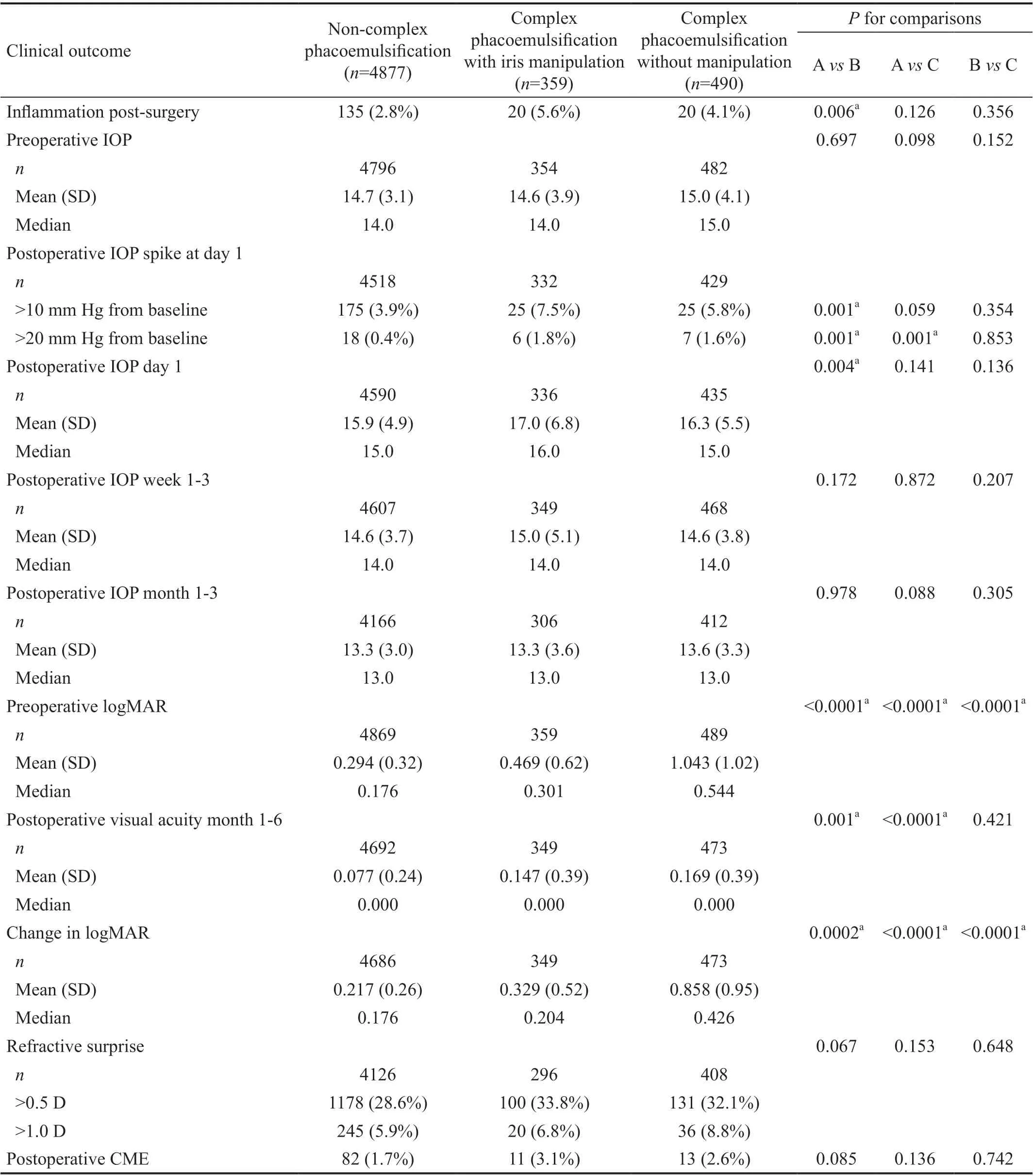
Table 3 Clinical outcomes of patient eyes
Our study demonstrated increased rates of intraoperative complications, specifically VL, zonular dialysis, and retained lens fragments in both complex phacoemulsification groups. The odds of any intraoperative complication when adjusting for baseline variables was increased in complex phacoemulsification with or without iris manipulation. In a prior study, iris manipulation was not statistically significantly associated with increased PCR or VL, although the rates of these complications were not similar between mechanical pupil expansion and no pupil expander groups[12]. In this same study by Nderitu and Ursell[12], there was increased rates of zonular dialysis, which they attributed to the increased complexity of the case, as opposed to direct use of mechanical pupil expanders. This appears consistent with our results given similar rates of intraoperative complications between complex phacoemulsification cases both with and without iris manipulation. It is worth noting that some surgeons may have used CTRs for rotational stability in cases with highly myopic eyes while inserting a toric intraocular lens, which would otherwise not necessarily be deemed a complex case.
Iris manipulation and its effect on pseudophakic CME is mixed in the literature. Similar to our study, Nderitu and Ursell[12]found no difference between the rate of pseudophakic CME in cases with and without iris manipulation. In contrast, Taipaleet al[16]found that cases with iris manipulation did increase the rate of pseudophakic CME when compared to non-complex phacoemulsification. In this study, we found similar rates of CME between all study groups, but the difference in CME between the noncomplex group (1.7%) and complex with iris manipulation (3.1%) was of borderline significance (P=0.085).The overall rate of CME however, was low in our cohort and postoperative macular OCTs were only ordered at the discretion of the surgeon. This limitation and small sample size could have impacted this finding.
Similar to other studies, we found that iris manipulation during phacoemulsification led to increased rates of IOP spike postoperatively[12]. Interestingly, rates of an IOP spike >20 mm Hg was found to be significantly increased in complex cases without iris manipulation. However, when comparing the mean IOP at postoperative day 1, this was only significantly different in cases with iris manipulation compared to non-complex cases. This effect was not long-term as there was no statistically significant difference between mean IOPs at postoperative weeks 1-3 or months 1-3 across all groups. Prior studies have shown transient IOP elevations after phacoemulsification, and have theorized inflammation, retained lens cortical material,pigmented debris of the iris, or remaining viscoelastic as possible etiologies for this IOP spike[18]. All of these etiologies are plausible explanations for the transient IOP rise that was found in cases with iris manipulation. Further study is needed to better elucidate which of these or other factors are truly causative.
While the above parameters are clinically relevant to postoperative care, the ultimate VA outcome is paramount when assessing outcomes after cataract surgery with or without iris manipulation. Preoperative VA was significantly worse in both complex groups compared to non-complex phacoemulsification. Similarly, the best postoperative VA was statistically significantly worse in both complex groups compared to non-complex, although the clinical difference was minor (20/23vs20/28vs20/29 for non-complex,complex with iris manipulation, and complex without iris manipulation, respectively). Thus, despite the increased rates of inflammation, complications, and IOP spike associated with iris manipulation, the visual outcomes remain generally very good in this group.
There are some notable limitations to this study. First is the retrospective design of the study. Second, there were limited data on possible iris manipulation maneuvers in non-complex phacoemulsification cases (i.e., sphincterotomy, pupilloplasty),which may have affected the overall power of the study comparisons. Additionally, there was no information regarding the extent of the surgery that trainees completed. Postoperative inflammation was subjectively graded according to the treating physician which may vary widely between different clinicians.As mentioned previously, the use of CTR may have been related to the placement of toric lenses in highly myopic eyes,although we believe this to be a significant minority of cases.Finally, the postoperative drop regimen was not standardized across surgeons during the study period.
In conclusion, both complex phacoemulsification with and without iris manipulation led to increased rates of intraoperative complications. However, only complex cases with iris manipulation led to increased rates of postoperative inflammation, which was true even when adjusting for multiple possible confounding variables. These results suggest that intraoperative complications are more likely related to the complexity of the case, as opposed to direct iris manipulation;whereas, postoperative inflammation is more likely related to direct iris manipulation, and less likely the mere complexity of the case. Surgeons should be aware of the increased risks associated with iris manipulation along with the possible need for longer or more intense anti-inflammatory regimens postoperatively. Future prospective, randomized studies are needed to confirm these findings.
ACKNOWLEDGEMENTS
Conflicts of Interest:Williams ER,None;Patnaik JL,None;Miller DC,None;Lynch AM,None;Davidson RS,None;Kahook MY,None;Seibold LK,None.
 International Journal of Ophthalmology2021年5期
International Journal of Ophthalmology2021年5期
- International Journal of Ophthalmology的其它文章
- Comprehensive evaluation of intravitreal conbercept versus half-dose photodynamic therapy for chronic central serous chorioretinopathy
- Lipid accumulation and protein modifications of Bruch’s membrane in age-related macular degeneration
- Via pars plana anterior iris enclavation lOL fixation
- Role of microRNA-25 in high glucose cultured Müller glia
- Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HlF-1/VEGFA pathway and promoting PEDF expression
- Expression levels of pro-inflammatory interleukin-8 and certain antimicrobial peptides in concurrent with bacterial conjunctivitis
