Role of microRNA-25 in high glucose cultured Müller glia
Yu Liu, Han Shen, Song-Tao Yuan, Qing-Huai Liu
Department of Ophthalmology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
Abstract
● KEYWORDS: microRNA-25; Müller glia; proliferation;apoptosis
INTRODUCTION
Diabetic retinopathy (DR) is a major microvascular complication of diabetes and one of the main causes of blindness in working-age adults[1]. Multiple studies have suggested that the pathogenesis of DR is closely involved in the dysfunction of Müller glia (MG), which occurs even before vascular injury, especially in the preclinical stage of DR[2-3].MGs are the major retinal supporting cells that participate in retinal metabolism, function, maintenance and protection by specifically expressing a variety of enzymes and ion channels. MGs also play an important role in the occurrence and development of retinal injury, making further investigation of the pathogenic role of MGs in DR of great significance for early diagnosis and treatment of DR[4-5].
MicroRNAs (miRNAs) are small non-coding RNAs which participate in posttranscriptional regulation of their target mRNAs and participates in physiological and pathological processes[6]. Accumulating evidence has shown that aberrant expressions of miRNAs are highly related to the onset and progression of various diseases, including cancer, congenital heart disease, and degeneration diseases[7]. Regarding ocular diseases, microRNA-25 (miR-25) has been reported to induce mature MGs to re-differentiate into retinal progenitor cells[8-9],but its role in DR has yet been reported. Here, we detected the expression difference of miR-25 between low and high glucose cultured Müller glia (LGMG and HGMG, respectively) and analyzed the effects of miR-25 on the HGMG. Moreover, we discussed its potential mechanism in the progress of DR in hope of identifying new targets for the treatment of DR.
MATERIALS AND METHODS
Culture of Mice Primary Müller GliaPrimary cultures of mice retinal microglia were conducted following the established protocol with minor modifications[10]. Briefly,postnatal day 6 to 7 mice were sacrificed under anesthesia and retina from 4-5 mice were harvested and digested in 70 U collagenase for 15min at 37℃. Cells were collected by centrifugation at 300×g for 5min and resuspended in Dulbecco’s modified Eagle media/nutrient mixture F-12(DMEM/F12) medium containing 10% fetal bovine serum(FBS), 1% penicillin and streptomycin.
Evaluation of the Purity of Primary Cultured Müller GliaImmunocytochemistry and flow cytometry were conducted to validate the purity of MG. Cells were seeded on 6-well cell culture plates and were fixed in 4% paraformaldehyde for 10min at room temperature. Cells were washed three times with PBS. Afterwards, 5% bovine serum albumin (BSA)solution was added for 60min, and samples were stained with primary antibodies (Abcam, ab16700 at 1/1000 dilution;ab64613 at 1/1000 dilution) and the second antibodies(1:200 dilution). Each sample was observed under a confocal microscope (MIC00223, LSM5 Live).
RT-PCRMG in different groups were harvested, and total RNA was extracted using Trizol reagent (Vazyme Biotech Co,Nanjing, China). The miRNA cDNA was synthesized using the kit (Ribo, Guangzhou, China) according to the manufacturer’s instructions. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed using miRNA RTqPCR Starter Kit (Ribo, Guangzhou, China). MiR-39-3p was served as the external reference, added before total RNA extraction. All amplifications were performed in triplicate.
Measurement of Müller Glia ProliferationCell proliferation was measured using the cell counting kit-8 (CCK8; MCE,USA) and the EdU cell proliferation kit (Ribo, Guangzhou,China). Forty-eight hours after transfection with miR-25 mimics or negative control, cell proliferation was detected using the CCK8 kit and EdU kit following the manufacturer’s instructions.
Detection of Müller Glia ApoptosisCell apoptosis assay was performed using the Annexin V-FITC/PI apoptosis detection kit (Vazyme Biotech Co, Nanjing, China) according to the manufacturer’s protocol. MGs in different experimental groups were collected by centrifugation at 300×gfor 5min followed by being washed with PBS for three times. The 300 μL of 1×binding buffer was added and samples were mixed for analyze with a fluorescence activating cell sorter (FACS; BD Biosciences).
Luciferase Activity AssayThe luciferase activity assay was conducted in 96-well plates. Wild-type Fbsw7 (Fbsw7-WT) or its mutation (Fbsw7- Mut) were co-transfected into MGs with miR-25 mimic or mimic NC (100 nmol/L) using Lipofectamine 3000 (Invitrogen Technologies). The 24h after transfection,luciferase activities of podocytes were ensured using a Dual-Luciferase Report Assay Kit (Promega, Madison, WI).
Western Blot AnalysisMGs (1×105cells) were lysed in RIPA buffer, followed by 12 000×g centrifugation for 15min and concentration quantification using BCA protein assay kit (Cell Signaling Technology, USA). After blocking with 5% BSA,0.45 μm polyvinylidene fluoride (PVDF) membranes were incubated with antibodies against Fbsw7 (Abcam, ab192328 at 1/500 dilution) and GAPDH (Abcam, ab8245 at 1/1000 dilution). Protein bands were detected with a Tanon 4100 Multi Flurescence Imager.
Statistical AnalysisAll data were expressed as mean ± standard deviation. Thet-test, two-way repeated-measure analysis ofvariance (ANOVA), or curve fit. All statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, USA).P-values<0.05 were considered statistically significant. All experiments were conducted in triplicate.
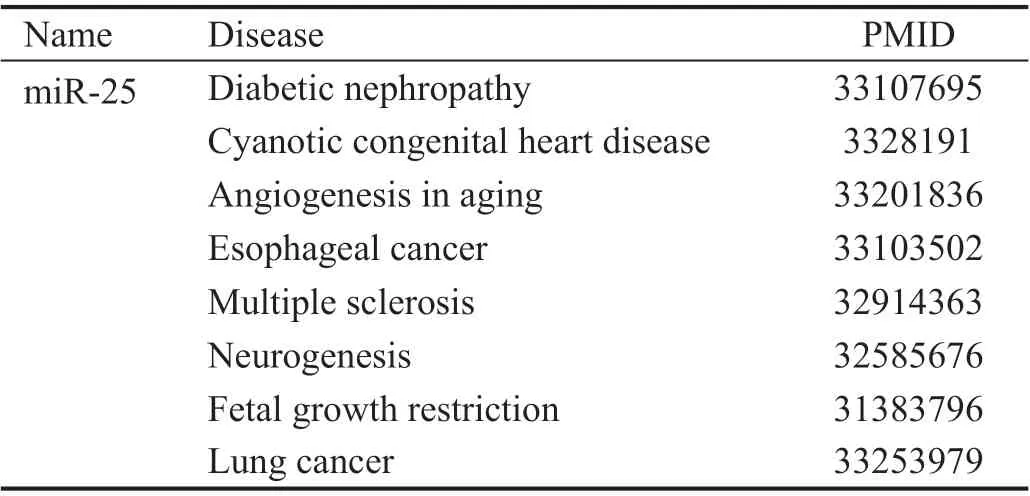
Table 1 Published miR-25 related diseases
RESULTS
Characterization of Cultured Retinal Müller GliaAs shown in Figure 1A, primary cultured MG exhibited typical morphological features of glia cells. Flow cytometry and immunocytochemistry both confirmed that glutamine synthetase (GS) and vimentin were positively expressed in primary cultured MG (Figure 1B and 1C). Nuclei were stained with DAPI. These results altogether confirmed our cultured cells were primarily MG.
miR-25 Downregulated in Müller Glia Under High GlucoseThe roles of miR-25 have been reported in multiple diseases including cancer, degeneration, and genesis of neural progenitor cells (Table 1). To evaluate the potential role of miR-25 in MGs, we investigated the expression level of miR-25 in MGs under high glucose. Results showed that the expression of miR-25 was significantly downregulated in high glucose (25 mmol/L) compared to its in low glucose medium(5 mmol/L;P<0.05, Figure 2A). These results indicate that the dysregulation of miR-25 expression in HGMG may have a regulatory role in the dysfunction of DR.
miR-25 Alleviated HG-induced Injury on Müller GliaIncreasing evidence confirmed that miRNAs regulate apoptosis of retina cells exposed to high glucose. Shiet al[11]and Huanget al[12]reported that M2 macrophage-derived exosomal miR-25-3p attenuated high glucose-induced podocytes injury in diabetic nephropathy. Herein, we investigated the effects of miR-25 on MGs proliferation and apoptosis in high glucose medium. The 100 nmol/L miR-25 mimic, and negative control miRNA-NC were transfected into cells using Lipofectamine 3000 for 48h. As shown in Figure 2B, flow cytometry analysis results showed that up-regulation of miR-25 significantly alleviate apoptosis rate of MG treated with high glucose compared with the high glucose treated alone group. Also,the EdU staining as well as CCK8 assay confirmed that MG proliferation was significantly downregulated in high glucose medium, while miR-25 mimics treated group showed no significant difference compared with NC group (P<0.001,Figure 2C and 2D). Our data reveal that MG were damaged in high glucose, while 500 nmol/L miR-25 could efficiently effectively attenuate high glucose-induced MG injury.

Figure 1 MG characterization A: Two days after seeding, light images were taken at 40× and 200× magnification; B: Immunocytochemistry confirmed that expressions of vimentin and glutamine synthetase (GS) in MG; C: Flow cytometric analysis of characteristic cell markers of MGs. Blue curves represent isotype controls for fluorescein isothiocyanate (FITC) and red curves represent measured markers (vimentin and GS,respectively). This experiment was repeated triplicate independently.
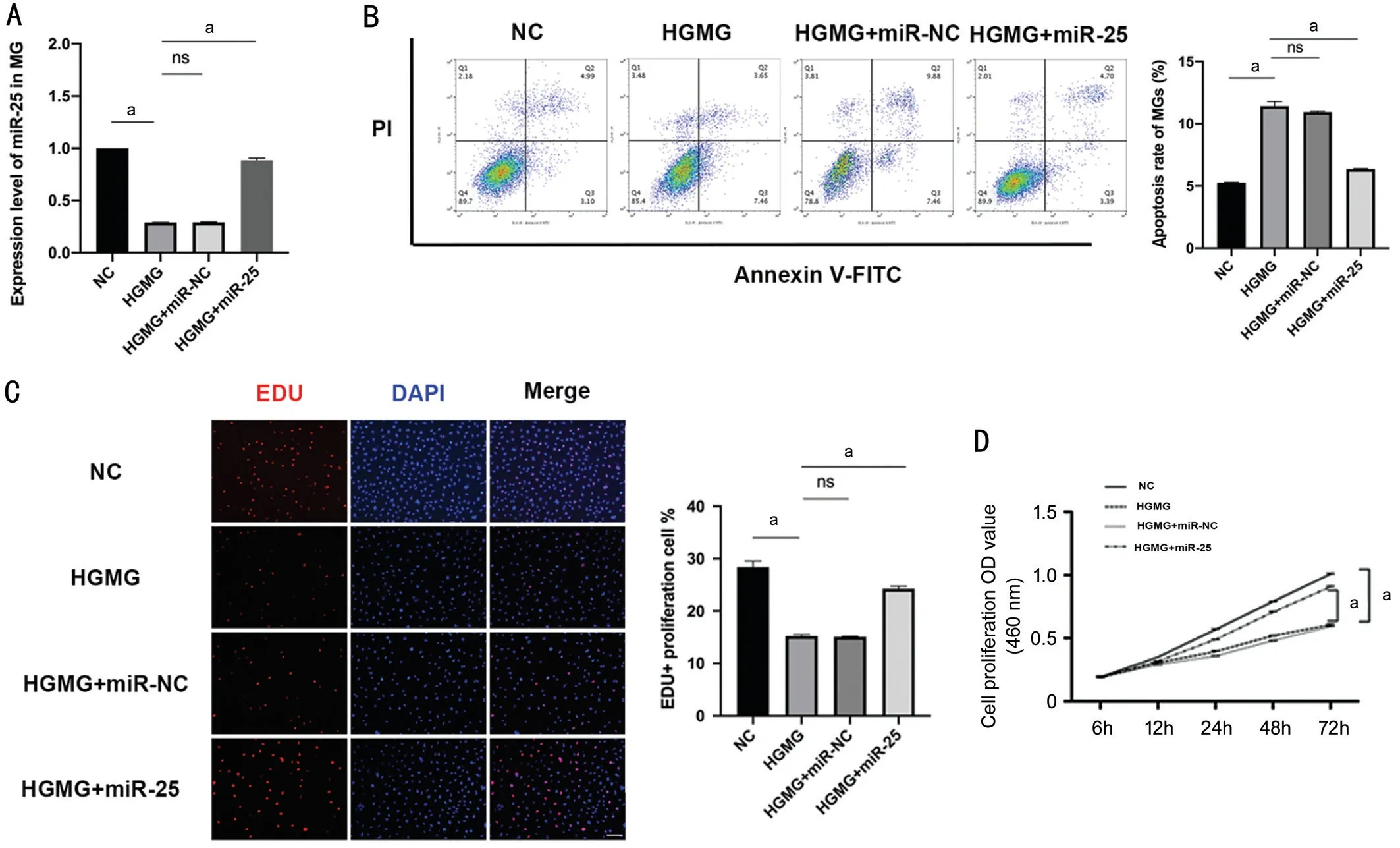
Figure 2 miR-25 attenuated HG-induced injury on MG A: RT-qPCR results showed that high glucose medium could significantly downregulated the expression level of miR-25 in MG, while 100 nmol/L miR-25 mimics was efficient to overexpress it; B: FACS results showed that miR-25 overexpression attenuated HG-induced MGs apoptosis; C and D: EdU staining and CCK8 assay both verified that miR-25 rescued MGs proliferation to a basal level. Scale bar=100 μm. aP<0.001, n=3.
miR-25 Alleviated HG-induced Müller Glia InjuryviamiR-25 Targeting Fbxw7Fbxw7, which encodes F-box and WD repeat domain-containing 7 protein, was characterized as the target of miR-25 based on two datasets (miRbase, and miRTarBase; Figure 3A). Dual luciferase report assay was conducted to investigate whether miR-25 directly targeted Fbxw7. Co-transfecting miR-25 and 3’UTR-Fbxw7-WT vectors significantly decreased Fbxw7 luciferase activity, while 3’UTR- Fbxw7-MUT did not affect the luciferase activity(Figure 3B). To determine whether above effects on MGs depend specifically on Fbxw7 suppression, we overexpressed Fbxw7. Western blot results showed greater expression of Fbxw7 in HG group than in miR-25 overexpression group(Figure 4A). FACS results showed that miR-25 overexpression attenuated HG-induced MGs apoptosis (Figure 4B). EdU staining as well as CCK8 assay showed that miR-25 mimics could protect MGs from HG-induced injury (Figure 4C and 4D). Our data suggest that after miR-25 overexpression, a decrease in Fbxw7 is essential for MGs to show increased proliferation and less apoptosis in high glucose medium(Figure 4B-4D).

Figure 3 Fbsw7 was characterized as the target of miR-25 A: Predicted duplex formation between mouse Fbsw7 3’UTR and miR-25. Seven genes were recognized as miR-25 targets verified by two different datasets (miRDB and miRTarBase). B: Dual luciferase reporter assay on Fbsw7-WT or Fbsw7-MUT transfected with NC or miR-25 mimics to explore the possible regulation machanisms. The luciferase activities were measured 48h later. aP<0.001, n=3.

Figure 4 miR-25 attenuated HG-induced MG injury via miR-25 downregulated Fbxw7 expression A: Western blot verified that the protein level of Fbxw7 was decreased in the miR-25 overexpression group (HGMG+miR-25) compared with HGMG group; B: Cell apoptosis were detected by FACS; C, D: Cell proliferation of MGs after PBS, HGMG, HGMG+miR-25 and HGMG+miR-25+Fbsw7 administration were measured by EDU staining and CCK8 assay. Scale bar=100 μm. aP<0.001, n=3.
DISCUSSION
The miRNAs play important roles in regulating the complex physiological and pathological processes by inhibiting the transcription and translation of the target mRNAs or promote its degradation by splicing the target mRNAs[13-14]. Numerous studies indicated the significant role of miRNAs in regulating the onset and progression of diabetes and its complications[15].However, few studies have yet reported the elusive relationship between miRNA levels and the pathological development of DR. In our study, we found that high glucose notably decreased the expression level of miR-25 in MG, indicating its potential role in modulating MG damages in DR. Furthermore,overexpression of miR-25 by miR-25 mimics (100 nmol/L)attenuated apoptosis and improved proliferation in MG under HG condition. Findings from our current study suggest that further investment in miR-25 may serve as a novel biomarker for the early detection of DR and shed light on the treatment for DR.
Ninget al[16]once reported that miR-25-3p could enhance the cell proliferation and migration of mouse glioma cells, impair tumorigenesis and regulate the invasion of gastric cancer cells.Also, miR-25-3p was verified to regulate cancer cell processes by targeting DKK3 and Fbsw7 genesviarelated pathways[17].Multiple studies have discussed the role of miR-25 in ocular diseases. Zhanget al[18]once reported that the increased miR-25 was confirmed to regulate the degeneration of retinal pigment epithelium in retinal degeneration disease. A previous research conducted by Wohlet al[8]demonstrated that increasing the level of miR-25 with a specific mimic causes an increase in MG-derived neuronal cells which means miR-25 may have a general role in maintaining the neural progenitor phenotype. Based on our data, miR-25 mimic was proven to protect MGs from high glucose induced damagesviadownregulating Fbxw7, making maintaining the miR-25 expression a useful strategy in preventing MGs death associated with DR.MGs are responsible for a broad variety of significant functions including uptake and recycling of neurotransmitters,maintaining the water balance, ionic environment, and supply of nutrients for the retinal neurons[19-20]. As the major glial cell type in the retina, MGs are closely related to DR[21-23]. To date,numerous studies indicate that dysfunction of the retinal MGs precedes the microvascular dysregulation, especially in early stage of DR[24]. Our results clearly showed that Müller cells are damaged in high glucose. Treatment of 25 mmol/L glucose resulted in a significant decrease in proliferation and increase in apoptosis compared with normal glucose. The apoptosis of MGs could initiate oxidative stress, producing reactive oxygen species[25].
To conclude, our current data demonstrate the function of miR-25 as a protective miRNA in MGs against HG-induced injury, while the specific mechanism of miR-25 exerting a rescue effect on MGs needs to be further investigated.
ACKNOWLEDGEMENTS
We thank colleagues in Jiangsu Province Hospital Core Facility Center for critical input and suggestions.
Foundation:Supported by National Key Research and Development Program of China (No.2017YFA0104101).
Conflicts of Interest:Liu Y,None;Shen H,None;Yuan ST,None;Liu QH,None.
 International Journal of Ophthalmology2021年5期
International Journal of Ophthalmology2021年5期
- International Journal of Ophthalmology的其它文章
- Comprehensive evaluation of intravitreal conbercept versus half-dose photodynamic therapy for chronic central serous chorioretinopathy
- Lipid accumulation and protein modifications of Bruch’s membrane in age-related macular degeneration
- Via pars plana anterior iris enclavation lOL fixation
- Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HlF-1/VEGFA pathway and promoting PEDF expression
- Expression levels of pro-inflammatory interleukin-8 and certain antimicrobial peptides in concurrent with bacterial conjunctivitis
- lris manipulation during phacoemulsification: intraoperative and postoperative complications
