Associations of lens thickness and axial length with outcomes of laser peripheral iridotomy
Ya-Meng Liu, Die Hu, Long-Fang Zhou, Jie Lan, Cheng-Cheng Feng, Xiao-Yun Wang,Xiao-Jing Pan
1Weifang Medical University, Weifang 261021, Shandong Province, China
2State Key Laboratory Cultivation Base, Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute,Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao 266071, Shandong Province,China
3Qingdao Eye Hospital of Shandong First Medical University,Qingdao 266071, Shandong Province, China
Abstract
● KEYWORDS: laser peripheral iridotomy; primary angleclosure glaucoma; lens thickness; axial length; anterior chamber angle metrics
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness disease in the world. It is characterized by progressive optic nerve atrophy and visual impairment. In China, primary angle-closure glaucoma (PACG) accounts for the majority of patients with glaucoma[1]. The stenosis or closure of the anterior chamber angle plays an important role in the elevation of intraocular pressure (IOP). Laser peripheral iridotomy(LPI), as a common treatment for primary angle closure (PAC)or PACG, could remove pupil block, widen angle, and reduce IOP. Many studies have confirmed the effectiveness of LPI[2-4]. However, LPI cannot completely control the progression of glaucoma[5]. Therefore, it is important to evaluate the postoperative effect after LPI. For patients with poor postoperative effect, other methods could control IOP and improve the success rate of glaucoma treatment. Patients with PAC generally have some abnormal anatomic characteristics such as thick lens, short axis, or forward lens[6]. Whether one eye with smaller lens, longer axis length and backward lens is better effective after LPI? The purpose of this study was to analyze the association of lens thickness (LT) and axial length (AL) with postoperative of LPI, and examine the factors associated with the widening of the angle.
SUBJECTS AND METHODS
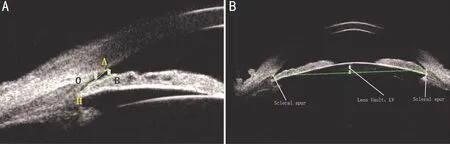
Figure 1 Parameter measurement of anterior chamber angle in UBM images A: Measurement of AOD, TIA, and ARA. O: Scleral spur; H:Deepest point of angle recess; AO: 750 μm; AOD: The length of AB (AO perpendicular to AB); TIA: The size of angle AOB; ARA: The area of A-H-B. B: Measurement of LV [the distance between the midpoint of bilateral scleral process and the intersection point (intersected by vertical line through the midpoint and the anterior surface of the lens)].
Ethical ApprovalThis study was approved by the Qingdao Eye Hospital, and was performed in accordance with the principles of the Declaration of Helsinki. All of the subjects signed informed consent.
SubjectsPatients diagnosed as primary angle-closure suspect(PACS), PAC or PACG at Qingdao Eye Hospital from May 2017 to December 2017 were recruited into this study.PACS was defined as one eye having at least 180 degrees iridotrabecular contact without visible pigmented trabecular meshwork by gonioscope examination at static state and without elevated intraocular pressure. PAC was defined as a PACS patient with IOP>21 mm Hg, and without optic nerve impairment. PACG was defined a PAC patient with optic nerve impairment[1]. Subjects were excluded if they were: 1) patients with eye operation history; 2) patients with eye trauma history;3) patients with dislocated lens, ciliary body cysts, or iris cysts;4) patients with iris atrophy or pupil stiffness induced by acute angle-closure glaucoma; 5) patients with retina or vitreous diseases or corneal diseases; 6) patients with uncontrolled severe diabetes mellitus and hypertension.
Clinical AssessmentAll subjects underwent a standardized ophthalmic examination at baseline. AL, anterior central chamber depth (ACD), and LT were obtained by the LENSTAR eye biometer from Haag-Streit before and 1wk after LPI. All subjects were examed by one skilled examination physician under the same condition of natural light illumination. Every eye was examined 3 times and the avarage value of AL, ACD,and LT were chosen for analysis.
Ultasound BiomicroscopyThe subjects were examined by a panoramic ultrasound biomicroscope SW-3200L (probe frequency 35 MHz, axial and lateral resolution ≤40 micron)produced by Tianjin Electronic Technology Co., Ltd. All examinations were performed by the same skilled examination physician under the same lighting environment. The patient lay on the examination bed. The appropriate eyecup loaded with saline solution was placed into eye after surface anaesthesia.To capture the image of central anterior, upper, lower, left, and right angles, patients rotated their eyeball forward, upward,downward, leftward, and rightward, respectively. The central anterior chamber collected horizontal and vertical images. The ultasound biomicroscopy (UBM) examination was performed before and 1wk after LPI.
Parameter Measurement of Anterior Chamber AngleImage J software was used to process images obtained from UBM (Figure 1). A circle was draw with scleral process(defined as the point O) as center and 750 μm as radius.The intersection point with the corneal endothelium defined as point A. The perpendicular line of AO intersected front surface of iris at point B. The length of AB was defined as the angle opening distance (AOD), and the size of angle AOB was defined as the trabecular iris angle (TIA). The area of the triangle composed of point H (deepest point of angle recess), the area of A-H-B was defined as the angle recess area (ARA; Figure 1A). All the tested eyes were measured in four directions (inferior, superior, nasal and temporal), and the average value was taken as the parameter value of chamber angle. The UBM images with eyes looking straight ahead were taken to determine the bilateral scleral process. The distance between the midpoint of bilateral scleral process and the intersection point (intersected by vertical line through the midpoint and the anterior surface of the lens) was defined as lens vault (LV). The values from horizontal and vertical images were averaged as the results (Figure 1B).
Laser Peripheral IridotomyLPI was performed with VISULAS YAG III laser (Zeiss, German) by a skilled physician. The 0.05% jaborandi alkali dropped eyes five times(every 5min). After miosis without light reflex, oxybuprocaine hydrochloride dropped eyes 3 times for local anesthesia,and then placed contact lens. Following laser beam vertical to contact lens, LPI was performed on the weak area of the iris. Following LPI, diclofenac sodium eyedrops and 0.1%flumilong eyedrops were applied to the eyes 4 times a day for 1wk. The patients were re-examined one day and one week after LPI to avoid complications such as anterior chamber bleeding and lens dislocation.
Statistical AnalysisAll data were analyzed using SPSS 20.0 statistical analysis software, and were expressed by mean±standard deviation (AL, LT, LV, ACD, AOD, TIA,ARA). A paired two-tailedttest followed by testing the normal distribution to assess the statistical significance. The difference values between the postoperative and preoperative parameter were defined as ΔACD, ΔAOD, ΔTIA, ΔARA, respectively.The difference values of PACS, PAC, and PACG groups were analyzed by one-way ANOVA. Multivariate regression analysis was performed to assess the effect of AL, LT, LV on ΔACD, ΔAOD, ΔTIA, and ΔARA. If the results had no statistically significant, AL was divided into group≥22.8 mm and group<22.8 mm, LT was divided into group≥4.7 mm and group<4.7 mm, and LV was divided into group≥1 mm and group<1 mm. Evaluating the effects of AL, LT and LV on ΔACD, ΔAOD, ΔTIA and ΔARA was performed by analyzing the changes of ΔACD, ΔAOD, ΔTIA, ΔARA between different groups.P<0.05 was considered significant.
RESULTS
A total of 69 patients (97 eyes) were included in this study. No postoperative complications such as anterior angle hemorrhage or lens dislocation occurred. All patients successfully complete the examination of AL, ACD, and LT. Some UBM results were removed due to unsatisfied eye position or unclear images, and thus 84 UBM images were analyzed in the end.
Sixty-eight eyes were diagnosed as PACS, 16 eyes were diagnosed as PAC, and 13 eyes were diagnosed as PACG. The mean age was 62.31±9.03 years old.
There is no statistically significant of ΔACD, ΔAOD, ΔTIA or ΔARA between PACS, PAC, and PACG (Table 1). Therefore,the data of anterior angle parameters were analyzed as a whole and not grouped in the subsequent analysis. The observation indexes before and after LPI were shown in Table 2.
The effect of AL, LT, LV on ΔACD were shown in Tables 3-5. Table 3 showed that LT was the influence factor of ΔACD (P=0.048), and the regression coefficients was -0.078.Compared with group LT<4.7 mm, ΔACD reduced by -0.078 mm in group LT≥4.7 mm. Tables 3 and 4 showed that there is no statistically significant of ΔACD between different AL group.AL was not the influence factors of ΔACD (P>0.05).
Table 5 showed that there is no statistically significant of ΔACD between different LV group. LV was not the influence factor of ΔACD (P>0.05).
The effects of LT on the angle parameters was shown in Table 6.Compared with group<4.7 mm, ΔAOD, ΔTIA, and ΔARA in group ≥4.7 mm all significantly increased (P<0.05). Thus, LT was the influence factors of ΔAOD, ΔTIA, and ΔARA.The effects of AL on the angle parameters was shown in Table 7. There was no statistically significant of ΔAOD,ΔTIA or ΔARA between different AL group. AL was not the influence factors of ΔACD.
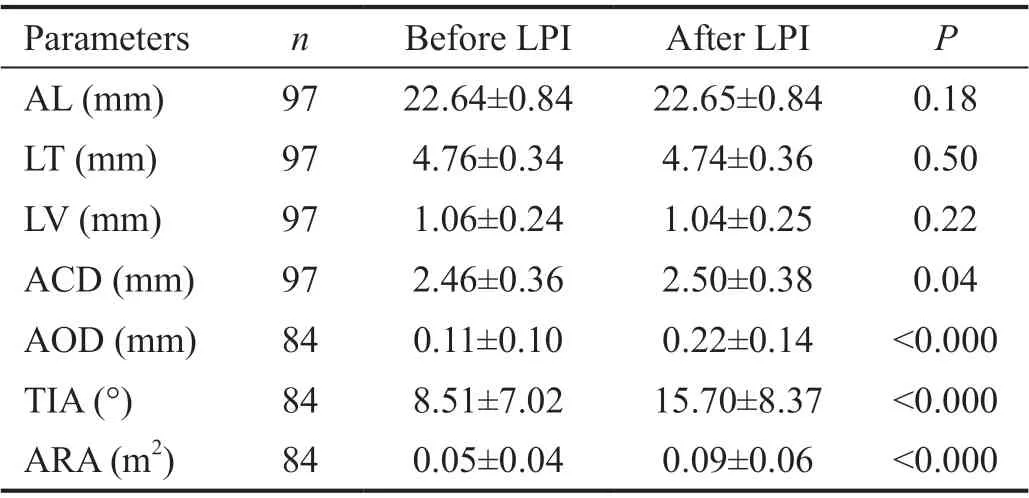
Table 2 Parameter of anterior segment morphology before and after LPI

Table 3 Linear regression analysis of ΔACD

Table 4 Comparison of ΔACD between different AL group

Table 5 Comparison of ΔACD between different LV group
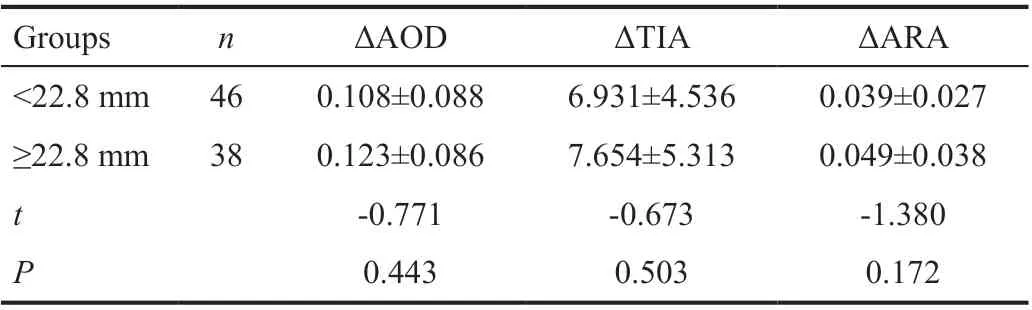
Table 7 Comparison of ΔAOD, ΔTIA or ΔARA between different AL group
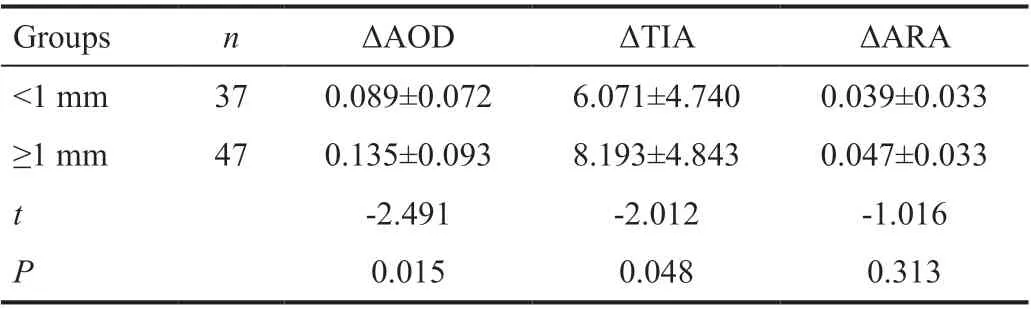
Table 8 Comparison of ΔAOD, ΔTIA or ΔARA between different LV group
The effects of LV on the angle parameters was shown in Table 8. Compared with group<1 mm, ΔAOD and ΔTIA in group≥1 mm were significantly increased (P<0.05). Thus, LV was the influence factors of ΔAOD and ΔTIA. There was no statistically significant of ΔARA between different LV group.LV was not the influence factors of ΔARA.
DISCUSSION
We previously demonstrated that the anterior chamber angle metrics such as ACD, AOD, TIA, and ARA were significantly increased after LPI. Through correlation analysis, we confirmed that greater LT and LV were associated with greater increases in anterior segment biometric parameters after LPI,while AL had no effect on postoperative outcomes.
PACG is an eye disease with progressive closure of anterior chamber angle and progressive IOP increase that ultimately leads to optic nerve impairment. It is classified into three categories according to epidemiological factors which is PACS, PAC, and PACG[1]. Most of PACG are characterized by shallow ACD, short AL, thick LT or large LV[6]. LV represents the degree of lens protrusion to anterior chamber in sclera level and the degree of pupillary block[6]. LPI is able to relieve pupillary block, widen angles, and thus has been accepted by most ophthalmologists as a treatment for all patients with narrow angles. In this study, all patients were subjected to LPI.The ACD, AOD, TIA, and ARA were all significantly increased compared with pre-operation, indicating the effectiveness of LPI in widening angles. No significant changes were observed in AL and LT after surgery, indicating almost no effect of LPI on the anatomic structure of eyeball and lens. The LV was slightly reduced without statistic significant, suggesting no substantial change in the relative position of lens following LPI[7]. There was no statistical difference of angle parameters between group PACS, PAC, and PACG, which showed that angle widen induced by LPI was not different in the three groups. These were similar to the results of Anget al’s study[7].Therefore, the data of anterior angle parameters were analyzed as a whole and not grouped in the subsequent analysis.
However, LPI cannot cure all the patients with narrow angles.At least one quadrant residual angle closure was observed in 30%-50% patients with PAC at one month after LPI[8-9].Long-term observation showed that the percentage of angle closure in two quadrants or above is as high as 81.8%[10]. Other therapeutic methods instead of LPI may be more effective for these patients with poor postoperative outcomes. Thus, it is necessary to predict postoperative LPI effect. In this study, we analyzed the association between AL, LT, LV and ACD, AOD,TIA, ARA by comparing the differences between preoperative and postoperative anterior angle parameters, and investigated whether AL, LT or LV could affect postoperative outcomes of LPI.
Our study suggested that LT could significantly affect the opening degree of angle after LPI. Compared with LT<4.7 mm group, the postoperative outcomes of LPI was more better in group with≥4.7 mm. Previous studies showed that age and iris curvature were the influencing factors of LPI, and both were positively correlated with postoperative angle[11]. Besides,studies also showed that postoperative angle significantly increased in patients with thinner iris thickness[12]. Moghimiet al[13]found that AOD was the influencing factor of LPI.Although the above studies and the current study have different observation, there are closely relationship. As the age increases, the thickness of the lens gradually increase[14]. The thickened lens moves forward the iris, resulting in the increase in iris curvature, more narrow of the anterior chamber angle,and the occurrence of pupil block. After LPI, the factor of pupil block is relieved, the pressure of the anterior and posterior chambers is balanced, the iris is more flat, and thus the opening degree of angle is larger[15].
LV represents the degree of lens protuberating into anterior chamber at scleral spur level. It is significantly increased in patients with PACS, PAC, and PACG than in normal subjects.The increased LV induces the forward protrusion of lens, the increase in contact area of iris and lens, the decrease in gap between iris and lens, and severity pupil block[16]. LPI is able to relieve pupillary block. AOD is significantly increased in patients with larger LV following LPI. Our study reflected that:the larger LV, the larger ΔAOD and ΔTIA. Previous studies also showed similar results[3,17]. However, the change of LV has nothing to do with ΔACD and ΔARA. The reason is that LPI could significantly widen the depth of the peripheral anterior chamber compared with the central anterior chamber[18].ΔAOD and ΔTIA are more sensitive than ΔARA, which result in LV has no significate correlation with ΔARA.In this study, AL had no effect on the parameters of the anterior angles following LPI, which was consistent with previous studies[13,17]. Therefore, AL does not affect the postoperative outcomes of LPI.
Our study had limitations. One limitation was the small sample size. More samples are necessary to further improve the reliability of the analysis. The second limitation was no performance of subgroups between objects. The third limitation was that the change of IOP was not analyzed in the study.
In conclusion, LPI widen the angle of the anterior chamber by removing the pupillary block. LPI may be more effective in patients with thicker LT or larger LV.
ACKNOWLEDGEMENTS
Foundations:Supported by the Natural Science Foundation of Shandong Province (No.ZR2020MH172); the Demonstration and Guidance Project of Science and Technology Benefiting People in Qingdao (No.20-3-4-39-nsh).
Conflicts of Interest:Liu YM,None;Hu D,None;Zhou LF,None;Lan J,None;Feng CC,None;Wang XY,None;Pan XJ,None.
 International Journal of Ophthalmology2021年5期
International Journal of Ophthalmology2021年5期
- International Journal of Ophthalmology的其它文章
- Comprehensive evaluation of intravitreal conbercept versus half-dose photodynamic therapy for chronic central serous chorioretinopathy
- Lipid accumulation and protein modifications of Bruch’s membrane in age-related macular degeneration
- Via pars plana anterior iris enclavation lOL fixation
- Role of microRNA-25 in high glucose cultured Müller glia
- Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HlF-1/VEGFA pathway and promoting PEDF expression
- Expression levels of pro-inflammatory interleukin-8 and certain antimicrobial peptides in concurrent with bacterial conjunctivitis
